Multisensory Texture Perception in Individuals with Williams Syndrome
Abstract
:1. Introduction
- haptic and visuo-haptic performances would not significantly differ for all participants;
- haptic and visuo-haptic performances would be inferior to visual performances for all participants;
- people with WS would show comparable performance to that of TD children matched on MA for the three tasks;
- people with WS would show inferior performance to that of individuals matched on CA for the three tasks.
2. Materials and Methods
2.1. Participants
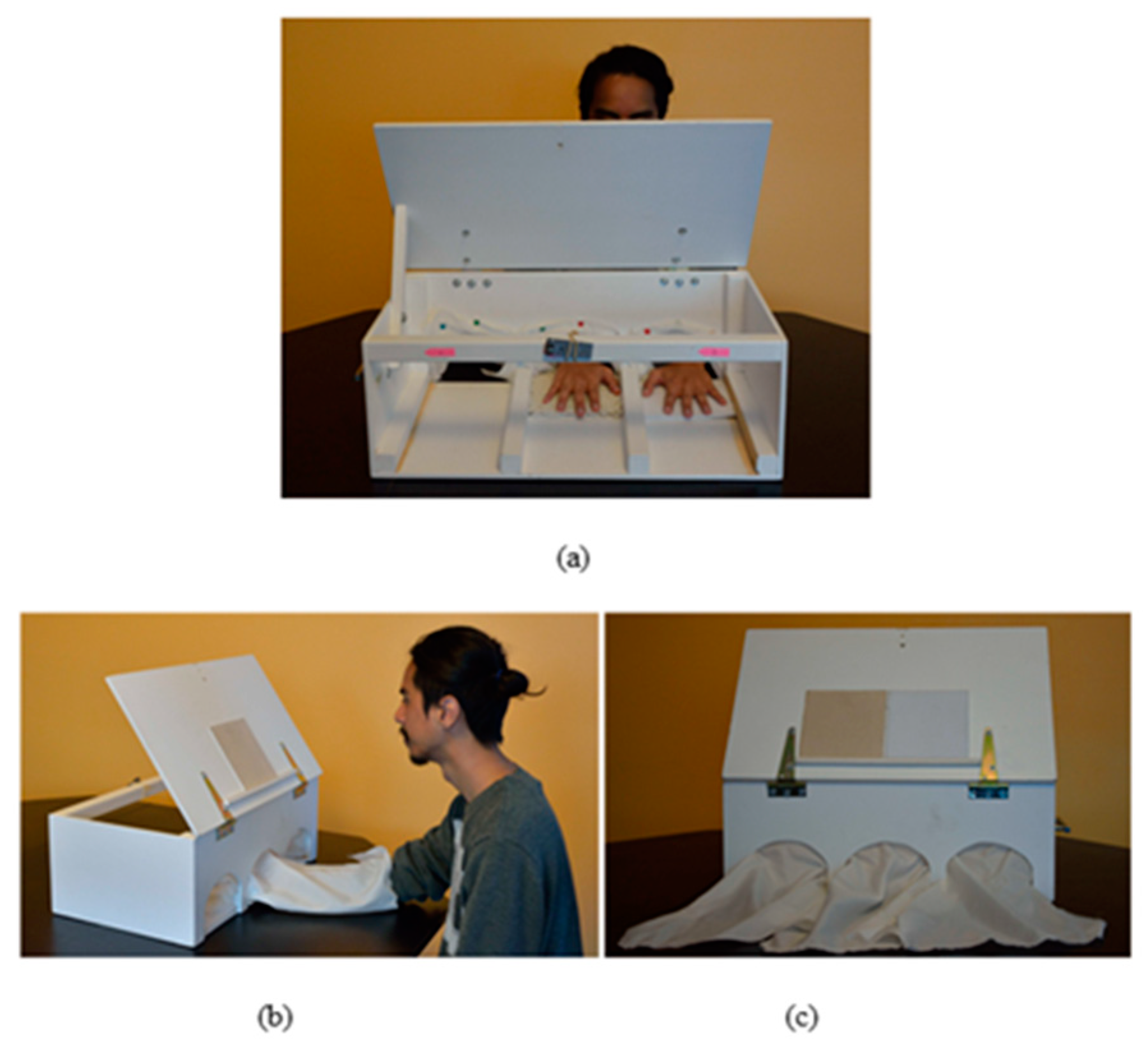
2.2. Stimuli
2.3. Procedure
2.4. Statistical Analyses
3. Results
3.1. Identical Pairs
3.1.1. WS Group versus TD-CA Group
3.1.2. WS Group versus TD-MA Group
3.1.3. Correlations between Matching Performances and Respective Chronological Age and RCPM Scores
3.2. Different Pairs
3.2.1. WS Group versus TD-CA Group
3.2.2. WS Group versus TD-MA Group
3.2.3. Correlations between Matching Performances and Respective Chronological Age and RCPM Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewart, A.K.; Morris, C.A.; Atkinson, D.; Jin, W.; Sternes, K.; Spallone, P.; Stock, A.D.; Leppert, M. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat. Genet. 1993, 5, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Morris, C. The dysmorphology, genetics, and natural history of Williams-Beuren syndrome. In Williams-Beuren Syndrome: Research, Evaluation, and Treatment; Morris, C., Lenhoff, H., Wang, P., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2006; pp. 3–17. [Google Scholar]
- Strømme, P.; Bjømstad, P.G.; Ramstad, K. Prevalence estimation of williams syndrome. J. Child Neurol. 2002, 17, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.A.; Wilson, S.J.; Reutens, D.C. Research Review: Williams syndrome: A critical review of the cognitive, behavioral, and neuroanatomical phenotype. J. Child Psychol. Psychiatry 2008, 49, 576–608. [Google Scholar] [CrossRef] [PubMed]
- Miezah, D.; Porter, M.; Rossi, A.; Kazzi, C.; Batchelor, J.; Reeve, J. Cognitive profile of young children with Williams syndrome. J. Intellect. Disabil. Res. 2021, 65, 784–794. [Google Scholar] [CrossRef]
- Bellugi, U.; Lichtenberger, L.; Jones, W.; Lai, Z.; George, M.S. The neurocognitive profile of williams syndrome: A complex pattern of strengths and weaknesses. J. Cogn. Neurosci. 2000, 12, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Heiz, J.; Barisnikov, K. Visual–motor integration, visual perception and motor coordination in a population with Williams syndrome and in typically developing children. J. Intellect. Disabil. Res. 2016, 60, 945–955. [Google Scholar] [CrossRef]
