Insights into Neonatal Cerebral Autoregulation by Blood Pressure Monitoring and Cerebral Tissue Oxygenation: A Qualitative Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. The Search Strategy and Study Selection Criteria
2.2. Search Strategy
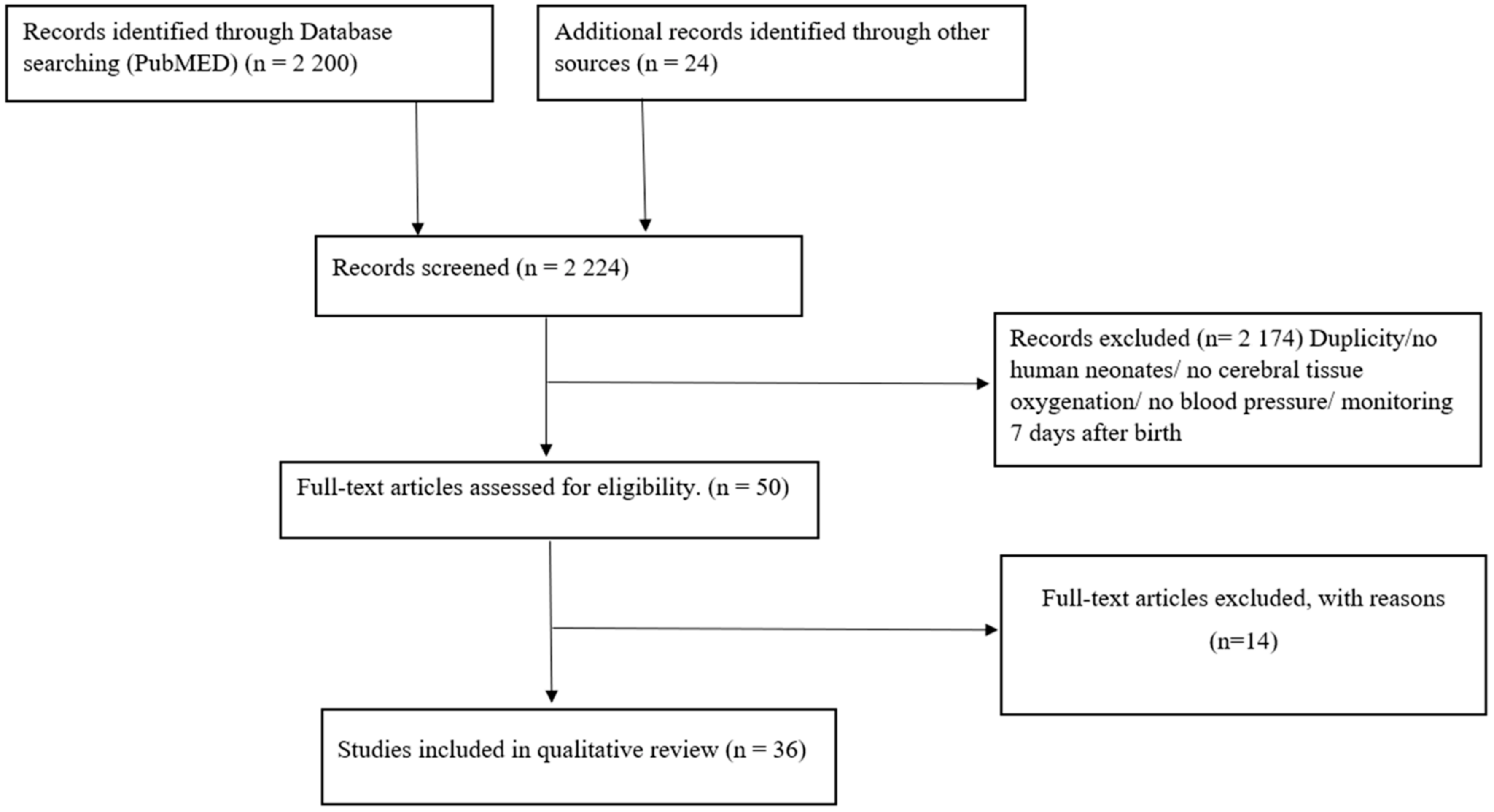
2.3. Study Selection
3. Results
4. Discussion
4.1. Immediate Transition
4.2. First Day after Birth
4.3. Beyond the First Day after Birth
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, S.U.; Brodsky, D. Fetal Physiology and the Transition to Extrauterine Life. Clin. Perinatol. 2016, 43, 395–407. [Google Scholar] [CrossRef]
- Hooper, S.B.; Pas, A.B.T.; Lang, J.; van Vonderen, J.J.; Roehr, C.C.; Kluckow, M.; Gill, A.W.; Wallace, E.M.; Polglase, G.R. Cardiovascular transition at birth: A physiological sequence. Pediatr. Res. 2015, 77, 608–614. [Google Scholar] [CrossRef]
- Saikia, D.; Mahanta, B. Cardiovascular and respiratory physiology in children. Indian J. Anaesth. 2019, 63, 690. [Google Scholar] [CrossRef]
- Askin, D.F. Complications in the Transition from Fetal to Neonatal Life. J. Obstet. Gynecol. Neonatal Nurs. 2002, 31, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Pas, A.T.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Lee, H.C.; Escobedo, M.B.; Hoover, A.V.; Kamath-Rayne, B.D.; Kapadia, V.S.; Magid, D.J.; Niermeyer, S.; Schmölzer, G.M.; Szyld, E.; et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142 (Suppl. S2), S524–S550. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; De Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142 (Suppl. S1), 329. [Google Scholar] [CrossRef]
- Baik, N.; Urlesberger, B.; Schwaberger, B.; Freidl, T.; Schmölzer, G.M.; Pichler, G. Cardiocirculatory Monitoring during Immediate Fetal-to-Neonatal Transition: A Systematic Qualitative Review of the Literature. Neonatology 2015, 107, 100–107. Available online: https://www.karger.com/Article/Fulltext/368042 (accessed on 18 November 2014). [CrossRef] [PubMed]
- Marx, G.F.; Cabe, C.M.; Kim, Y.I.; Eidelman, A.I. Neonatal blood pressures. Anaesthesist 1976, 25, 318–322. [Google Scholar] [CrossRef]
- Shah, S.; Kaul, A.; Khandare, J.; Dhalait, S. Comparison of Invasive Arterial Blood Pressure Monitoring vs. Non-Invasive Blood Pressure Monitoring in Preterm Infants <37 Weeks in the Neonatal Intensive Care Unit—A Prospective Observational Study. J. Trop. Pediatr. 2021, 67, 1058. [Google Scholar] [CrossRef]
- Sharma, D.; Farahbakhsh, N.; Shastri, S.; Sharma, P. Neonatal hypertension. J. Matern. Fetal Neonatal Med. 2016, 30, 540–550. [Google Scholar] [CrossRef]
- Dionne, J.M.; Bremner, S.A.; Baygani, S.K.; Batton, B.; Ergenekon, E.; Bhatt-Mehta, V.; Dempsey, E.; Kluckow, M.; Koplowitz, L.P.; Apele-Freimane, D.; et al. Method of Blood Pressure Measurement in Neonates and Infants: A Systematic Review and Analysis. J. Pediatr. 2020, 221, 23–31.e5. [Google Scholar] [CrossRef]
