Pain Evaluation and Treatment in Children: A Practical Approach
Abstract
1. Introduction
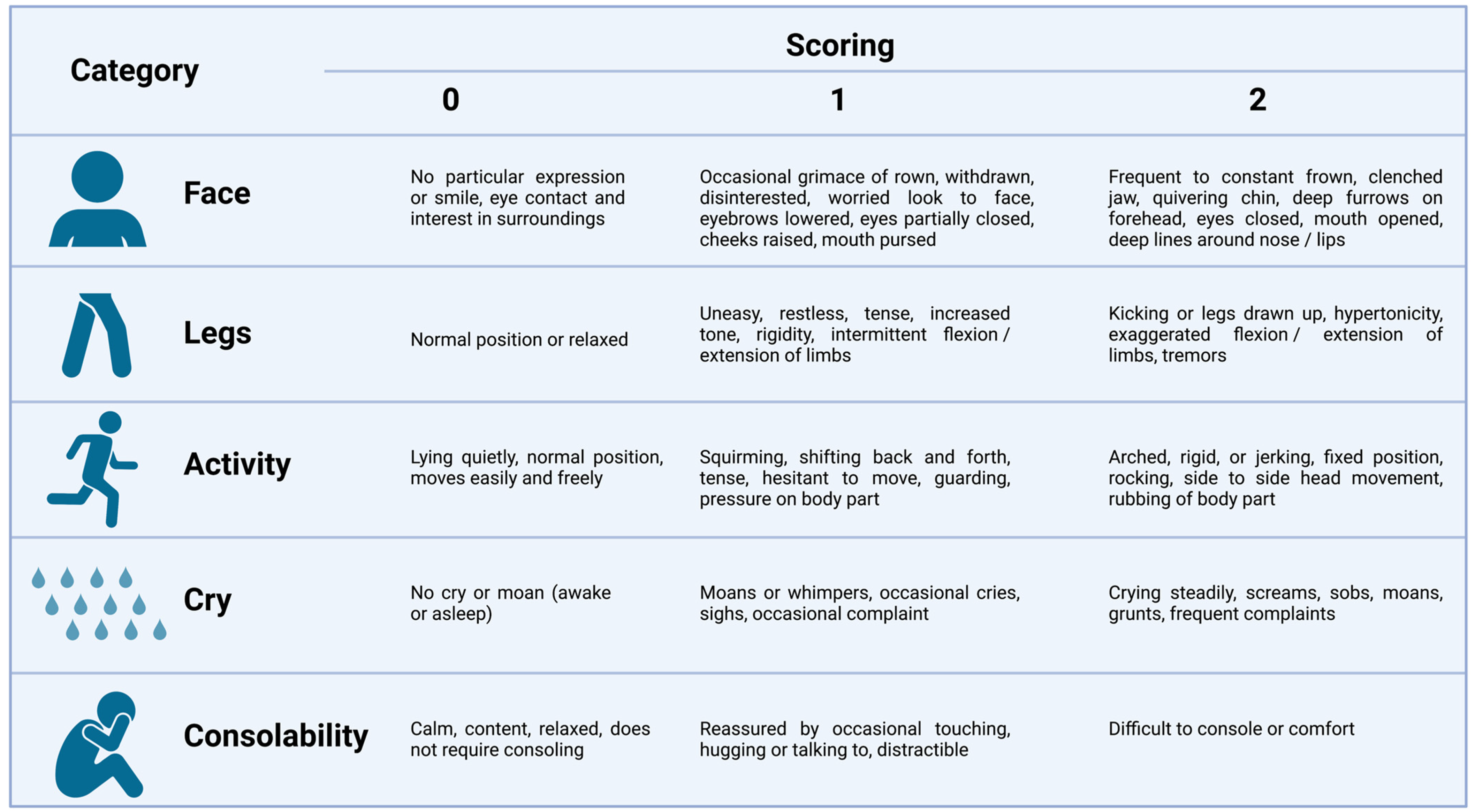
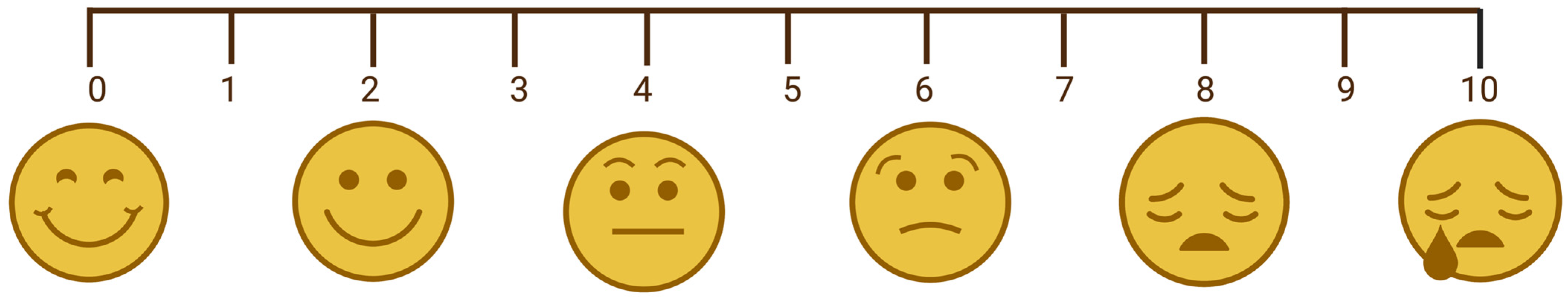
2. Pain Assessment
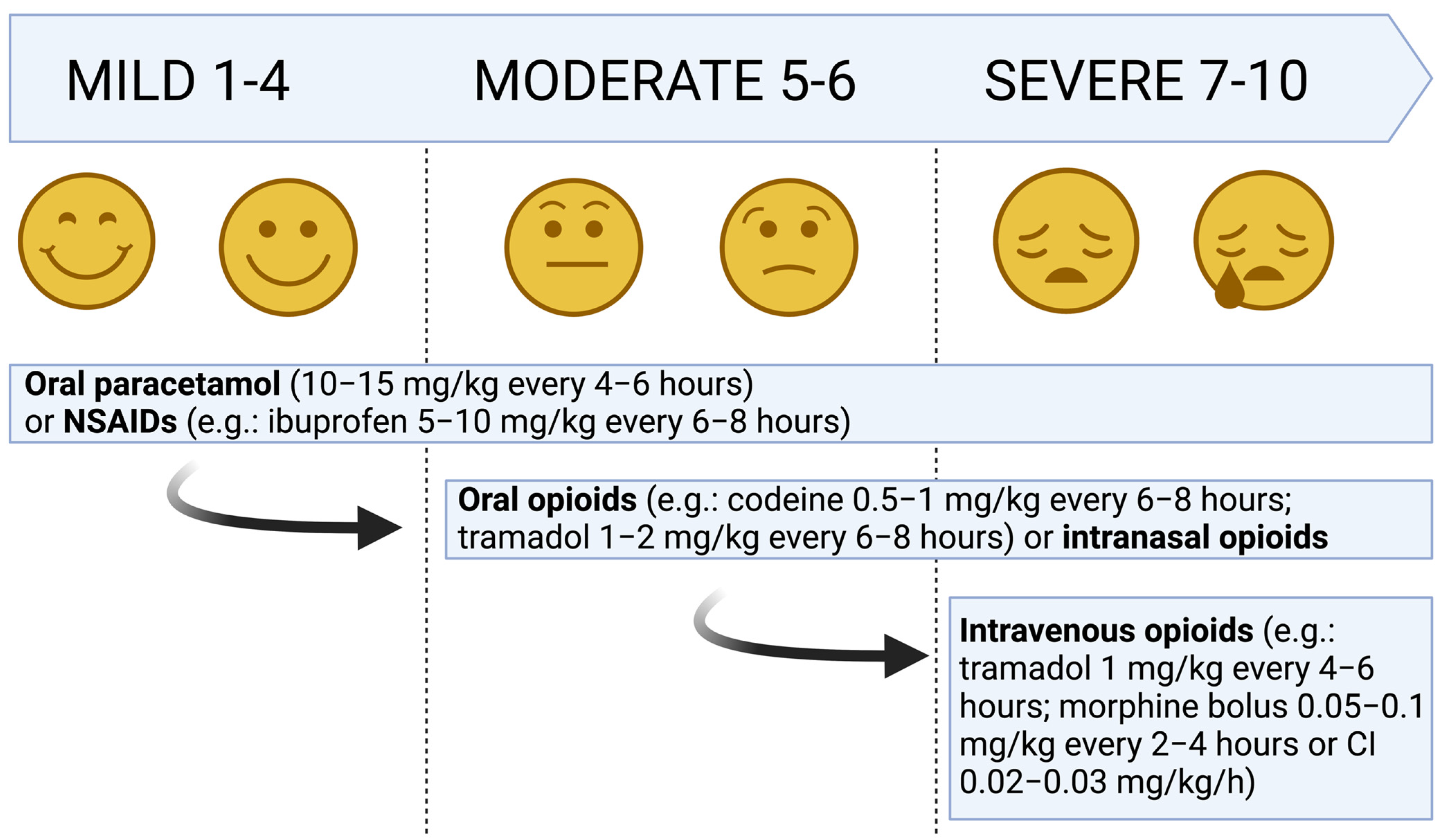
3. Pharmacological Treatment of Acute Pain
3.1. Acetaminophen
3.2. NSAIDs
3.2.1. Ibuprofen
3.2.2. Naproxen
3.2.3. Diclofenac
3.2.4. Ketoprofen
3.2.5. Ketorolac
3.3. Opioids
3.3.1. Morphine
3.3.2. Codeine
3.3.3. Fentanyl
3.3.4. Tramadol
3.3.5. Ketamine
3.4. Nitrous Oxide
4. Non-Pharmacological Treatment of Pain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krauss, B.S.; Calligaris, L.; Green, S.M.; Barbi, E. Current Concepts in Management of Pain in Children in the Emergency Department. Lancet 2016, 387, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Fisher, E.; Howard, R.F.; Slater, R.; Forgeron, P.; Palermo, T.M.; Birnie, K.A.; Anderson, B.J.; Chambers, C.T.; Crombez, G.; et al. Delivering Transformative Action in Paediatric Pain: A Lancet Child & Adolescent Health Commission. Lancet Child. Adolesc. Health 2021, 5, 47–87. [Google Scholar] [CrossRef]
- Mak, W.Y.; Yuen, V.; Irwin, M.; Hui, T. Pharmacotherapy for Acute Pain in Children: Current Practice and Recent Advances. Expert. Opin. Pharmacother. 2011, 12, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The Sensors of the Pain Pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Djouhri, L.; Lawson, S.N. Abeta-Fiber Nociceptive Primary Afferent Neurons: A Review of Incidence and Properties in Relation to Other Afferent A-Fiber Neurons in Mammals. Brain Res. Brain Res. Rev. 2004, 46, 131–145. [Google Scholar] [CrossRef]
- Zeltzer, L.K.; Bush, J.P.; Chen, E.; Riveral, A. A Psychobiologic Approach to Pediatric Pain: Part 1. History, Physiology, and Assessment Strategies. Curr. Probl. Pediatr. 1997, 27, 225–253. [Google Scholar] [CrossRef]
- Garland, E.L. Pain Processing in the Human Nervous System: A Selective Review of Nociceptive and Biobehavioral Pathways. Prim. Care 2012, 39, 561–571. [Google Scholar] [CrossRef]