- Mervis, C.B.; John, A.E. Cognitive and behavioral characteristics of children with Williams syndrome: Implications for intervention approaches. Am. J. Med. Genet. 2010, 154C, 229–248. [Google Scholar] [CrossRef]
- Royston, R.; Waite, J.; Howlin, P. Williams syndrome: Recent advances in our understanding of cognitive, social and psychological functioning. Curr. Opin. Psychiatry 2019, 32, 60–66. [Google Scholar] [CrossRef]
- Glod, M.; Riby, D.M.; Rodgers, J. Sensory Processing in Williams Syndrome: A Narrative Review. Rev. J. Autism. Dev. Disord. 2020, 7, 32–45. [Google Scholar] [CrossRef]
- Glod, M.; Riby, D.M.; Rodgers, J. Sensory processing profiles and autistic symptoms as predictive factors in autism spectrum disorder and Williams syndrome. J. Intellect. Disabil. Res. 2020, 64, 657–665. [Google Scholar] [CrossRef]
- Baker, A.E.Z.; ALane; Angley, M.T.; Young, R.L. The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. J. Autism. Dev. Disord. 2008, 38, 867–875. [Google Scholar] [CrossRef] [PubMed]
- John, A.E.; Mervis, C.B. Sensory modulation impairments in children with Williams syndrome. Am. J. Med. Genet. 2010, 154C, 266–276. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, D.N.; Miller, L.J.; Shyu, V.; Dunn, W. Short Sensory Profile; Psychological Corporation: New York, NY, USA, 1999. [Google Scholar]
- Powell, B.; Van Herwegen, J. Sensory processing in Williams Syndrome: Individual differences and changes over time. Rev. J. Autism. Dev. Disord. 2022, 52, 3129–3141. [Google Scholar] [CrossRef] [PubMed]
- Farran, E.K.; Wilmut, K. Texture segmentation in Williams syndrome. Neuropsychologia 2007, 45, 1009–1018. [Google Scholar] [CrossRef]
- Farran, E.K. Perceptual grouping ability in Williams syndrome: Evidence for deviant patterns of performance. Neuropsychologia 2005, 43, 815–822. [Google Scholar] [CrossRef]
- Farran, E.K.; Brown, J.H.; Cole, V.L.; Houston-Price, C.; Karmiloff-Smith, A. The development of perceptual grouping in infants with Williams syndrome. Int. J. Dev. Sci. 2007, 1, 253–271. [Google Scholar] [CrossRef]
- Yoshioka, T.; Dillon, M.R.; Beck, G.C.; Rapp, B.; Landau, B. Tactile localization on digits and hand: Structure and development. Psychol. Sci. 2013, 24, 1653–1663. [Google Scholar] [CrossRef]
