Mediation Analysis of Waist Circumference in the Association of Gut Microbiota with Insulin Resistance in Children
Abstract
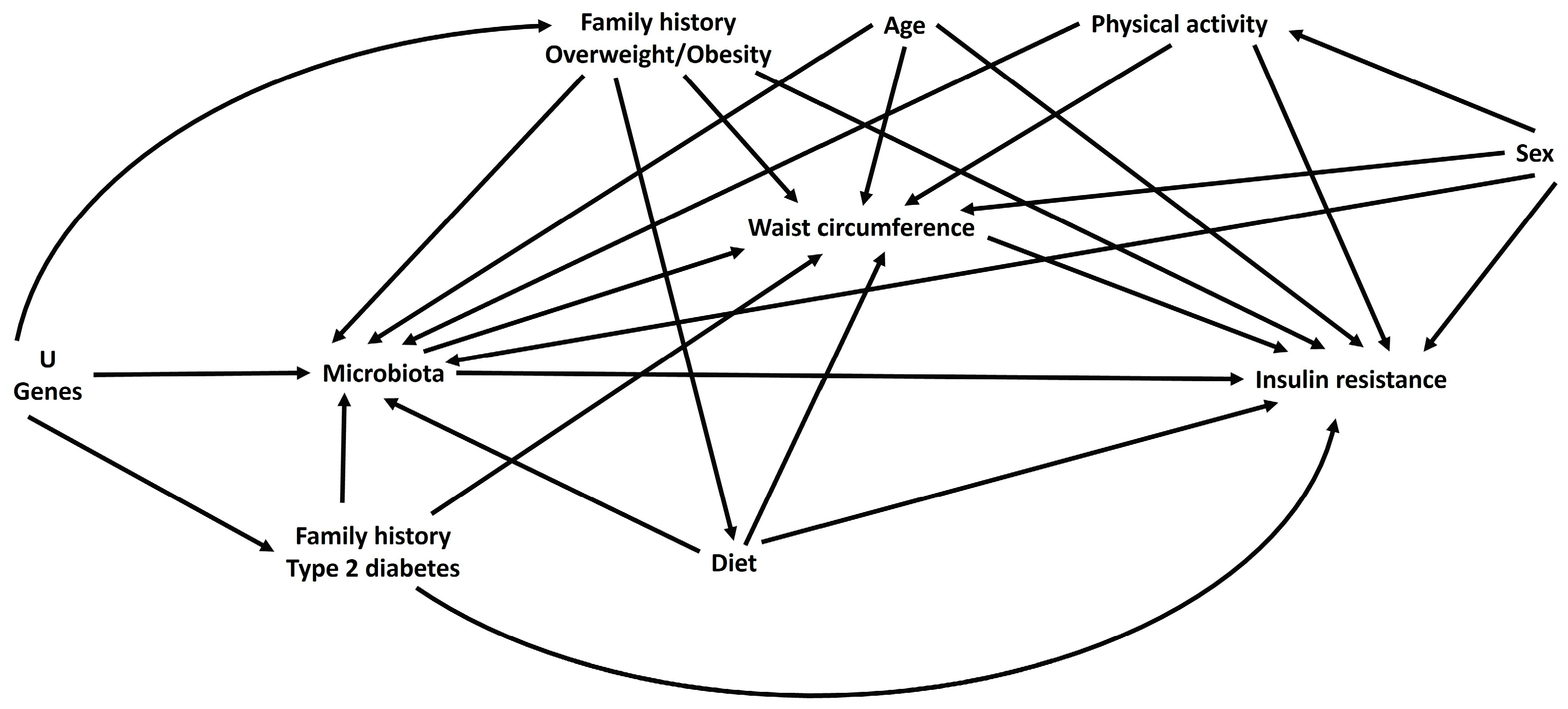
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Gut Microbiota
2.3. Waist Circumference and Body Mass Index
2.4. Biochemical Determination
2.5. Insulin Resistance
2.6. Hereditary Family History and Sociodemographic Data
2.7. Physical Activity
2.8. Diet
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brunetti, A.; Chiefari, E. Recent advances in the molecular genetics of type 2 diabetes mellitus. World J. Diabetes 2014, 5, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Morandi, A. Body composition and insulin resistance in children. Eur. J. Clin. Nutr. 2018, 72, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandhyala, S.M.; Talukdar, R. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Buccigrossi, V.; Nicastro, E. Functions of intestinal microflora in children. Curr. Opin. Gastroenterol. 2013, 29, 31–38. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Hawrelak, J.A.; Myers, S.P. The causes of intestinal dysbiosis: A review. Altern. Med. Rev. 2004, 9, 180–197. [Google Scholar]
- Pascale, A.; Marchesi, N. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, W. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Drissi, F.; Merhej, V. Comparative genomics analysis of Lactobacillus species associated with weight gain or weight protection. Nutr. Diabetes 2014, 24, e109. [Google Scholar] [CrossRef] [Green Version]
- Castañeda-Márquez, A.C.; Díaz-Benítez, C.E.; Bahena-Roman, M.; Campuzano-Benítez, G.E.; Galván-Portillo, M.; Campuzano-Rincón, J.C.; Lagunas-Martínez, A.; Bermudez-Morales, V.H.; Orbe-Orihuela, Y.C.; Peralta-Romero, J.; et al. Lactobacillus paracasei as a protective factor of obesity induced by an unhealthy diet in children. Obes. Res. Clin. Pract. 2020, 14, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Khalili, L.; Alipour, B. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran Biomed. J. 2019, 23, 68–77. [Google Scholar]
- Toejing, P.; Khampithum, N. Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods 2021, 10, 1455. [Google Scholar]
- Venegas, D.P.; De La Fuente, M.K. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliomäki, M.; Collado, M.C. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, B.G.; Stach, C.S. Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: Possible role in diabetes. mBio 2015, 24, e02554. [Google Scholar]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Velasco, B.I.; Cruz, M. La obesidad infantil como consecuencia de la interacción entre firmicutes y el consumo de alimentos con alto contenido energético [Childhood obesity is associated to the interaction between firmicutes and high energy food consumption]. Nutr. Hosp. 2014, 31, 1074–1081. [Google Scholar] [PubMed]
