Association between Vitamin D Levels, Puberty Timing, and Age at Menarche
Abstract
:1. Introduction
2. Methods
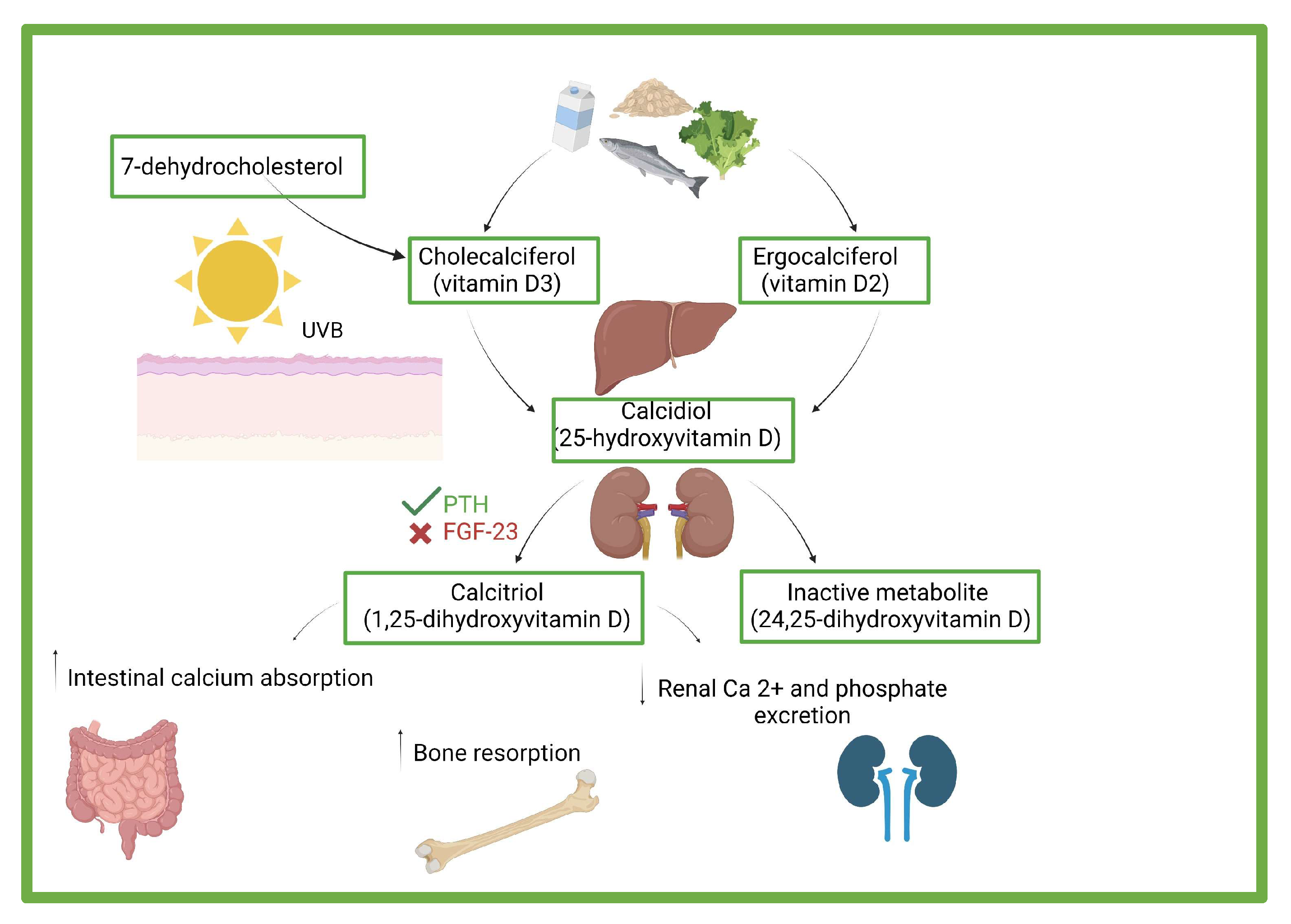
3. Vitamin D: Effects, Sources, and Deficiency
4. Assessment of Vitamin D and Risk Factors Associated to Deficiency
5. Vitamin D Levels and Precocious Puberty
6. Vitamin D Levels and Age of Menarche
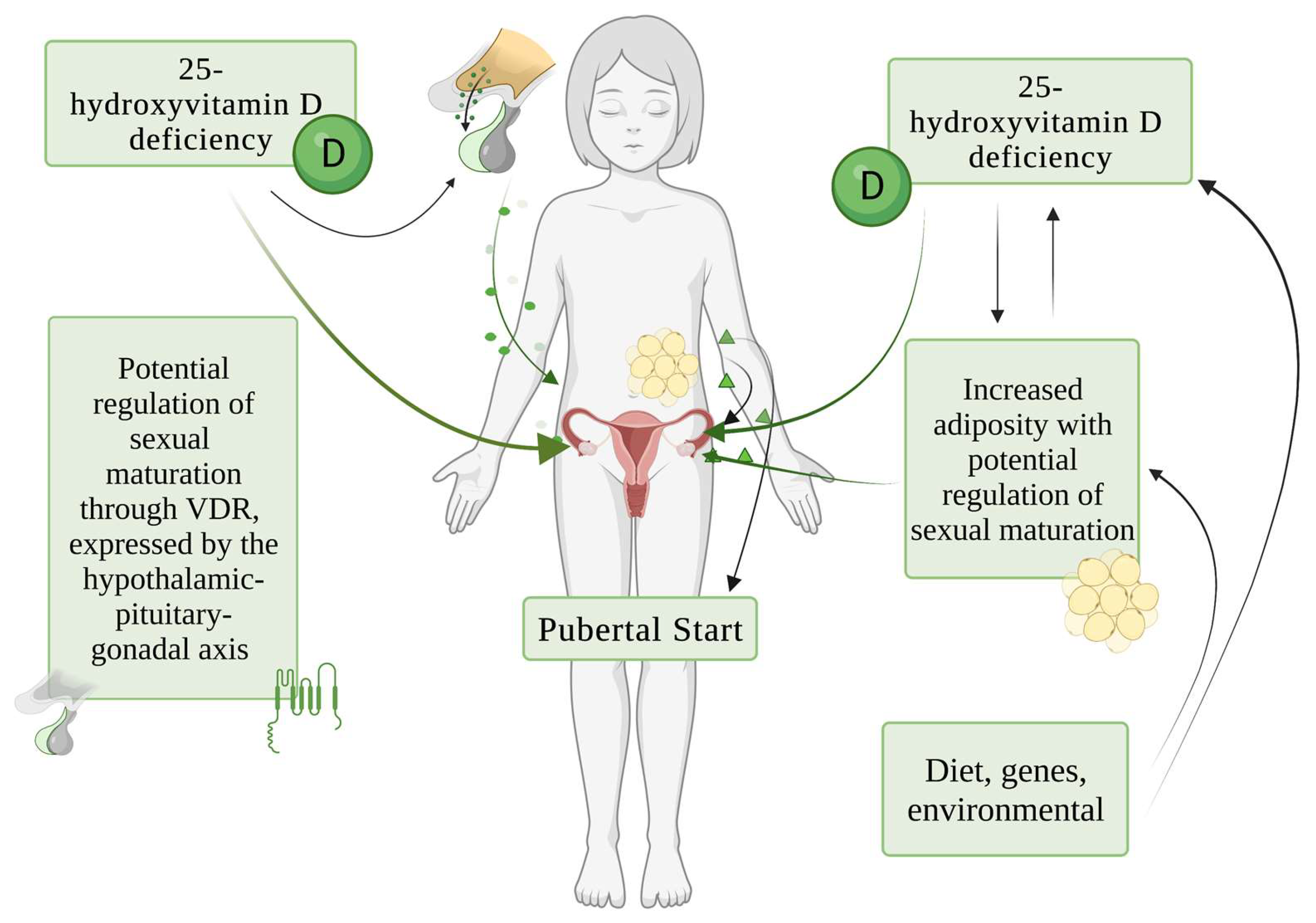
7. Potential Pathogenic Mechanisms of Role of Vitamin D in Sexual Maturation
8. Long-Term Consequences of Early Pubertal Development in Females
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abrams, S.A.; Tiosano, D. Update on vitamin D during childhood. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Gori, M.; Carlone, G.; Erba, P.; Massimetti, G.; Federico, G.; Saggese, G. Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: A cross-sectional study. Eur. J. Pediatr. 2013, 172, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Yan, F.; Cui, Y.; Song, Y.; Yan, S.; Cui, W. Does vitamin D have a potential role in precocious puberty? A meta-analysis. Food Funct. 2023, 14, 5301–5310. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, X.; Wang, Y.; Yan, S.; Li, D.; Cui, W. The association between vitamin D levels and precocious puberty: A meta-analysis. J. Pediatr. Endocrinol. Metab. 2020, 33, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.L.; Lane, L.C.; Cheetham, T. Puberty: Normal physiology (brief overview). Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101265. [Google Scholar] [CrossRef]
- Prentice, A. Vitamin D deficiency: A global perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)₂vitamin D₃: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.R.; Karras, S.N.; März, W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public. Health 2018, 15, 2241. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; He, Y.; Beck, J.; Silva Teixeira, S.; Harrison, K.; Xu, Y.; Sisley, S. Defining vitamin D receptor expression in the brain using a novel VDRCre mouse. J. Comp. Neurol. 2021, 529, 2362–2375. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; de’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Hawk, J.L.M.; Honig, P.; Giam, Y.C.; Hoath, S.; Mack, M.C.; Stamatas, G.N. New insights about infant and toddler skin: Implications for sun protection. Pediatrics 2011, 128, 92–102. [Google Scholar] [CrossRef]
- Golden, N.H.; Abrams, S.A. Committee on Nutrition Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [Green Version]
- Winzenberg, T.; Jones, G. Vitamin D and bone health in childhood and adolescence. Calcif. Tissue Int. 2013, 92, 140–150. [Google Scholar] [CrossRef]
- Frost, H.M.; Schönau, E. The “muscle-bone unit” in children and adolescents: A 2000 overview. J. Pediatr. Endocrinol. Metab. 2000, 13, 571–590. [Google Scholar] [CrossRef]