- Dempsey, E.M.; Barrington, K.J.; Marlow, N.; O’Donnell, C.P.F.; Miletin, J.; Naulaers, G.; Cheung, P.-Y.; Corcoran, J.D.; El-Khuffash, A.F.; Boylan, G.B.; et al. Hypotension in Preterm Infants (HIP) randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E. Challenges in Treating Low Blood Pressure in Preterm Infants. Children 2015, 2, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.M. What Should We Do about Low Blood Pressure in Preterm Infants. Neonatology 2017, 111, 402–407. [Google Scholar] [CrossRef]
- Pichler, G.; Cheung, P.-Y.; Binder, C.; O’reilly, M.; Schwaberger, B.; Aziz, K.; Urlesberger, B.; Schmölzer, G.M. Time Course Study of Blood Pressure in Term and Preterm Infants Immediately after Birth. PLoS ONE 2014, 9, e114504. [Google Scholar] [CrossRef]
- Suppan, E.; Pichler, G.; Binder-Heschl, C.; Schwaberger, B.; Urlesberger, B. Three Physiological Components That Influence Regional Cerebral Tissue Oxygen Saturation. Front. Pediatr. 2022, 10, 913223. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Schmölzer, G.M.; Urlesberger, B. Cerebral Tissue Oxygenation during Immediate Neonatal Transition and Resuscitation. Front. Pediatr. 2017, 5, 260. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Cheung, P.-Y.; Aziz, K.; Urlesberger, B.; Schmölzer, G.M. How to Monitor the Brain during Immediate Neonatal Transition and Resuscitation: A Systematic Qualitative Review of the Literature. Neonatology 2014, 105, 205–210. [Google Scholar] [CrossRef]
- Alderliesten, T.; Dix, L.; Baerts, W.; Caicedo, A.; van Huffel, S.; Naulaers, G.; Groenendaal, F.; van Bel, F.; Lemmers, P. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr. Res. 2016, 79, 55–64. Available online: https://pubmed.ncbi.nlm.nih.gov/26389823/ (accessed on 21 September 2015). [CrossRef]
- Lemmers, P.M.A.; Toet, M.; van Schelven, L.J.; van Bel, F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: The impact of respiratory distress syndrome. Exp. Brain Res. 2006, 173, 458–467. [Google Scholar] [CrossRef]
- Pfurtscheller, D.; Wolfsberger, C.H.; Höller, N.; Schwaberger, B.; Mileder, L.; Baik-Schneditz, N.; Urlesberger, B.; Pichler, G. Correlation between arterial blood pressures and regional cerebral oxygen saturation in preterm neonates during postnatal transition-an observational study. Front. Pediatr. 2022, 10, 1244. [Google Scholar] [CrossRef]
- Tsuji, M.; Saul, J.P.; du Plessis, A.; Eichenwald, E.; Sobh, J.; Crocker, R.; Volpe, J.J. Cerebral Intravascular Oxygenation Correlates with Mean Arterial Pressure in Critically Ill Premature Infants. Pediatrics 2000, 106, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.M.; Stone, B.S.; Shepard, J.A.; Czosnyka, M.; Easley, R.B.; Brady, K.M. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J. Perinatol. 2011, 31, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Vesoulis, Z.A.; Liao, S.M.; Mathur, A.M. Gestational age-dependent relationship between cerebral oxygen extraction and blood pressure. Pediatr. Res. 2017, 82, 934–939. [Google Scholar] [CrossRef]
- Eriksen, V.R.; Hahn, G.H.; Greisen, G. Cerebral autoregulation in the preterm newborn using near-infrared spectroscopy: A comparison of time-domain and frequency-domain analyses. J. Biomed. Opt. 2015, 20, 37009. [Google Scholar] [CrossRef]