- Schoemann, A.M.; Boulton, A.J. Determining Power and Sample Size for Simple and Complex Mediation Models. Soc. Psychol. Personal. Sci. 2017, 8, 379–386. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Vilela, B.S.; Vasques, A.C. The HOMA-Adiponectin (HOMA-AD) Closely Mirrors the HOMA-IR Index in the Screening of Insulin Resistance in the Brazilian Metabolic Syndrome Study (BRAMS). PLoS ONE 2016, 11, e0158751. [Google Scholar] [CrossRef] [Green Version]
- Hernández, B.; Gortmaker, S.L. Validez y reproducibilidad de un cuestionario de actividad e inactividad física para escolares de la ciudad de México [Validity and reproducibility of a questionnaire on physical activity and non-activity for school children in Mexico City]. Salud Publica Mex. 2000, 42, 315–323. [Google Scholar] [CrossRef] [Green Version]
- de Onis, M.; Onyango, A.W. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.J. The extracellular domain of Staphylococcus aureus LtaS binds insulin and induces insulin resistance during infection. Nat. Microbiol. 2018, 3, 622–631. [Google Scholar] [CrossRef]
- Ayala-García, J.C.; Lagunas-Martínez, A. High Relative Abundance of Staphylococcus aureus and Serum Cytokines Are Associated with Cardiometabolic Abnormalities in Children. Metab. Syndr. Relat. Disord. 2022, 20, 303–311. [Google Scholar]
- Collado, M.C.; Isolauri, E. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Isolauri, E. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Van Hul, M. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ni, Y. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, e2100536. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.L.; Onnerfält, J. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity 2012, 20, 2257–2261. [Google Scholar] [PubMed]
- Cani, P.D.; Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [PubMed] [Green Version]
- Roberfroid, M.; Gibson, G.R. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. S2), S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Oca, P.; Robles-Vera, I. Gut DYSBIOSIS and altered barrier function precedes the appearance of metabolic syndrome in a rat model of nutrient-induced catch-up growth. J. Nutr. Biochem. 2020, 81, 108383. [Google Scholar]
- Stadlbauer, V.; Engertsberger, L. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef]