- Bhattoa, H.P.; Konstantynowicz, J.; Laszcz, N.; Wojcik, M.; Pludowski, P. Vitamin D: Musculoskeletal health. Rev. Endocr. Metab. Disord. 2017, 18, 363–371. [Google Scholar] [CrossRef]
- Galthen-Sørensen, M.; Andersen, L.B.; Sperling, L.; Christesen, H.T. Maternal 25-hydroxyvitamin D level and fetal bone growth assessed by ultrasound: A systematic review. Ultrasound Obstet. Gynecol. 2014, 44, 633–640. [Google Scholar] [CrossRef] [Green Version]
- SACN Vitamin D and Health Report. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 14 June 2023).
- Young, B.E.; McNanley, T.J.; Cooper, E.M.; McIntyre, A.W.; Witter, F.; Harris, Z.L.; O’Brien, K.O. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am. J. Clin. Nutr. 2012, 95, 1103–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banajeh, S.M. Nutritional rickets and vitamin D deficiency--association with the outcomes of childhood very severe pneumonia: A prospective cohort study. Pediatr. Pulmonol. 2009, 44, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Muhe, L.; Lulseged, S.; Mason, K.E.; Simoes, E.A. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 1997, 349, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Tekin, M.; Konca, C.; Celik, V.; Almis, H.; Kahramaner, Z.; Erdemir, A.; Gulyuz, A.; Uckardes, F.; Turgut, M. The Association between Vitamin D Levels and Urinary Tract Infection in Children. Horm. Res. Paediatr. 2015, 83, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Hogan, P.G.; Hunstad, D.A.; Fritz, S.A. Vitamin D sufficiency and Staphylococcus aureus infection in children. Pediatr. Infect. Dis. J. 2015, 34, 544–545. [Google Scholar] [CrossRef] [Green Version]
- Thornton, K.A.; Marín, C.; Mora-Plazas, M.; Villamor, E. Vitamin D deficiency associated with increased incidence of gastrointestinal and ear infections in school-age children. Pediatr. Infect. Dis. J. 2013, 32, 585–593. [Google Scholar] [CrossRef]
- Bucak, I.H.; Ozturk, A.B.; Almis, H.; Cevik, M.Ö.; Tekin, M.; Konca, Ç.; Turgut, M.; Bulbul, M. Is there a relationship between low vitamin D and rotaviral diarrhea? Pediatr. Int. 2016, 58, 270–273. [Google Scholar] [CrossRef]
- Cusick, S.E.; Opoka, R.O.; Lund, T.C.; John, C.C.; Polgreen, L.E. Vitamin D insufficiency is common in Ugandan children and is associated with severe malaria. PLoS ONE 2014, 9, e113185. [Google Scholar] [CrossRef]
- Aydemir, G.; Cekmez, F.; Kalkan, G.; Fidanci, M.K.; Kaya, G.; Karaoglu, A.; Meral, C.; Arzıman, İ.; Karademir, F.; Ayar, G.; et al. High serum 25-hydroxyvitamin D levels are associated with pediatric sepsis. Tohoku J. Exp. Med. 2014, 234, 295–298. [Google Scholar] [CrossRef]
- Daley, P.; Jagannathan, V.; John, K.R.; Sarojini, J.; Latha, A.; Vieth, R.; Suzana, S.; Jeyaseelan, L.; Christopher, D.J.; Smieja, M.; et al. Adjunctive vitamin D for treatment of active tuberculosis in India: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 528–534. [Google Scholar] [CrossRef]
