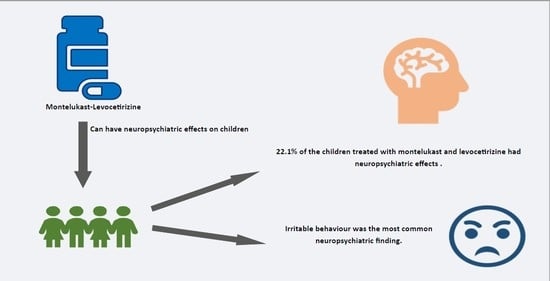
Evaluation of Neuropsychiatric Effects of Montelukast–Levocetirizine Combination Therapy in Children with Asthma and Allergic Rhinitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Type, and Sample
2.2. Evaluations
2.2.1. Test for Respiratory and Asthma Control in Kids (TRACK)
2.2.2. Rhino Conjunctivitis Scoring System (RCSS)
2.3. Statistical Analysis
2.4. Ethics
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. Allergic diseases and asthma: A major global health concern. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 39–41. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 3 June 2023).
- Padem, N.; Glick Robison, R. The infant and toddler with wheezing. Allergy Asthma Proc. 2019, 40, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Data from Yearly National Health Interview Survey. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 6 June 2023).
- Sipahi, S.; Tamay, Z. Beş yaş ve altı çocuklarda astım tedavisine güncel yaklaşım. Klin. Tıp Pediatri Derg. 2018, 10, 33–37. [Google Scholar]
- Global Initiative for Asthma. 2022 GINA Main Report. Available online: https://ginasthma.org/gina-reports/ (accessed on 8 June 2023).
- Casale, T.B.; Dykewicz, M.S. Clinical implications of the allergic rhinitis-asthma link. Am. J. Med. Sci. 2004, 327, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Varshney, J.; Varshney, H. Allergic rhinitis: An overview. Indian J. Otolaryngol. Head Neck Surg. 2015, 67, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Mandhane, S.N.; Shah, J.H.; Thennati, R. Allergic rhinitis: An update on disease, present treatments and future prospects. Int. Immunopharmacol. 2011, 11, 1646–1662. [Google Scholar] [CrossRef]
- Canonica, G.; Bousquet, J.; Mullol, J.; Scadding, G.; Virchow, J. A survey of the burden of allergic rhinitis in Europe. Allergy 2007, 62, 17–25. [Google Scholar] [CrossRef]
- Zuberi, F.F.; Haroon, M.A.; Haseeb, A.; Khuhawar, S.M. Role of Montelukast in asthma and allergic rhinitis patients. Pak. J. Med. Sci. 2020, 36, 1517. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Mohd Noor, N.; Mat Lazim, N.; Abdullah, B. Efficacy of montelukast in allergic rhinitis treatment: A systematic review and meta-analysis. Drugs 2020, 80, 1831–1851. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administeration. Drug Approval Package. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/020829s000_SingulairTOC.cfm (accessed on 20 June 2023).
- Murphy, R.C.; Gijón, M.A. Biosynthesis and metabolism of leukotrienes. Biochem. J. 2007, 405, 379–395. [Google Scholar] [CrossRef] [Green Version]
- McMillan, R. Leukotrienes in respiratory disease. Paediatr. Respir. Rev. 2001, 2, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Jo-Watanabe, A.; Okuno, T.; Yokomizo, T. The role of leukotrienes as potential therapeutic targets in allergic disorders. Int. J. Mol. Sci. 2019, 20, 3580. [Google Scholar] [CrossRef] [Green Version]
- Busse, W. The role of leukotrienes in asthma and allergic rhinitis. Clin. Exp. Allergy 1996, 6, 868–879. [Google Scholar] [CrossRef]
- Glockler-Lauf, S.D.; Finkelstein, Y.; Zhu, J.; Feldman, L.Y.; To, T. Montelukast and neuropsychiatric events in children with asthma: A nested case–control study. J. Pediatr. 2019, 209, 176–182.e4. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Toennesen, L.L.; Eklöf, J.; Sivapalan, P.; Meteran, H.; Bønnelykke, K.; Ulrik, C.S.; Stæhr Jensen, J.U. Psychiatric adverse effects of montelukast—A nationwide cohort study. J Allergy Clin. Immunol. Pract. 2023, 11, 2096–2103.e1. [Google Scholar] [CrossRef]
- Ekhart, C.; van Hunsel, F.; Sellick, V.; de Vries, T. Neuropsychiatric reactions with the use of montelukast. BMJ 2022, 376, e067554. [Google Scholar] [CrossRef]
- Park, H.J. Scientific update of singulair-focused on neuropsychiatric disorder. Obstr. Lung Dis. 2021, 9, 59–63. [Google Scholar]
- Yilmaz Bayer, O.; Turktas, I.; Ertoy Karagol, H.I.; Soysal, S.; Yapar, D. Neuropsychiatric adverse drug reactions induced by montelukast impair the quality of life in children with asthma. J. Asthma 2022, 59, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Benard, B.; Bastien, V.; Vinet, B.; Yang, R.; Krajinovic, M.; Ducharme, F.M. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur. Respir. J. 2017, 50, 1700148. [Google Scholar] [CrossRef] [Green Version]
- Aldea Perona, A.; García-Sáiz, M.; Sanz Álvarez, E. Psychiatric disorders and montelukast in children: A disproportionality analysis of the VigiBase®. Drug Saf. 2016, 39, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kovesi, T. Neuropsychiatric side effects of montelukast. J. Pediatr. 2019, 212, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administeration. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug (accessed on 20 June 2023).