- Schumann, R.R. Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: A short review. Res. Immunol. 1992, 143, 11–15. [Google Scholar]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [Green Version]
- De Loera-Rodriguez, C.O.; Delgado-Rizo, V. Over-expression of TLR4-CD14, pro-inflammatory cytokines, metabolic markers and NEFAs in obese non-diabetic Mexicans. J. Inflamm. 2014, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Patimah, I. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Oliva-Olivera, W. Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E319–E332. [Google Scholar] [CrossRef]
- Cândido, A.P.C.; Geloneze, B. Adiponectin, HOMA-Adiponectin, HOMA-IR in Children and Adolescents: Ouro Preto Study. Indian J. Pediatr. 2021, 88, 336–344. [Google Scholar] [PubMed]
- Makni, E.; Moalla, W. The Homeostasis Model Assessment-adiponectin (HOMA-AD) is the most sensitive predictor of insulin resistance in obese children. Ann. D’endocrinol. 2012, 73, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yeckel, C.W.; Ram, W. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J. Clin. Endocrinol. Metab. 2004, 89, 1096–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conwell, L.S.; Trost, S.G. Indexes of insulin resistance and secretion in obese children and adolescents: A validation study. Diabetes Care 2004, 27, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005, 115, e500–e503. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Neeland, I.J. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar]
- VanderWeele, T.J. Invited commentary: Structural equation models and epidemiologic analysis. Am. J. Epidemiol. 2012, 176, 608–612. [Google Scholar]
- Li, W.Z.; Stirling, K. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes 2020, 11, 293–308. [Google Scholar]
- Collado, M.C.; Derrien, M. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haarman, M.; Knol, J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchetti De Gregoris, T.; Aldred, N. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods 2011, 86, 351–356. [Google Scholar] [CrossRef] [PubMed]
| Characteristics a n = 533 | Normal Weight | Overweight/Obesity | p-Value |
|---|---|---|---|
| n = 265 (51%) | n = 268 (49%) | ||
| Age (years) | 9 (7–10) | 9 (8–10) | 0.013 |
| Physical activity (METs/hour/week) | 301 (150–577) | 337 (162–625) | 0.330 |
| Sex | |||
| Boy (%) | 52 | 48 | 0.368 |
| Girl (%) | 48 | 52 | |
| Family History of OW/OB | |||
| Yes (%) | 43 | 57 | 0.001 |
| No (%) | 58 | 42 | |
| Family History of T2D | |||
| Yes (%) | 37 | 63 | 0.020 |
| No (%) | 52 | 48 | |
| Macronutrient Consumption | |||
| Carbohydrates (g/day) | 272.01 (210.27–339.53) | 272.64 (211.07–357.14) | 0.571 |