- Moodley, A.; Qin, M.; Singh, K.K.; Spector, S.A. Vitamin D-related host genetic variants alter HIV disease progression in children. Pediatr. Infect. Dis. J. 2013, 32, 1230–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltayeb, A.A.; Abdou, M.A.A.; Abdel-aal, A.M.; Othman, M.H. Vitamin D status and viral response to therapy in hepatitis C infected children. World J. Gastroenterol. 2015, 21, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Gergen, P.J.; Teach, S.J.; Mitchell, H.E.; Freishtat, R.F.; Calatroni, A.; Matsui, E.; Kattan, M.; Bloomberg, G.R.; Liu, A.H.; Kercsmar, C.; et al. Lack of a relation between serum 25-hydroxyvitamin D concentrations and asthma in adolescents. Am. J. Clin. Nutr. 2013, 97, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Brehm, J.M.; Acosta-Pérez, E.; Klei, L.; Roeder, K.; Barmada, M.; Boutaoui, N.; Forno, E.; Kelly, R.; Paul, K.; Sylvia, J.; et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am. J. Respir. Crit. Care Med. 2012, 186, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Dimeloe, S.; Richards, D.F.; Chambers, E.S.; Black, C.; Urry, Z.; Ryanna, K.; Xystrakis, E.; Bush, A.; Saglani, S.; et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax 2014, 69, 508–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazianas, M.; Butcher, G.P.; Subhani, J.M.; Finch, P.J.; Ang, L.; Collins, C.; Heaney, R.P.; Zaidi, M.; Maxwell, J.D. Calcium absorption and bone mineral density in celiacs after long term treatment with gluten-free diet and adequate calcium intake. Osteoporos. Int. 2005, 16, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Soles, C.M. Epidemiologic evidence supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Dermatoendocrinology 2009, 1, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Kinney, D.K.; Barch, D.H.; Chayka, B.; Napoleon, S.; Munir, K.M. Environmental risk factors for autism: Do they help cause de novo genetic mutations that contribute to the disorder? Med. Hypotheses 2010, 74, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Cannell, J.J.; Grant, W.B. What is the role of vitamin D in autism? Dermatoendocrinology 2013, 5, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Bener, A.; Khattab, A.O.; Al-Dabbagh, M.M. Is high prevalence of Vitamin D deficiency evidence for autism disorder?: In a highly endogamous population. J. Pediatr. Neurosci. 2014, 9, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Pathania, M.; Dhar, M.; Kumar, A.; Saha, S.; Malhotra, R. Association of Vitamin D Status with Metabolic Syndrome and Its Individual Risk Factors: A Cross-Sectional Study. Cureus 2023, 15, e38344. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Cena, H.; Biino, G.; Grazi, R.; Bortoni, G.; Braschi, V.; Tomasinelli, C.E.; Schneider, L.; Zuccotti, G. Screening Questionnaire for Vitamin D Insufficiency in Children with Obesity. Children 2022, 9, 1685. [Google Scholar] [CrossRef]