- Zieliński, J.; Morawska-Kochman, M.; Zatoński, T. Pain Assessment and Management in Children in the Postoperative Period: A Review of the Most Commonly Used Postoperative Pain Assessment Tools, New Diagnostic Methods and the Latest Guidelines for Postoperative Pain Therapy in Children. Adv. Clin. Exp. Med. 2020, 29, 365–374. [Google Scholar] [CrossRef]
- van Dijk, M.; de Boer, J.B.; Koot, H.M.; Tibboel, D.; Passchier, J.; Duivenvoorden, H.J. The Reliability and Validity of the COMFORT Scale as a Postoperative Pain Instrument in 0 to 3-Year-Old Infants. Pain 2000, 84, 367–377. [Google Scholar] [CrossRef]
- Wilson, G.A.; Doyle, E. Validation of Three Paediatric Pain Scores for Use by Parents. Anaesthesia 1996, 51, 1005–1007. [Google Scholar] [CrossRef]
- Suraseranivongse, S.; Kaosaard, R.; Intakong, P.; Pornsiriprasert, S.; Karnchana, Y.; Kaopinpruck, J.; Sangjeen, K. A Comparison of Postoperative Pain Scales in Neonates. Br. J. Anaesth. 2006, 97, 540–544. [Google Scholar] [CrossRef]
- Gaglani, A.; Gross, T. Pediatric Pain Management. Emerg. Med. Clin. N. Am. 2018, 36, 323–334. [Google Scholar] [CrossRef]
- Bailey, B.; Trottier, E.D. Managing Pediatric Pain in the Emergency Department. Paediatr. Drugs 2016, 18, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Hachimi-Idrissi, S.; Coffey, F.; Hautz, W.E.; Leach, R.; Sauter, T.C.; Sforzi, I.; Dobias, V. Approaching Acute Pain in Emergency Settings: European Society for Emergency Medicine (EUSEM) Guidelines-Part 1: Assessment. Intern. Emerg. Med. 2020, 15, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J. Paracetamol (Acetaminophen): Mechanisms of Action. Paediatr. Anaesth. 2008, 18, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Gibb, I.A.; Anderson, B.J. Paracetamol (Acetaminophen) Pharmacodynamics: Interpreting the Plasma Concentration. Arch. Dis. Child. 2008, 93, 241–247. [Google Scholar] [CrossRef]
- Cuzzolin, L.; Antonucci, R.; Fanos, V. Paracetamol (Acetaminophen) Efficacy and Safety in the Newborn. Curr. Drug Metab. 2013, 14, 178–185. [Google Scholar]