- Riera, J.; Cabañas, F.; Serrano, J.J.; Bravo, M.C.; López-Ortego, P.; Sánchez, L.; Madero, R.; Pellicer, A. New Time-Frequency Method for Cerebral Autoregulation in Newborns: Predictive Capacity for Clinical Outcomes. J. Pediatr. 2014, 165, 897–902.e1. [Google Scholar] [CrossRef]
- Victor, S.; Marson, A.G.; Appleton, R.E.; Beirne, M.; Weindling, A.M. Relationship Between Blood Pressure, Cerebral Electrical Activity, Cerebral Fractional Oxygen Extraction, and Peripheral Blood Flow in Very Low Birth Weight Newborn Infants. Pediatr. Res. 2006, 59, 314–319. [Google Scholar] [CrossRef]
- Victor, S.; Appleton, R.E.; Beirne, M.; Marson, A.G.; Weindling, A.M. The Relationship between Cardiac Output, Cerebral Electrical Activity, Cerebral Fractional Oxygen Extraction and Peripheral Blood Flow in Premature Newborn Infants. Pediatr. Res. 2006, 60, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.B.; Hahn, G.H.; Leung, T.S.; Greisen, G. Precision of coherence analysis to detect cerebral autoregulation by near-infrared spectroscopy in preterm infants. J. Biomed. Opt. 2010, 15, 37002. [Google Scholar] [CrossRef]
- Bonestroo, H.J.C.; Lemmers, P.M.A.; Baerts, W.; van Bel, F. Effect of Antihypotensive Treatment on Cerebral Oxygenation of Preterm Infants Without PDA. Pediatrics 2011, 128, e1502–e1510. [Google Scholar] [CrossRef] [PubMed]
- Hahn, G.H.; Maroun, L.L.; Larsen, N.; Hougaard, D.M.; Sorensen, L.C.; Lou, H.C.; Greisen, G. Cerebral autoregulation in the first day after preterm birth: No evidence of association with systemic inflammation. Pediatr. Res. 2012, 71, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Stammwitz, A.; von Siebenthal, K.; Bucher, H.U.; Wolf, M. Can the Assessment of Spontaneous Oscillations by Near Infrared Spectrophotometry Predict Neurological Outcome of Preterm Infants? In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016. [Google Scholar]
- Da Costa, C.S.; Czosnyka, M.; Smielewski, P.; Austin, T. Optimal Mean Arterial Blood Pressure in Extremely Preterm Infants within the First 24 Hours of Life. J. Pediatr. 2018, 203, 242–248. [Google Scholar] [CrossRef] [PubMed]
- a Costa, C.S.; Cardim, D.; Molnar, Z.; Kelsall, W.; Ng, I.; Czosnyka, M.; Smielewski, P.; Austin, T. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr. Res. 2019, 86, 247–253. [Google Scholar] [CrossRef]
- O’Leary, H.; Gregas, M.C.; Limperopoulos, C.; Zaretskaya, I.; Bassan, H.; Soul, J.S.; Di Salvo, D.N.; du Plessis, A.J. Elevated Cerebral Pressure Passivity Is Associated with Prematurity-Related Intracranial Hemorrhage. Pediatrics 2009, 124, 302–309. [Google Scholar] [CrossRef]
- Wong, F.Y.; Silas, R.; Hew, S.; Samarasinghe, T.; Walker, A.M.; Morty, R.E. Cerebral Oxygenation Is Highly Sensitive to Blood Pressure Variability in Sick Preterm Infants. PLoS ONE 2012, 7, e43165. [Google Scholar] [CrossRef]
- Chock, V.Y.; Kwon, S.H.; Ambalavanan, N.; Batton, B.; Nelin, L.D.; Chalak, L.F.; Tian, L.; Van Meurs, K.P. Cerebral Oxygenation and Autoregulation in Preterm Infants (Early NIRS Study). J. Pediatr. 2020, 227, 94–100.e1. [Google Scholar] [CrossRef]