- Gayathri, E.; Sowmya, P.; Punnagai, K.; Mahalakshmi, V. Comparative study on the efficacy and safety of bepotastine besilate versus levocetirizine in chronic spontaneous urticaria: A randomised, open-label, parallel study. Indian J. Dermatol. Venereol Leprol. 2023, 1–8. [Google Scholar] [CrossRef]
- Bian, S.; Li, L.; Wan, Z.; Cui, L.; Xu, Y.; Guan, K.; Zhao, B.; Wang, L.; Yin, J. Neuropsychiatric side reactions of leukotriene receptor antagonist, antihistamine, and inhaled corticosteroid: A real-world analysis of the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS). World Allergy Organ. J. 2021, 14, 100594. [Google Scholar] [PubMed]
- Murphy, K.R.; Zeiger, R.S.; Kosinski, M.; Chipps, B.; Mellon, M.; Schatz, M.; Lampl, K.; Hanlon, J.T.; Ramachandran, S. Test for respiratory and asthma control in kids (TRACK): A caregivercompleted questionnaire for preschool-aged children. J. Allergy Clin. Immunol. 2009, 123, 833–939.e9. [Google Scholar] [CrossRef]
- Büyüktiryaki, A.B. Okul Öncesi Çağı Astımlı Türk Çocuklarında Çocuklar İçin Solunum ve Astım Kontrol Testi (ÇİSAKT)’nin Geçerlilik, Güvenilirlik ve Değişime Duyarlılığı. Subspecialty Thesis, University of Hacettepe, Ankara, Turkey, 2013. [Google Scholar]
- Florack, J.; Brighetti, M.A.; Perna, S.; Pizzulli, A.; Pizzulli, A.; Tripodi, S.; Costa, C.; Travaglini, A.; Pelosi, S.; Bianchi, A.; et al. Comparison of six disease severity scores for allergic rhinitis against pollen counts a prospective analysis at population and individual level. Pediatr. Allergy Immunol. 2016, 27, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquali, M.; Baiardini, I.; Rogkakou, A.; Riccio, A.M.; Gamalero, C.; Descalzi, D.; Folli, C.; Passalacqua, G.; Canonica, G.W. Levocetirizine in persistent allergic rhinitis and asthma: Effects on symptoms, quality of life and inflammatory parameters. Clin. Exp. Allergy 2006, 36, 1161–1167. [Google Scholar] [CrossRef]
- Ammari, S.; Berraies, A.; Hamdi, B.; Jemia, E.B.; Ammar, J.; Hamzaoui, A. Montelukast: Neuropsychiatric adverse drug reactions in Tunisian asthmatic children. Eur. Respir. Soc. 2018, 52, PA4610. [Google Scholar]
- Al-Shamrani, A.; Alharbi, S.; Kobeisy, S.; AlKhater, S.A.; Alalkami, H.; Alahmadi, T.; Almutairi, A.; Alharbi, A.S.; Yousef, A.A. Adverse drug reactions (ADRs) of montelukast in children. Children 2022, 9, 1783. [Google Scholar] [CrossRef]
- Thompson, A.; Sardana, N.; Craig, T.J. Sleep impairment and daytime sleepiness in patients with allergic rhinitis: The role of congestion and inflammation. Ann. Allergy Asthma Immunol. 2013, 111, 446–451. [Google Scholar] [CrossRef]
| Median (Min–Max) | |
|---|---|
| WBC * (103/uL) | 8480.0 (3330.0–21,440.0) |
| Neutrophil (103/uL) | 3450.0 (1080.0–14,500.0) |
| Eosinophil (absolute)(103/uL) | 260.0 (0–1760.0) |