- Kumar, J.; Muntner, P.; Kaskel, F.J.; Hailpern, S.M.; Melamed, M.L. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 2009, 124, e362–e370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Montagnana, M.; Targher, G. Vitamin D deficiency among Italian children. Can. Med Assoc. J. 2007, 177, 1529–1530, author reply 1530. [Google Scholar] [CrossRef] [Green Version]
- Marrone, G.; Rosso, I.; Moretti, R.; Valent, F.; Romanello, C. Is vitamin D status known among children living in Northern Italy? Eur. J. Nutr. 2012, 51, 143–149. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D deficiency during pregnancy: An ongoing epidemic. Am. J. Clin. Nutr. 2006, 84, 273. [Google Scholar] [CrossRef]
- Lee, J.M.; Smith, J.R.; Philipp, B.L.; Chen, T.C.; Mathieu, J.; Holick, M.F. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin. Pediatr. 2007, 46, 42–44. [Google Scholar] [CrossRef]
- Greer, F.R. Fat-soluble vitamin supplements for enterally fed preterm infants. Neonatal. Netw. 2001, 20, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A. Vitamin D and the breastfed infant. J. Obs. Gynecol. Neonatal. Nurs. 2005, 34, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [CrossRef] [PubMed] [Green Version]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Dawson-Hughes, B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults. Am. J. Med. 2004, 116, 634–639. [Google Scholar] [CrossRef]
- Bowman, S.A. Beverage choices of young females: Changes and impact on nutrient intakes. J. Am. Diet. Assoc. 2002, 102, 1234–1239. [Google Scholar] [CrossRef]
- Roth, D.E.; Martz, P.; Yeo, R.; Prosser, C.; Bell, M.; Jones, A.B. Are national vitamin D guidelines sufficient to maintain adequate blood levels in children? Can. J. Public. Health 2005, 96, 443–449. [Google Scholar] [CrossRef]
- Lehmann, B.; Rudolph, T.; Pietzsch, J.; Meurer, M. Conversion of vitamin D3 to 1alpha,25-dihydroxyvitamin D3 in human skin equivalents. Exp. Dermatol. 2000, 9, 97–103. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Harel, Z.; Flanagan, P.; Forcier, M.; Harel, D. Low vitamin D status among obese adolescents: Prevalence and response to treatment. J. Adolesc. Health 2011, 48, 448–452. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, E.; Grandone, A.; Cirillo, G.; Capristo, C.; Marzuillo, P.; Di Sessa, A.; Umano, G.R.; Ruggiero, L.; Perrone, L. Bioavailable Vitamin D in Obese Children: The Role of Insulin Resistance. J. Clin. Endocrinol. Metab. 2015, 100, 3949–3955. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef] [Green Version]
- Thacher, T.D.; Levine, M.A. CYP2R1 mutations causing vitamin D-deficiency rickets. J. Steroid Biochem. Mol. Biol. 2017, 173, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kaplan, L.E.; Perwad, F.; Huang, N.; Sharma, A.; Choi, Y.; Miller, W.L.; Portale, A.A. Vitamin D 1alpha-hydroxylase gene mutations in patients with 1alpha-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2007, 92, 3177–3182. [Google Scholar] [CrossRef] [Green Version]