- Kooi, E.M.; van der Laan, M.E.; Verhagen, E.A.; van Braeckel, K.N.; Bos, A.F. Volume Expansion Does Not Alter Cerebral Tissue Oxygen Extraction in Preterm Infants with Clinical Signs of Poor Perfusion. Neonatology 2013, 103, 308–314. [Google Scholar] [CrossRef]
- Eriksen, V.R.; Hahn, G.H.; Greisen, G. Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr. 2014, 103, 1221–1226. Available online: https://pubmed.ncbi.nlm.nih.gov/25266994/ (accessed on 23 October 2014). [CrossRef]
- Alderliesten, T.; Lemmers, P.M.; Smarius, J.J.; van de Vosse, R.E.; Baerts, W.; van Bel, F. Cerebral Oxygenation, Extraction, and Autoregulation in Very Preterm Infants Who Develop Peri-Intraventricular Hemorrhage. J. Pediatr. 2013, 162, 698–704.e2. [Google Scholar] [CrossRef]
- Wong, F.Y.; Leung, T.S.; Austin, T.; Wilkinson, M.; Meek, J.H.; Wyatt, J.S.; Walker, A.M. Impaired Autoregulation in Preterm Infants Identified by Using Spatially Resolved Spectroscopy. Pediatrics 2008, 121, e604–e611. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, A.; De Smet, D.; Naulaers, G.; Ameye, L.; Vanderhaegen, J.; Lemmers, P.; van Bel, F.; Van Huffel, S. Cerebral Tissue Oxygenation and Regional Oxygen Saturation Can Be Used to Study Cerebral Autoregulation in Prematurely Born Infants. Pediatr. Res. 2011, 69, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chan, G.S.H.; Tracy, M.B.; Lee, Q.Y.; Hinder, M.; Savkin, A.V.; Lovell, N.H. Cerebral Near-Infrared Spectroscopy Analysis in Preterm Infants with Intraventricular Hemorrhage. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: Piscataway, NJ, USA, 2011. [Google Scholar]
- Mitra, S.; Czosnyka, M.; Smielewski, P.; O’Reilly, H.; Brady, K.; Austin, T. Heart rate passivity of cerebral tissue oxygenation is associated with predictors of poor outcome in preterm infants. Acta Paediatr. 2014, 103, e374–e382. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, E.A.; Hummel, L.A.; Bos, A.F.; Kooi, E.M. Near-infrared spectroscopy to detect absence of cerebrovascular autoregulation in preterm infants. Clin. Neurophysiol. 2014, 125, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Traub, T.M.; Grabowski, R.; Rais-Bahrami, K. Pilot study of cerebral and somatic autoregulation using NIRS in preterm neonates. J. Neonatal Perinat. Med. 2021, 14, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Smet, D.D.; Vanderhaegen, J.; Naulaers, G.; van Huffel, S. New Measurements for Assessment of Impaired Cerebral Autoregulation Using Near-Infrared Spectroscopy. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2009. [Google Scholar]
- Demel, A.; Feilke, K.; Schöning, M.; Wolf, M.; Poets, C.F.; Franz, A.R. Healthy term and moderately preterm infants have similar cerebral oxygen saturation and cerebral blood flow volumes during early post-natal transition. Acta Paediatr. 2015, 104, e330–e336. Available online: https://pubmed.ncbi.nlm.nih.gov/25867534/ (accessed on 14 May 2015).