- Close, R.; Bale, P.; Armon, K. Use of Non-Steroidal Anti-Inflammatory Drugs in Paediatrics. Arch. Dis. Child. Educ. Pract. Ed. 2021, 106, 47–52. [Google Scholar] [CrossRef]
- Poddighe, D.; Brambilla, I.; Licari, A.; Marseglia, G.L. Ibuprofen for Pain Control in Children: New Value for an Old Molecule. Pediatr. Emerg. Care 2019, 35, 448–453. [Google Scholar] [CrossRef] [PubMed]
- de Martino, M.; Chiarugi, A.; Boner, A.; Montini, G.; De’ Angelis, G.L. Working Towards an Appropriate Use of Ibuprofen in Children: An Evidence-Based Appraisal. Drugs 2017, 77, 1295–1311. [Google Scholar] [CrossRef] [PubMed]
- Korpela, R.; Silvola, J.; Laakso, E.; Meretoja, O.A. Oral Naproxen but Not Oral Paracetamol Reduces the Need for Rescue Analgesic after Adenoidectomy in Children. Acta Anaesthesiol. Scand. 2007, 51, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.F. Pain Management in Children: NSAID Use in the Perioperative and Emergency Department Settings. Paediatr. Drugs 2021, 23, 361–372. [Google Scholar] [CrossRef]
- Stone, S.B. Ketorolac in Postoperative Neonates and Infants: A Systematic Review. J. Pediatr. Pharmacol. Ther. 2021, 26, 240–247. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Mastrangelo, S.; Capozza, M.A.; Triarico, S.; Attinà, G.; Maurizi, P.; Romano, A.; Ruggiero, A. Opioid Transdermal Delivery System: A Useful Method for Pain Management in Children. Ann. Transl. Med. 2021, 9, 185. [Google Scholar] [CrossRef]
- Rodieux, F.; Piguet, V.; Desmeules, J.; Samer, C.F. Safety Issues of Pharmacological Acute Pain Treatment in Children. Clin. Pharmacol. Ther. 2019, 105, 1130–1138. [Google Scholar] [CrossRef]
- Rieder, M.J.; Jong, G.’t. The Use of Oral Opioids to Control Children’s Pain in the Post-Codeine Era. Paediatr. Child. Health 2021, 26, 120–127. [Google Scholar] [CrossRef]
- Duedahl, T.H.; Hansen, E.H. A Qualitative Systematic Review of Morphine Treatment in Children with Postoperative Pain. Paediatr. Anaesth. 2007, 17, 756–774. [Google Scholar] [CrossRef]
- Wille, C.; Bocquet, N.; Cojocaru, B.; Leis, A.; Chéron, G. Oral morphine administration for children’s traumatic pain. Arch. Pediatr. 2005, 12, 248–253. [Google Scholar] [CrossRef]