- Çelik, N.; Doğan, H.O.; Zararsiz, G. Different threshold levels of circulating total and free 25-hydroxyvitamin D for the diagnosis of vitamin D deficiency in obese adolescents. Eur. J. Pediatr. 2021, 180, 2619–2627. [Google Scholar] [CrossRef]
- Herman-Giddens, M.E.; Steffes, J.; Harris, D.; Slora, E.; Hussey, M.; Dowshen, S.A.; Wasserman, R.; Serwint, J.R.; Smitherman, L.; Reiter, E.O. Secondary Sexual Characteristics in Boys: Data From the Pediatric Research in Office Settings Network. Pediatrics 2012, 130, e1058–e1068. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.S.; Schubert, C.M.; Chumlea, W.C.; Roche, A.F.; Kulin, H.E.; Lee, P.A.; Himes, J.H.; Ryan, A.S. National Estimates of the Timing of Sexual Maturation and Racial Differences among US Children. Pediatrics 2002, 110, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, V.; Verduci, E.; Magenes, V.C.; Pascuzzi, M.C.; Rossi, V.; Sangiorgio, A.; Bosetti, A.; Zuccotti, G.; Mameli, C. The Role of Pediatric Nutrition as a Modifiable Risk Factor for Precocious Puberty. Life 2021, 11, 1353. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.J.; Shim, Y.S.; Jeong, H.R.; Kwon, E.; Hwang, J.S. Associations between serum vitamin D levels and precocious puberty in girls. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 91. [Google Scholar] [CrossRef] [Green Version]
- Villamor, E.; Marin, C.; Mora-Plazas, M.; Baylin, A. Vitamin D deficiency and age at menarche: A prospective study. Am. J. Clin. Nutr. 2011, 94, 1020–1025. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Long, W.; Du, C.; Yang, H.; Wu, S.; Ning, Q.; Luo, X. Prevalence of vitamin D deficiency in girls with idiopathic central precocious puberty. Front. Med. 2018, 12, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.-M.; Fang, J.; Zhang, P.-P.; Zhao, Y.-D.; Xu, Y.-N. Serum 25-hydroxyvitamin D levels and the risk of idiopathic central precocious puberty in girls. Clinics 2023, 78, 100244. [Google Scholar] [CrossRef] [PubMed]
- Durá-Travé, T.; Gallinas-Victoriano, F. Vitamin D status and parathyroid hormone assessment in girls with central precocious puberty. J. Endocrinol. Investig. 2022, 45, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Duhil De Bénazé, G.; Brauner, R.; Souberbielle, J.-C. There is no association between vitamin D status and characteristics of central precocious puberty in girls. Eur. J. Pediatr. 2017, 176, 1677–1680. [Google Scholar] [CrossRef]
- Hua, A. Vitamin D Status And Age of Menarche. Master’s Thesis, Yale University, New Haven, CT, USA, 2016. Public Health Theses. Available online: https://elischolar.library.yale.edu/ysphtdl/1132 (accessed on 10 July 2023).