- Baik, N.; Urlesberger, B.; Schwaberger, B.; Avian, A.; Mileder, L.; Schmölzer, G.M.; Pichler, G. Blood Pressure during the Immediate Neonatal Transition: Is the Mean Arterial Blood Pressure Relevant for the Cerebral Regional Oxygenation? Neonatology 2017, 112, 97–102. [Google Scholar] [CrossRef]
- Takami, T.; Sunohara, D.; Kondo, A.; Mizukaki, N.; Suganami, Y.; Takei, Y.; Miyajima, T.; Hoshika, A. Changes in cerebral perfusion in extremely low birth weight infants during the first 72 hours after birth. Pediatr. Res. 2010, 68, 435–439. [Google Scholar] [CrossRef]
- Bruckner, M.; Binder-Heschl, C.; Schwaberger, B.; Mileder, L.P.; Baik-Schneditz, N.; Koestenberger, M.; Avian, A.; Urlesberger, B.; Pichler, G. Cerebral and peripheral tissue oxygenation in stable neonates: Absent influence of cardiac function. Acta Paediatr. 2020, 109, 1560–1569. [Google Scholar] [CrossRef]
- Pichler, G.; Höller, N.; Baik-Schneditz, N.; Schwaberger, B.; Mileder, L.; Stadler, J.; Avian, A.; Pansy, J.; Urlesberger, B. Avoiding Arterial Hypotension in Preterm Neonates (AHIP)—A Single Center Randomised Controlled Study Investigating Simultaneous Near Infrared Spectroscopy Measurements of Cerebral and Peripheral Regional Tissue Oxygenation and Dedicated Interventions. Front. Pediatr. 2018, 6, 289. [Google Scholar] [CrossRef]
- Pellicer, A.; Valverde, E.; Elorza, M.D.; Madero, R.; Gayá, F.; Quero, J.; Cabañas, F. Cardiovascular Support for Low Birth Weight Infants and Cerebral Hemodynamics: A Randomized, Blinded, Clinical Trial. Pediatrics 2005, 115, 1501–1512. [Google Scholar] [CrossRef]
- Binder-Heschl, C.; Urlesberger, B.; Schwaberger, B.; Koestenberger, M.; Pichler, G. Borderline hypotension: How does it influence cerebral regional tissue oxygenation in preterm infants? J. Matern. Fetal Neonatal Med. 2015, 29, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Naulaers, G. Cerebral tissue oxygenation index in very premature infants. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, 189F–192F. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.L.; Meskell, S.; Falk, M.C.; Shadbolt, B. Normative blood pressure data in non-ventilated premature neonates from 28–36 weeks gestation. Pediatr. Nephrol. 2009, 24, 141–146. [Google Scholar] [CrossRef]
- Feng, S.Y.S.; Samarasinghe, T.; Phillips, D.J.; Alexiou, T.; Hollis, J.H.; Yu, V.Y.H.; Walker, A.M. Acute and chronic effects of endotoxin on cerebral circulation in lambs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R760–R766. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, A.A.; Kimimasa, T.; Bell, M.J.; MacPherson, T.A.; Keller, B.B.; Yanowitz, T.D. Placental Inflammation and Fetal Hemodynamics in a Rat Model of Chorioamnionitis. Pediatr. Res. 2010, 68, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.C.; Lassen, N.A.; Friis-Hansen, B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J. Pediatr. 1979, 94, 118–121. [Google Scholar] [CrossRef]
- Baik-Schneditz, N.; Pichler, G.; Schwaberger, B.; Binder-Heschl, C.; Mileder, L.; Reiss, I.K.; Avian, A.; Greimel, P.; Klaritsch, P.; Urlesberger, B. Effect of Intrauterine Growth Restriction on Cerebral Regional Oxygen Saturation in Preterm and Term Neonates during Immediate Postnatal Transition. Neonatology 2020, 117, 324–330. [Google Scholar] [CrossRef]
- Levene, M.; Chiswick, M.; Field, D.; Forsyth, S.; Gamsu, H.; Grant, A. Development of audit measures and guidelines for good practice in the management of neonatal respiratory distress syndrome. Report of a Joint Working Group of the British Association of Perinatal Medicine and the Research Unit of the Royal College of Physicians. Arch. Dis. Child. 1992, 67, 1221–1227. [Google Scholar] [CrossRef]