- Poonai, N.; Bhullar, G.; Lin, K.; Papini, A.; Mainprize, D.; Howard, J.; Teefy, J.; Bale, M.; Langford, C.; Lim, R.; et al. Oral Administration of Morphine versus Ibuprofen to Manage Postfracture Pain in Children: A Randomized Trial. CMAJ 2014, 186, 1358–1363. [Google Scholar] [CrossRef]
- Thigpen, J.C.; Odle, B.L.; Harirforoosh, S. Opioids: A Review of Pharmacokinetics and Pharmacodynamics in Neonates, Infants, and Children. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 591–609. [Google Scholar] [CrossRef]
- Wong, C.; Lau, E.; Palozzi, L.; Campbell, F. Pain Management in Children: Part 2—A Transition from Codeine to Morphine for Moderate to Severe Pain in Children. Can. Pharm. J. (Ott) 2012, 145, 276–279.e1. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; O’Sullivan, R.; Wakai, A.; Grant, T.S.; Barrett, M.J.; Cronin, J.; McCoy, S.C.; Hom, J.; Kandamany, N. Intranasal Fentanyl for the Management of Acute Pain in Children. Cochrane Database Syst. Rev. 2014, 2014, CD009942. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.D. Acute Pain Management in Infants and Children-Part 1: Pain Pathways, Pain Assessment, and Outpatient Pain Management. Pediatr. Ann. 2014, 43, e163–e168. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Silva, L.; Lee, J.Y.; Bellolio, F.; Homme, J.L.; Anderson, J.L. Intranasal Ketamine for Acute Pain Management in Children: A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 2020, 38, 1860–1866. [Google Scholar] [CrossRef]
- Burnweit, C.; Diana-Zerpa, J.A.; Nahmad, M.H.; Lankau, C.A.; Weinberger, M.; Malvezzi, L.; Smith, L.; Shapiro, T.; Thayer, K. Nitrous Oxide Analgesia for Minor Pediatric Surgical Procedures: An Effective Alternative to Conscious Sedation? J. Pediatr. Surg. 2004, 39, 495–499; discussion 495–499. [Google Scholar] [CrossRef] [PubMed]
- Babl, F.E.; Oakley, E.; Seaman, C.; Barnett, P.; Sharwood, L.N. High-Concentration Nitrous Oxide for Procedural Sedation in Children: Adverse Events and Depth of Sedation. Pediatrics 2008, 121, e528–e532. [Google Scholar] [CrossRef]