| Lipids (g/day) | 74.84 (59.28–93.39) | 74.81 (58.18–93.66) | 0.989 |
| Proteins (g/day) | 69.66 (55.15–83.43) | 68.13 (53.48–86.88) | 0.963 |
| Exposure Variables | |||
| S. aureus (RA) | 0.0000368 (7.54 × 10−6–0.0002222) | 0.0000558 (9.83 × 10−6–0.000277) | 0.140 |
| L. paracasei (RA) | 0.0002953 (0.0000239–0.0025059) | 0.0003333 (0.0000212–0.0044035) | 0.622 |
| L. casei (RA) | 0.0004417 (0.0000628–0.0064059) | 0.0004487 (0.0000457–0.0075155) | 0.858 |
| L. reuteri (RA) | 0.0001371 (0.0000198–0.0009797) | 0.0001141 (0.0000202–0.0007388) | 0.521 |
| A. muciniphila (RA) | 0.0033518 (0.0000323–0.0585953) | 0.0034792 (0.0000413–0.0742626) | 0.539 |
| Mediator Variable | |||
| Waist circumference (cm) | 56.7 (53.5–61.2) | 74.4 (67.7–79.9) | <0.001 |
| Outcome Variables | |||
| HOMA-IR | 0.36 (0.28–0.53) | 0.81 (0.41–1.55) | <0.001 |
| HOMA-AD | 0.06 (0.05–0.10) | 0.17 (0.06–0.21) | <0.001 |
| Waist Circumference ≥ 63.6 cm | |||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| RA of Staphylococcus aureus * | |||
| Medium tertile | 2.30 | 1.44, 3.65 | <0.001 |
| High tertile | 1.72 | 1.08, 2.73 | 0.022 |
| RA of Lactobacillus reuteri * | |||
| Medium tertile | 1.19 | 0.76, 1.89 | 0.447 |
| High tertile | 1.21 | 0.77, 1.90 | 0.417 |
| RA of Lactobacillus paracasei ** | |||
| Low tertile | 0.82 | 0.52, 1.29 | 0.393 |
| Medium tertile | 0.89 | 0.57, 1.41 | 0.639 |
| RA of Lactobacillus casei ** | |||
| Low tertile | 0.78 | 0.49, 1.23 | 0.283 |
| Medium tertile | 0.63 | 0.40, 1.00 | 0.050 |
| RA of Akkermansia muciniphila ** | |||
| Low tertile | 0.62 | 0.39, 0.99 | 0.046 |
| Medium tertile | 0.50 | 0.32, 0.80 | 0.004 |
| HOMA-IR | HOMA-AD | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| Waist circumference ≥ 63.6 cm * | 0.62 | 0.49, 0.75 | <0.001 | 0.16 | 0.13, 0.20 | <0.001 |
| RA of Staphylococcus aureus ** | ||||||
| Medium tertile | 0.15 | −0.01, 0.31 | 0.066 | 0.03 | −0.01, 0.07 | 0.111 |
| High tertile | 0.04 | −0.12, 0.20 | 0.616 | 0.01 | −0.03, 0.06 | 0.459 |
| RA of Lactobacillus reuteri ** | ||||||
| Medium tertile | 0.05 | −0.11, 0.21 | 0.544 | 0.006 | −0.03, 0.05 | 0.766 |
| High tertile | −0.08 | −0.24, 0.08 | 0.345 | −0.005 | −0.05, 0.04 | 0.801 |
| RA of Lactobacillus paracasei *** | ||||||
| Low tertile | −0.10 | −0.27, 0.06 | 0.210 | −0.02 | −0.06, 0.02 | 0.407 |
| Medium tertile | 0.13 | −0.03, 0.29 | 0.105 | 0.03 | −0.01, 0.07 | 0.141 |
| RA of Lactobacillus casei *** | ||||||
| Low tertile | −0.06 | −0.22, 0.11 | 0.492 | −0.01 | −0.05, 0.03 | 0.585 |
| Medium tertile | −0.07 | −0.23, 0.10 | 0.445 | −0.02 | −0.06, 0.02 | 0.289 |
| RA of Akkermansia muciniphila *** | ||||||