| Author (Reference) (Year) of Publication | Neonates, n | Gestational Age, Weeks | NIRS Device | Arterial Blood Pressure | Duration | Initiation | Study Aim | Main Results Concerning BP and NIRS |
|---|---|---|---|---|---|---|---|---|
| Baik [50] (2017) | Term n = 292, preterm n = 186 | Term 38.9 ± 0.8, preterm 31.0 ± 3.5 | INVOS 5100c | MABP (oscillometric) | 15 min | 0–1 min | Impact of MABP on the cerebral regional oxygen saturation | In preterm neonates, MABP correlated negatively with cFTOE |
| Pfurtscheller [22] (2022) | Preterm n = 47 | 34.4 ± 1.6 (resp. support, n = 25) 34.5 ± 1.5 (stable, n = 22) | INVOS 5100c | MABP (oscillometric) | 15 min | 0–1 min | Impact of MABP and NIRS parameters in compromised neonates | In compromised preterm neonates, MABP correlated negatively with cFTOE and positively with crSO2 |
| Author (Reference) (Year) of Publication | Neonates, n | Gestational Age, Weeks | NIRS Device | Arterial Blood Pressure Evaluation | Duration | Initiation | Study Aim | Main Results Concerning BP and NIRS |
|---|---|---|---|---|---|---|---|---|
| Naulaers [56] (2002) | Preterm n = 15 | 28.0 (25.0–30.0) | NIRO 300 | n.a. | 48 h | <6.0 h | To describe normal values of cTOI in premature infants | cTOI, MABP, and CBF increased in the first 3 days in preterm neonates |
| Pellicer [54] (2005) | Preterm n = 59 | 28.3 ± 2.3 | Critikon | MABP (oscillometric and invasive) | 80 min | 5.3 ± 3.7 h | Effect of two catecholamines on brain hemodynamics in LBW neonates | Epinephrin and dopamine increased BP, CBF, and HbD, whereas cerebral circulation is still pressure passive |
| Lemmers [21] (2006) | Preterm n = 83 | 26.6 ± 1.32 (with RDS, n = 18) 29.3 ± 1.74 (without RDS, n = 20) | INVOS 4100 | MABP (invasive) | 72 h | 1.0–2.0 h | The influence of RDS on arterial blood pressure in preterm neonates with and without RDS | RDS neonates showed impaired CA with positive MABP-crSO2 and negative MABP–cFTOE correlations |
| Victor [29] (2006) | Preterm n = 40 | 27.0 (23.0–30.0) | NIRO 500 | MABP (invasive) | 96 h | <24.0 h | Association between cardiocirculatory values and cerebral oxygenation | Stable very premature neonates showed intact CA without correlation of MABP-cFTOE and CO-cFTOE |
| Victor [28] (2006) | Preterm n = 35 | 27.0 (24.0–34.0) | NIRO 500 | MABP (invasive) and echo | 96 h | <24.0 h | Association between cardiocirculatory values and cerebral monitoring | aEEG and cFTOE maintained normal above MABP of 23 mmHg |
| O’Leary [36] (2009) | Preterm n = 88 | 26.0 (23.0–30.0) | NIRO 500 | MABP (invasive) | 96 h | 11.0 h | Association between CA and outcome | MAP-HbD gain reflecting cerebral pressure passivity was associated with IVH or PVL |
| Hahn [30] (2010) | Preterm n = 22 | 27.5 (24.0–29.0) | NIRO 300 | MABP (invasive) | 1.3–3.7 h | 17.4 h | Increasing precision of coherence analysis by adding MABP | CA measurements took hours and can be improved by adding MABP |
| Takami [51] (2010) | Preterm n = 16 | 25.2 ± 1.6 | NIRO 200 NIRO 300 | MABP (oscillometric and invasive) and echo | 72 h | 3.0–6.0 h | Detailed analyses of cerebral oxygenation and cardiac function | cTOI decreased initially, then increased, while FTOE showed the opposite pattern; MABP increased gradually |
| Bonestroo [31] (2011) | Preterm n = 142 | 30.0 (26.0–31.6) (volume, n = 33) (control 1, n = 33) 29.4 (25.9–31.6) (dopamine, n = 38) (control 2, n = 38) | INVOS 4100–5100 | MABP (invasive) | 1 h | 15 min before treatment | Effect of volume expansion and dopamine in hypotensive preterm neonates | No significant changed in rScO2 and cFTOE |
| Gilmore [24] (2011) | Preterm n = 23 | 26 ± 1 | Foresight | MABP (invasive) | 24–96 h | 14.4 ± 14.4 h | Relationship between CA and blood pressure | Correlation between MABP and impaired CA |
| Hahn [32] (2012) | Preterm n = 60 | 27 ± 1 | NIRO 300 | MABP (invasive) | 2.3 h | 2.3 ± 0.5 h | Neonates with inflammation and CA | Impairment of CA measured with OI worsened with lower MABP |