| Eosinophil (%) | 3.0 (0–16.9) |
| Lymphocyte (103/uL) | 3840.0 (1140.0–13,510.0) |
| Platelet (103/uL) | 334,000.0 (167,000.0–743,000.0) |
| Total IgE (IU/mL) | 91.0 (2.0–2735.0) |
| Allergy Test Positivity | n | % |
|---|---|---|
| House dust mite | 14 | 20.6 |
| Egg | 5 | 7.4 |
| Cat | 3 | 4.4 |
| Nuts | 3 | 4.4 |
| Pollen | 2 | 2.9 |
| Cow’s milk | 2 | 2.9 |
| Median | Minimum | Maximum | p Value | |
|---|---|---|---|---|
| TRACK-Before | 40.0 | 5.0 | 75.0 | <0.001 |
| TRACK-After | 87.5 | 25.0 | 100.0 | |
| RCSS-Before | 2.17 | 0.83 | 3.0 | <0.001 |
| RCSS-After | 0 | 0 | 3.0 |
| Findings | Before | After | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Irritable behavior | 56 | 82.4 | 43 | 63.2 | 0.004 |
| Hallucination | 8 | 11.8 | 5 | 7.4 | 0.508 |
| Headache | 14 | 20.6 | 2 | 2.9 | <0.001 |
| Nightmares | 35 | 51.5 | 17 | 25.0 | <0.001 |
| Sleep disorder | 43 | 63.2 | 22 | 32.4 | <0.001 |
| Behavioral and mood disorders | 25 | 36.8 | 15 | 22.1 | 0.013 |
| Restlessness | 31 | 45.6 | 16 | 23.5 | <0.001 |
| Depression | 0 | 0 | 0 | 0 | - |
| Findings | Development of Neuropsychiatric Findings | Improvement in Findings | ||
|---|---|---|---|---|
| n | % | n | % | |
| Irritable behavior | 9 | 13.2 | 25 | 36.8 |
| Hallucination | 3 | 4.4 | 7 | 10.3 |
| Headache | 0 | 0 | 12 | 17.6 |
| Nightmares | 5 | 7.4 | 24 | 35.3 |
| Sleep disorder | 3 | 4.4 | 31 | 45.6 |
| Behavioral and mood disorders | 4 | 5.9 | 18 | 26.5 |
| Restlessness | 2 | 2.9 | 19 | 27.9 |
| Depression | 0 | 0 | 0 | 0 |
| At least one neuropsychiatric finding | 15 | 22.1 | 52 | 76.5 |
| p Value | OR | 95% C.I. for OR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 0.754 | 1.210 | 0.368 | 3.983 |
| Age | 0.966 | 1.013 | 0.558 | 1.839 |
| TRACK-Before treatment | 0.806 | 1.006 | 0.962 | 1.051 |
| RCSS-Before treatment | 0.514 | 0.706 | 0.248 | 2.008 |
| Allergy test positivity | 0.438 | 1.649 | 0.466 | 5.834 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altaş, U.; Altaş, Z.M.; Öz, F.; Özkars, M.Y. Evaluation of Neuropsychiatric Effects of Montelukast–Levocetirizine Combination Therapy in Children with Asthma and Allergic Rhinitis. Children 2023, 10, 1301. https://doi.org/10.3390/children10081301
Altaş U, Altaş ZM, Öz F, Özkars MY. Evaluation of Neuropsychiatric Effects of Montelukast–Levocetirizine Combination Therapy in Children with Asthma and Allergic Rhinitis. Children. 2023; 10(8):1301. https://doi.org/10.3390/children10081301
Chicago/Turabian StyleAltaş, Uğur, Zeynep Meva Altaş, Fırat Öz, and Mehmet Yaşar Özkars. 2023. "Evaluation of Neuropsychiatric Effects of Montelukast–Levocetirizine Combination Therapy in Children with Asthma and Allergic Rhinitis" Children 10, no. 8: 1301. https://doi.org/10.3390/children10081301