- Al-Taiar, A.; Al-Sabah, R.; Shaban, L.; Sharaf Alddin, R.; Durgampudi, P.K.; Galadima, H. Is age of menarche directly related to vitamin D levels? Am. J. Hum. Biol. 2022, 34, e23731. [Google Scholar] [CrossRef]
- Parent, A.-S.; Teilmann, G.; Juul, A.; Skakkebaek, N.E.; Toppari, J.; Bourguignon, J.-P. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003, 24, 668–693. [Google Scholar] [CrossRef] [Green Version]
- Chew, A.; Harris, S.S. Does vitamin D affect timing of menarche? Nutr. Rev. 2013, 71, 189–193. [Google Scholar] [CrossRef]
- Dicken, C.L.; Israel, D.D.; Davis, J.B.; Sun, Y.; Shu, J.; Hardin, J.; Neal-Perry, G. Peripubertal Vitamin D3 Deficiency Delays Puberty and Disrupts the Estrous Cycle in Adult Female Mice1. Biol. Reprod. 2012, 87, 51. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Al-Awadhi, N.; Al-Kandari, N.; Al-Hasan, T.; Almurjan, D.; Ali, S.; Al-Taiar, A. Age at menarche and its relationship to body mass index among adolescent girls in Kuwait. BMC Public Health 2013, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Van Lenthe, F.; Kemper, C.; Van Mechelen, W. Rapid maturation in adolescence results in greater obesity in adulthood: The Amsterdam Growth and Health Study. Am. J. Clin. Nutr. 1996, 64, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Matkovic, V.; Ilich, J.Z.; Skugor, M.; Badenhop, N.E.; Goel, P.; Clairmont, A.; Klisovic, D.; Nahhas, R.W.; Landoll, J.D. Leptin Is Inversely Related to Age at Menarche in Human Females*. J. Clin. Endocrinol. Metab. 1997, 82, 3239–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholas, C.; Davis, J.; Fisher, T.; Segal, T.; Petti, M.; Sun, Y.; Wolfe, A.; Neal-Perry, G. Maternal Vitamin D Deficiency Programs Reproductive Dysfunction in Female Mice Offspring through Adverse Effects on the Neuroendocrine Axis. Endocrinology 2016, 157, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Kitagawa, Y.; Kawase, Y.; Nagaya, T.; Tokudome, S. Advanced onset of menarche and higher bone mineral density depending on vitamin D receptor gene polymorphism. Eur. J. Endocrinol. 1998, 139, 522–527. [Google Scholar] [CrossRef]
- Panda, D.K.; Miao, D.; Tremblay, M.L.; Sirois, J.; Farookhi, R.; Hendy, G.N.; Goltzman, D. Targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 7498–7503. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, M.; Von Versen-Höynck, F. Vitamin D—roles in women’s reproductive health? Reprod. Biol. Endocrinol. 2011, 9, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzesiak, M. Vitamin D3 Action within the Ovary—An Updated Review. Physiol. Res. 2020, 69, 371–378. [Google Scholar] [CrossRef]
- Cheng, T.S.; Ong, K.K.; Biro, F.M. Adverse Effects of Early Puberty Timing in Girls and Potential Solutions. J. Pediatr. Adolesc. Gynecol. 2022, 35, 532–535. [Google Scholar] [CrossRef]
- Bayley, N.; Pinneau, S.R. Tables for predicting adult height from skeletal age: Revised for use with the Greulich-Pyle hand standards. J. Pediatr. 1952, 40, 423–441. [Google Scholar] [CrossRef]
- Zachmann, M.; Sobradillo, B.; Frank, M.; Frisch, H.; Prader, A. Bayley-Pinneau, Roche-Wainer-Thissen, and Tanner height predictions in normal children and in patients with various pathologic conditions. J. Pediatr. 1978, 93, 749–755. [Google Scholar] [CrossRef]
- Karlberg, P.; Taranger, J.; Engström, I.; Karlberg, J.; Landström, T.; Lichtenstein, H.; Lindstrom, B.; Svennberg-Redegren, I.I. physical growth from birth to 16 years and longitudinal outcome of the study during the same age period. Acta Paediatr. Scand. Suppl. 1976, 65, 7–76. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Linder, B.; Sobel, E.H.; Saenger, P.; DiMartino-Nardi, J. Bayley-Pinneau method of height prediction in girls with central precocious puberty: Correlation with adult height. J. Pediatr. 1995, 126, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Bahlburg, H.F.; Ehrhardt, A.A.; Bell, J.J.; Cohen, S.F.; Healey, J.M.; Feldman, J.F.; Morishima, A.; Baker, S.W.; New, M.I. Idiopathic precocious puberty in girls: Psychosexual development. J. Youth Adolesc. 1985, 14, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R.; Forouhi, N.G.; Sharp, S.J.; Luben, R.; Bingham, S.A.; Khaw, K.-T.; Wareham, N.J.; Ong, K.K. Early age at menarche associated with cardiovascular disease and mortality. J. Clin. Endocrinol. Metab. 2009, 94, 4953–4960. [Google Scholar] [CrossRef]