| Wong [37] (2012) | Preterm n = 32 | 26.3 ± 1.5 | NIRO 200 | MABP (invasive) | 57.0 ± 5.9 h | 12 ± 5.8 h | Relationship between cerebral autoregulatory capacity and blood pressure | Sick infants exhibited blood pressure-dependent variations in crSO2 |
| Alderliesten [41] (2013) | Preterm n = 90 | 27.9 (26.2–30.0) (with IVH, n = 30) 27.5 (25.4–31.0) (without IVH, n = 60) | INVOS 4100–5100 | MABP (invasive) | 24 h after IVH | 21.0 h | Association between CA and IVH | IVH infants exhibited increased crSO2, decreased cFTOE, and passive brain perfusion indicated by MABP–crSO2 correlation |
| Kooi [39] (2013) | Preterm n = 14 | 27.6 (25.0–28.7) | INVOS 5100C | MABP (invasive) | 1 h after volume therapy | 16.8 h | Effect of volume therapy in hypotensive neonates | Volume did not improve cFTOE in preterm neonates |
| Eriksen [40] (2014) | Preterm n = 60 | 26.2 ± 1.5 (dopamine, n = 13) 26.7 ± 1.2 (no dopamine, n = 47) | NIRO 300 | MABP (invasive) | 2.3 ± 0.5 h | 18 ± 9.4 h | Effect of dopamine therapy in terms of CA | Dopamine therapy was associated with decreased CA |
| Riera [27] (2014) | Preterm n = 54 | 27 ± 2 | NIRO 200NX | MABP (invasive) | 9.5 h | <24.0 h | To identify impaired hemodynamics | BIAR COH (a specific time–frequency analysis consisting of MABP and TOI) identified cerebral hypoperfusion |
| Binder-Heschl [55] (2015) | Preterm n = 46 | 33.4 ± 1.9 (hypotensive, n = 17) 33.3 ± 1.3 (normotensive, n = 29) | INVOS 5100 | MABP (oscillometric and invasive) and echo | 24 h | <6.0 h | CA during hypotension | There were no significant differences in mean 24-h crSO2 and cFTOE between hypotensive and normotensive neonates |
| Demel [49] (2015) | Term n = 7, Preterm n = 16 | 39.9 (37.0–40.2) (term, n = 7) 34.0 (32.2–35.6) (preterm, n = 16) | Oxiplex TS 3.1 | MABP (oscillometric) | 72 h | 7.0–11.0 h term 1.5–2.0 h preterm | Feasibility of NIRS and Doppler sonography | Measurements of crSO2 using frequency domain NIRS was feasible |
| Eriksen [26] (2015) | Preterm n = 60 | 27 ± 1 | NIRO 300 | MABP (invasive) | 2.3 ± 0.5 h | 18.0 ± 9.4 h | Comparison of two conventional methods used to describe CA | Time domain analysis using TOI and MABP appeared more robust in describing CA |
| Stammwitz [33] (2016) | Preterm n = 31 | 27.3 (26.0–32.0) | Critikon | MABP (invasive) | 68–76 h | <6.0 h | Association between CA and outcome | Higher variability of TOI was associated with IVH and death |
| Vesoulis [25] (2017) | Preterm n = 68 | 25.5 ± 1.3 | Foresight | MABP (invasive) | 72 h | 17.8 ± 9.7 h | Evaluation of the interaction between BP, changes in oxygen extraction, and maturity | In extreme preterm neonates, MABP and cFTOE showed a positive correlation, indicating immature autoregulation |
| Da Costa [34] (2018) | Preterm n = 44 | 25.0 (23.0–27.0) | NIRO 200NX | MABP (invasive) | 24 h | 3.1–12.6 h | To define optimal MABP using NIRS | Optimal MABP gained by TOI and HR identified risk patients |
| Pichler [53] (2018) | Preterm n = 98 | 33.1 (32.0–34.0) (with NIRS, n = 49) 33.4 (32.3–34.3) (without NIRS, n = 49) | NIRO 200NX | MABP (oscillometer and invasive) | 48 h | 2.0 (1.5–3.5) h (with NIRS) 2.5 (2.0–4.0) h (without NIRS) | Reduction of hypotensive episodes by using NIRS | cTOI measurements led to a non-significant reduction in arterial hypotension |
| Da Costa [35] (2019) | Preterm n = 43 | 25.7 (23.6–31.0) | NIRO 200NX | MABP (invasive) and echo | 48 h | 6.0 h | Association of MABP and IVH in preterm neonates | crSO2 was lower in neonates with IVH before and during the event |
| Bruckner [52] (2020) | Term n = 13, preterm n = 47 | 34.0 (33.0–35.0) (whole cohort) | INVOS 5100 | MABP (oscillometric and invasive) and echo | 24 h | 4.0–6.0 h | Association between cardiac function and crSO2 | In stable term and preterm neonates, crSO2 and cFTOE did not correlate with CO |