| Low tertile | 0.04 | −0.12, 0.21 | 0.611 | 0.02 | −0.02, 0.06 | 0.410 |
| Medium tertile | 0.05 | −0.11, 0.21 | 0.527 | 0.002 | −0.04, 0.04 | 0.907 |
| HOMA-IR | HOMA-AD | |||||
|---|---|---|---|---|---|---|
| Path Coefficient (PC) | 95% CI | p-Value | Path Coefficient (PC) | 95% CI | p-Value | |
| Direct effect | ||||||
| RA of Staphylococcus aureus * | ||||||
| Medium tertile | 0.043 | −0.11, 0.19 | 0.574 | 0.0048 | −0.03, 0.04 | 0.807 |
| High tertile | −0.03 | −0.18, 0.12 | 0.696 | −0.0033 | −0.04, 0.03 | 0.868 |
| RA of Lactobacillus reuteri * | ||||||
| Medium tertile | 0.025 | −0.12, 0.17 | 0.744 | −0.00042 | −0.04, 0.04 | 0.983 |
| High tertile | −0.11 | −0.25, 0.04 | 0.165 | −0.013 | −0.05, 0.02 | 0.518 |
| RA of Lactobacillus paracasei ** | ||||||
| Low tertile | −0.076 | −0.22, 0.07 | 0.317 | −0.01 | −0.04, 0.03 | 0.598 |
| Medium tertile | 0.15 | 0.002, 0.30 | 0.047 | 0.036 | −0.002, 0.07 | 0.066 |
| RA of Lactobacillus casei ** | ||||||
| Low tertile | −0.036 | −0.19, 0.11 | 0.639 | −0.0023 | −0.04, 0.04 | 0.906 |
| Medium tertile | −0.0016 | −0.15, 0.15 | 0.983 | −0.0063 | −0.04, 0.03 | 0.748 |
| RA of Akkermansia muciniphila ** | ||||||
| Low tertile | 0.11 | −0.04, 0.26 | 0.158 | 0.035 | −0.003, 0.07 | 0.074 |
| Medium tertile | 0.15 | −0.003, 0.30 | 0.056 | 0.027 | −0.01, 0.06 | 0.164 |
| Indirect effect | ||||||
| RA of Staphylococcus aureus * | ||||||
| Medium tertile | 0.11 | 0.04, 0.17 | 0.001 | 0.03 | 0.01, 0.05 | 0.001 |
| High tertile | 0.07 | 0.01, 0.13 | 0.024 | 0.02 | 0.002, 0.03 | 0.024 |
| RA of Lactobacillus reuteri * | ||||||
| Medium tertile | 0.02 | −0.04, 0.09 | 0.417 | 0.007 | −0.009, 0.02 | 0.417 |
| High tertile | 0.03 | −0.03, 0.09 | 0.387 | 0.007 | −0.01, 0.02 | 0.386 |
| RA of Lactobacillus paracasei ** | ||||||
| Low tertile | −0.03 | −0.09, 0.03 | 0.373 | −0.007 | −0.02, 0.009 | 0.373 |
| Medium tertile | −0.02 | −0.08, 0.04 | 0.610 | −0.004 | −0.02, 0.01 | 0.610 |
| RA of Lactobacillus casei ** | ||||||
| Low tertile | −0.03 | −0.10, 0.03 | 0.260 | −0.009 | −0.03, 0.007 | 0.260 |
| Medium tertile | −0.06 | −0.01, 0.0006 | 0.052 | −0.02 | −0.03, 0.0001 | 0.052 |
| RA of Akkermansia muciniphila ** | ||||||
| Low tertile | −0.06 | −0.13, −0.0013 | 0.045 | −0.02 | −0.03, −0.0004 | 0.045 |
| Medium tertile | −0.09 | −0.16, −0.03 | 0.005 | −0.02 | −0.04, −0.007 | 0.005 |
| Total effect | ||||||
| RA of Staphylococcus aureus * | ||||||
| Medium tertile | 0.15 | −0.01, 0.31 | 0.063 | 0.035 | −0.007, 0.07 | 0.108 |
| High tertile | 0.04 | −0.12, 0.20 | 0.612 | 0.017 | −0.02, 0.06 | 0.455 |
| RA of Lactobacillus reuteri * | ||||||
| Medium tertile | 0.05 | −0.11, 0.21 | 0.514 | 0.006 | −0.03, 0.05 | 0.764 |