- Prentice, P.; Viner, R.M. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. Int. J. Obes. 2013, 37, 1036–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritte, R.; Lukanova, A.; Tjønneland, A.; Olsen, A.; Overvad, K.; Mesrine, S.; Fagherazzi, G.; Dossus, L.; Teucher, B.; Steindorf, K.; et al. Height, age at menarche and risk of hormone receptor-positive and -negative breast cancer: A cohort study. Int. J. Cancer 2013, 132, 2619–2629. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Yang, C.; Chen, X. Early age at menarche is associated with insulin resistance: A systemic review and meta-analysis. Postgrad. Med. 2019, 131, 144–150. [Google Scholar] [CrossRef]
- Bubach, S.; De Mola, C.L.; Hardy, R.; Dreyfus, J.; Santos, A.C.; Horta, B.L. Early menarche and blood pressure in adulthood: Systematic review and meta-analysis. J. Public Health 2018, 40, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Chan, I.I.; Kwok, M.K.; Schooling, C.M. Timing of Pubertal Development and Midlife Blood Pressure in Men and Women: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e386–e393. [Google Scholar] [CrossRef]
- Cheng, T.S.; Day, F.R.; Lakshman, R.; Ong, K.K. Association of puberty timing with type 2 diabetes: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003017. [Google Scholar] [CrossRef] [Green Version]
- Luijken, J.; Van Der Schouw, Y.T.; Mensink, D.; Onland-Moret, N.C. Association between age at menarche and cardiovascular disease: A systematic review on risk and potential mechanisms. Maturitas 2017, 104, 96–116. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Sun, X.; Yin, Z.; Li, H.; Liu, X.; Zhang, D.; Cheng, C.; Liu, L.; Liu, F.; et al. Age at menarche and risk of all-cause and cardiovascular mortality: A systematic review and dose–response meta-analysis. Menopause 2019, 26, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Werneck, A.O.; Coelho-e-Silva, M.J.; Padilha, C.S.; Ronque, E.R.V.; Cyrino, E.S.; Szwarcwald, C.L.; Silva, D.R. Age at menarche and cancer risk at adulthood. Ann. Hum. Biol. 2018, 45, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Clavel-Chapelon, F.; The E3N-EPIC Group. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br. J. Cancer 2002, 86, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.S.; Kang, S.; Park, S. Association of Estrogen-Related Polygenetic Risk Scores with Breast Cancer and Interactions with Alcohol Intake, Early Menarche, and Nulligravida. Asian Pac. J. Cancer Prev. 2022, 23, 13–24. [Google Scholar] [CrossRef]
- Westling, E.; Andrews, J.A.; Hampson, S.E.; Peterson, M. Pubertal Timing and Substance Use: The Effects of Gender, Parental Monitoring and Deviant Peers. J. Adolesc. Health 2008, 42, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Mrug, S.; Elliott, M.N.; Davies, S.; Tortolero, S.R.; Cuccaro, P.; Schuster, M.A. Early Puberty, Negative Peer Influence, and Problem Behaviors in Adolescent Girls. Pediatrics 2014, 133, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Mendle, J.; Ryan, R.M.; McKone, K.M.P. Age at Menarche, Depression, and Antisocial Behavior in Adulthood. Pediatrics 2018, 141, e20171703. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.M.; Killen, J.D.; Hayward, C.; Robinson, T.N.; Hammer, L.D.; Kraemer, H.C.; Varady, A.; Taylor, C.B. Timing and rate of sexual maturation and the onset of cigarette and alcohol use among teenage girls. Arch. Pediatr. Adolesc. Med. 1994, 148, 789–795. [Google Scholar] [CrossRef]
- Dick, D.M.; Rose, R.J.; Viken, R.J.; Kaprio, E.J. Pubertal timing and substance use: Associations between and within families across late adolescence. Dev. Psychol. 2000, 36, 180–189. [Google Scholar] [CrossRef]