| Chock [38] (2020) | Preterm n = 103 | 26.2 ± 1.7 | INVOS 5100C | MABP (invasive) | 96 h | 8.0–21.0 h | Association between CA and outcome | MABP and crSO2 correlated in neonates with adverse outcome |
| Author (Reference) (Year) of Publication | Neonates, n | Gestational Age, Weeks | NIRS Device | Arterial Blood Pressure Evaluation | Duration | Initiation | Study Aim | Main Results Concerning BP and NIRS |
|---|---|---|---|---|---|---|---|---|
| Tsuji [23] (2000) | Preterm n = 32 | 26 (23.0–31.0) | NIRO 500 | MABP (invasive) | 30 min | <72 h | Association between CA and outcome | Concordant changes in HbD and MABP suggest impaired cerebrovascular function |
| Wong [42] (2008) | Preterm n = 24 | 26 ± 2 | NIRO 300 | MABP (invasive) | 3 h | 28 h | Association between CA and outcome | High coherence between MABP and cTOI indicates impaired CA in sick preterm neonates |
| De Smet [48] (2009) | Term and preterm n = 20 | 28.7 (24.0–39.0) | NIRO 300 | MABP (invasive) | 1.5–23.5 h | <72 h | To assess whether cTOI may replace HbD for measuring CA | cTOI and HbD showed similar results; both may be used for calculating CA |
| Caicedo [43] (2011) | Preterm n = 53 | 29 ± 2 | INVOS 4100 and NIRO 300 | MABP (invasive) | 6–70 h | 24–72 h | To assess whether cTOI and crSO2 may replace HbD for measuring CA | cTOI, crSO2, and HbD showed similar results; all three may be used for calculating CA |
| Zhang [44] (2011) | Preterm n = 17 | 26.4 (24.0–29.0) | NIRO 300 | MABP (invasive) | 72 h | 24–72 h | Association between CA and outcome | Neonates with IVH showed higher TOI, lower cFTOE, and reduced coherence between MABP and HbD |
| Mitra [45] (2014) | Preterm n = 31 | 26.1 (23.7–32.6) | NIRO 200NX | MABP (invasive) | 2 h | 48 h | Association between cardio-circulatory values and CBF in sick preterm neonates | cTOI and HR, reflecting cerebrovascular reactivity, showed a correlation with MABP |
| Verhagen [46] (2014) | Preterm n = 25 | 29.1 (25.4–31.7) | INVOS 4100–5100 | MABP (invasive) | 24 h | <72 h | Association between clinical variables and CA | Negative correlation between MABP and cFTOE suggests the absence of CA |
| Traub [47] (2021) | Preterm n = 17 | 26.5 (23.0–33.2) | Foresight | MABP (invasive) | 24 h | 88.8 h | To determine whether NIRS helps to identify neonates at risk | Neonates maintain intact CA within normal MABP ranges |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfurtscheller, D.; Baik-Schneditz, N.; Schwaberger, B.; Urlesberger, B.; Pichler, G. Insights into Neonatal Cerebral Autoregulation by Blood Pressure Monitoring and Cerebral Tissue Oxygenation: A Qualitative Systematic Review. Children 2023, 10, 1304. https://doi.org/10.3390/children10081304
Pfurtscheller D, Baik-Schneditz N, Schwaberger B, Urlesberger B, Pichler G. Insights into Neonatal Cerebral Autoregulation by Blood Pressure Monitoring and Cerebral Tissue Oxygenation: A Qualitative Systematic Review. Children. 2023; 10(8):1304. https://doi.org/10.3390/children10081304
Chicago/Turabian StylePfurtscheller, Daniel, Nariae Baik-Schneditz, Bernhard Schwaberger, Berndt Urlesberger, and Gerhard Pichler. 2023. "Insights into Neonatal Cerebral Autoregulation by Blood Pressure Monitoring and Cerebral Tissue Oxygenation: A Qualitative Systematic Review" Children 10, no. 8: 1304. https://doi.org/10.3390/children10081304
APA StylePfurtscheller, D., Baik-Schneditz, N., Schwaberger, B., Urlesberger, B., & Pichler, G. (2023). Insights into Neonatal Cerebral Autoregulation by Blood Pressure Monitoring and Cerebral Tissue Oxygenation: A Qualitative Systematic Review. Children, 10(8), 1304. https://doi.org/10.3390/children10081304