- Babl, F.E.; Oakley, E.; Puspitadewi, A.; Sharwood, L.N. Limited Analgesic Efficacy of Nitrous Oxide for Painful Procedures in Children. Emerg. Med. J. 2008, 25, 717–721. [Google Scholar] [CrossRef] [PubMed]
| Nociceptive Pain | Neuropathic Pain | ||
|---|---|---|---|
| Somatic Pain | Visceral Pain | ||
| Origin | Superficial receptors | Visceral receptors | Nerves |
| Location and distribution |
|
|
|
| Transmission | A-delta-fibers | C-fibers | Dermatomal (periphery) or non-dermatomal (central) |
| Type of pain reported | Pinprick, sharp or stabbing | Pressure, sharp or ache | Tingling, prickling, lancinating or burning |
| Acronym | Age Range |
|---|---|
| CHIPPS | Under 5 years |
| CHEOPS | 1–7 years |
| FLACC | 2 months–7 years |
| OPS and MOPS | 8 months–13 years |
| Poker Chip Tool | From 3 years |
| Oucher Scale | 3–12 years |
| Wong–Baker FACES® Pain Rating Scale | From 3 years |
| FPS-R | From 4 years |
| VAS | From 5 years |
| NRS | From 8 years |
| Route | Newborns | Children < 40 kg | Children ≥ 40 kg | Maximum Dose |
|---|---|---|---|---|
| Intravenous | 20 to 40 mg/kg/day | 7.5–15 mg/kg every 4–6 h | 325–650 mg every 4–6 h. May give up to 1000 mg in one loading dose | 75 mg/kg/day for infants 100 mg/kg/day for children < 40 kg. 3–4 g/day for ≥40 kg. |
| Oral | 25 to 30 mg/kg/day in preterms of 30 weeks of gestational age; 45 mg/kg/day in preterms of 34 weeks of gestational age; 60 mg/kg/day in term neonates | 10–15 mg/kg every 4–6 h | ||
| Rectal | Loading dose of 30 mg/kg; then, 15–20 mg/kg every 6 h |
| Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) | Route | Dose |
|---|---|---|
| Ibuprofen | Oral | 5 to 10 mg/kg, every 6–8 h |
| Naproxene | Oral | 5 to 7.5 mg/kg, every 12 h |
| Diclofenac | Oral Intravenous | 0.3 to 1 mg/kg, every 8 h 0.3 to 1 mg/kg, every 12–24 h |
| Ketoprofen | Oral Intravenous | 0.3 to 2 mg/kg, every 8–12 h 0.3 to 2 mg/kg, every 8–12 h |
| Ketorolac | Intravenous | 0.5 mg/kg, every 6–8 h |
| Class | Drug | Route | Dose |
|---|---|---|---|