| High tertile | −0.08 | −0.24, 0.08 | 0.385 | −0.005 | −0.05, 0.04 | 0.799 |
| RA of Lactobacillus paracasei ** | ||||||
| Low tertile | −0.10 | −0.26, 0.05 | 0.205 | −0.02 | −0.06, 0.02 | 0.402 |
| Medium tertile | 0.13 | −0.03, 0.29 | 0.101 | 0.03 | −0.01, 0.07 | 0.136 |
| RA of Lactobacillus casei ** | ||||||
| Low tertile | −0.07 | −0.24, 0.09 | 0.387 | −0.01 | −0.05, 0.03 | 0.582 |
| Medium tertile | −0.06 | −0.22, 0.10 | 0.441 | −0.02 | −0.06, 0.02 | 0.284 |
| RA of Akkermansia muciniphila ** | ||||||
| Low tertile | 0.04 | −0.12, 0.20 | 0.607 | 0.02 | −0.02, 0.06 | 0.406 |
| Medium tertile | 0.05 | −0.11, 0.21 | 0.523 | 0.002 | −0.04, 0.04 | 0.906 |
| HOMA-IR | HOMA-AD | |||||
|---|---|---|---|---|---|---|
| Path Coefficient (PC) | 95% CI | p-Value | Path Coefficient (PC) | 95% CI | p-Value | |
| Direct effect | ||||||
| Gut microbiota | ||||||
| Profile 1 | −1.2 | −4.6, 2.1 | 0.479 | −0.4 | −1.5, 0.76 | 0.500 |
| Profile 2 | −0.98 | −2.45, 0.49 | 0.190 | −0.092 | −0.42, 0.24 | 0.588 |
| Indirect effect | ||||||
| Gut microbiota | ||||||
| Profile 1 | 0.63 | 0.50, 0.78 | <0.001 | 0.17 | 0.13, 0.20 | <0.001 |
| Profile 2 | 0.66 | 0.51, 0.81 | <0.001 | 0.17 | 0.13, 0.20 | <0.001 |
| Total effect | ||||||
| Gut microbiota | ||||||
| Profile 1 | −0.57 | −3.9, 2.8 | 0.736 | −0.23 | −1.38, 0.93 | 0.699 |
| Profile 2 | −0.32 | −1.76, 1.12 | 0.664 | 0.07 | −0.24, 0.40 | 0.642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-García, J.C.; Díaz-Benítez, C.E.; Lagunas-Martínez, A.; Orbe-Orihuela, Y.C.; Castañeda-Márquez, A.C.; Ortiz-Panozo, E.; Bermúdez-Morales, V.H.; Cruz, M.; Burguete-García, A.I. Mediation Analysis of Waist Circumference in the Association of Gut Microbiota with Insulin Resistance in Children. Children 2023, 10, 1382. https://doi.org/10.3390/children10081382
Ayala-García JC, Díaz-Benítez CE, Lagunas-Martínez A, Orbe-Orihuela YC, Castañeda-Márquez AC, Ortiz-Panozo E, Bermúdez-Morales VH, Cruz M, Burguete-García AI. Mediation Analysis of Waist Circumference in the Association of Gut Microbiota with Insulin Resistance in Children. Children. 2023; 10(8):1382. https://doi.org/10.3390/children10081382
Chicago/Turabian StyleAyala-García, Juan Carlos, Cinthya Estefhany Díaz-Benítez, Alfredo Lagunas-Martínez, Yaneth Citlalli Orbe-Orihuela, Ana Cristina Castañeda-Márquez, Eduardo Ortiz-Panozo, Víctor Hugo Bermúdez-Morales, Miguel Cruz, and Ana Isabel Burguete-García. 2023. "Mediation Analysis of Waist Circumference in the Association of Gut Microbiota with Insulin Resistance in Children" Children 10, no. 8: 1382. https://doi.org/10.3390/children10081382