- Zabin, L.S.; Kantner, J.F.; Zelnik, M. The risk of adolescent pregnancy in the first months of intercourse. Fam. Plann. Perspect. 1979, 11, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Lacomba-Trejo, L.; Casaña-Granell, S.; Valero-Moreno, S.; Perez-Marin, M. e inmediatamente. Estrés y apego del cuidador principal como predictores de las dificultades emocionales y de relación en pacientes pediátricos con talla baja. Calid. Vida Salud 2018, 11, 2–9. [Google Scholar]
- Kim, E.Y.; Lee, M.I. Psychosocial aspects in girls with idiopathic precocious puberty. Psychiatry Investig. 2012, 9, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Striegel-Moore, R.H.; Franko, D.L.; Padgett, J.; Bean, J.A. Self-Esteem in Adolescent Females. J. Adolesc. Health 2006, 39, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, J.; Gonzales, N.A.; Christopher, F.S.; Roosa, M.W.; Millsap, R.E. Early Puberty and Adolescent Pregnancy: The Influence of Alcohol Use. Pediatrics 2005, 116, 1451–1456. [Google Scholar] [CrossRef] [Green Version]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
| Reference | Type of Study | Study Population | Conclusions |
|---|---|---|---|
| Lee at al. [70] | Cross-sectional study | 60 girls with CPP vs. 30 controls | Significant difference in the mean serum 25OHD concentration (17.1 ± 4.5 ng/mL vs. 21.2 ± 5.0 ng/mL) |
| Zhao et al. [72] | Cross-sectional study | 280 girls with CPP vs. 188 normal girls | The girls with CPP had significantly lower mean levels of 25OHD (19.36 ± 6.15 vs. 20.98 ± 7.60) |
| Liu et al. [4] | Meta-analysis | 3016 PP patients vs. 8296 healthy individuals | Vitamin-D-deficient subjects were more likely to develop PP (OR = 2.02 [95% confidence interval 1.65–2.46]) |
| Wu et al. [3] | Meta-analysis | 10,755 subjects | The average serum vitamin D concentration of subjects with precocious puberty was 1.16 ng/mL, which was lower than that of the control group |
| Gan et al. [73] | Meta-analysis | 221 girls with ICPP vs. 144 healthy girls | Serum 25(OH)D levels in the ICPP group were significantly lower than those in healthy controls (p < 0.001) |
| Duhil de Bénazé et al. [75] | Retrospective study | 145 girls monitored for idiopathic CPP | The mean 25OHD concentration was 27.6 ± 17.3 ng/mL, without any correlation with puberty characteristics of the subjects |
| Durà-Travè et al. [74] | Cross-sectional study | 78 girls with CPP vs. 137 prepubertal girls | No significant differences in 25OHD concentrations between CPP and control groups |
| Reference | Type of Study | Study Population | Conclusions |
|---|---|---|---|
| Villamor et al. [71] | Prospective study | 242 girls | Girls with low vitamin D levels had earlier menarche that persisted after adjusting for their increased body mass index |
| Al Taiar et al. [77] | Cross-sectional study | 598 middleschool girls | No evidence for association between 25OHD level or status and age of menarche |
| Hua et al. [76] | Cross-sectional study | 3572 females | Vitamin D status was not associated with early age of menarche after adjusting for potential confounders |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Magenes, V.C.; Tagi, V.M.; Grazi, R.; Bianchi, A.; Cena, H.; Zuccotti, G.; Fabiano, V. Association between Vitamin D Levels, Puberty Timing, and Age at Menarche. Children 2023, 10, 1243. https://doi.org/10.3390/children10071243
Calcaterra V, Magenes VC, Tagi VM, Grazi R, Bianchi A, Cena H, Zuccotti G, Fabiano V. Association between Vitamin D Levels, Puberty Timing, and Age at Menarche. Children. 2023; 10(7):1243. https://doi.org/10.3390/children10071243
Chicago/Turabian StyleCalcaterra, Valeria, Vittoria Carlotta Magenes, Veronica Maria Tagi, Roberta Grazi, Alice Bianchi, Hellas Cena, Gianvincenzo Zuccotti, and Valentina Fabiano. 2023. "Association between Vitamin D Levels, Puberty Timing, and Age at Menarche" Children 10, no. 7: 1243. https://doi.org/10.3390/children10071243