| Weak opioids | Codeine | Oral/Rectal | 0.5–1 mg/kg, every 6–8 h |
| Tramadol | Oral | 1–2 mg/kg, every 6–8 h | |
| Intravenous | 1 mg/kg, every 4–6 h | ||
| Strong opioids | Morphine sulphate | Oral | 0.15–0.3 mg/kg, every 4 h |
| Morphine hydrochloride | Intravenous | Bolus: 0.05–0.1 mg/kg, every 2–4 h CI: 0.02–0.03 mg/kg/h | |
| Fentanyl | Intravenous | Bolus: 0.001–0.002 mg/kg CI: 0.001 mg/kg/h | |
| Intranasal | 0.0015–0.002 mg/kg/dose |
| Approach | Type | Indication |
|---|---|---|
| Psychological | Sharing information about procedures | Procedural pain Postoperative pain |
| Distractive techniques | Active distraction
| Procedural pain Postoperative pain |
Passive distraction
| Procedural pain Postoperative pain | |
| Non-distractive techniques | Transcutaneous Electrical Nerve Stimulation | Postoperative pain |
| Cognitive Behavioral Therapy | Postoperative pain Procedural pain | |
| Acupressure | Minor trauma | |
| Cryotherapy and heat therapy | Musculoskeletal injuries | |
| Traction and bracing | Fractures Traumas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansone, L.; Gentile, C.; Grasso, E.A.; Di Ludovico, A.; La Bella, S.; Chiarelli, F.; Breda, L. Pain Evaluation and Treatment in Children: A Practical Approach. Children 2023, 10, 1212. https://doi.org/10.3390/children10071212
Sansone L, Gentile C, Grasso EA, Di Ludovico A, La Bella S, Chiarelli F, Breda L. Pain Evaluation and Treatment in Children: A Practical Approach. Children. 2023; 10(7):1212. https://doi.org/10.3390/children10071212
Chicago/Turabian StyleSansone, Lorenzo, Cristina Gentile, Eleonora Agata Grasso, Armando Di Ludovico, Saverio La Bella, Francesco Chiarelli, and Luciana Breda. 2023. "Pain Evaluation and Treatment in Children: A Practical Approach" Children 10, no. 7: 1212. https://doi.org/10.3390/children10071212
APA StyleSansone, L., Gentile, C., Grasso, E. A., Di Ludovico, A., La Bella, S., Chiarelli, F., & Breda, L. (2023). Pain Evaluation and Treatment in Children: A Practical Approach. Children, 10(7), 1212. https://doi.org/10.3390/children10071212