- Schulte, T.; Müller-Oehring, E.M. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol. Rev. 2010, 20, 174–190. [Google Scholar] [CrossRef]
- Luders, E.; Di Paola, M.; Tomaiuolo, F.; Thompson, P.M.; Toga, A.W.; Vicari, S.; Petrides, M.; Caltagirone, C. Callosal morphology in Williams syndrome: A new evaluation of shape and thickness. Neuroreport 2007, 18, 203–207. [Google Scholar] [CrossRef]
- Martens, M.A.; Wilson, S.J.; Chen, J.; Wood, A.G.; Reutens, D.C. Handedness and corpus callosal morphology in Williams syndrome. Dev. Psychopathol. 2013, 25, 253–260. [Google Scholar] [CrossRef]
- Sampaio, A.; Bouix, S.; Sousa, N.; Vasconcelos, C.; Férnandez, M.; Shenton, M.E.; Gonçalves, Ó.F. Morphometry of corpus callosum in Williams syndrome: Shape as an index of neural development. Brain Struct. Funct. 2013, 218, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.E.; Eliez, S.; Warsofsky, I.S.; Bellugi, U.; Reiss, A.L. Corpus callosum morphology of Williams syndrome: Relation to genetics and behavior. Dev. Med. Child Neurol. 2001, 43, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Rondan, C.; Mancini, J.; Deruelle, C. Behavioural indexes of callosal functioning in Williams syndrome. J. Neuropsychol. 2007, 1, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hatwell, Y. Intermodal coordinations in children and adults. In Touching for Knowing. Cognitive Psychology of Haptic Manual Perception; Hatwell, Y., Streri, A., Gentaz, E., Eds.; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2003; pp. 207–219. [Google Scholar]
- Sathian, K.; Lacey, S. Cross-modal interactions of the tactile system. Curr. Dir. Psychol. Sci. 2022, 31, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-F. Cross-modal contextual coherence of information integration in people with Williams syndrome. Res. Dev. Disabil. 2013, 34, 4319–4327. [Google Scholar] [CrossRef]
- Hsu, C.-F. Is the contextual effect weak in people with Williams syndrome? An investigation of information integration ability using pictures. Res. Dev. Disabil. 2013, 34, 932–939. [Google Scholar] [CrossRef]
- Hsu, C.-F. Modality effect on contextual integration in people with Williams syndrome. Res. Dev. Disabil. 2014, 35, 1571–1578. [Google Scholar] [CrossRef]
- Järvinen-Pasley, A.; Vines, B.W.; Hill, K.J.; Yam, A.; Grichanik, M.; Mills, D.; Reiss, A.L.; Korenberg, J.R.; Bellugi, U. Cross-modal influences of affect across social and non-social domains in individuals with Williams syndrome. Neuropsychologia 2010, 48, 456–466. [Google Scholar] [CrossRef]
- Böhning, M.; Campbell, R.; Karmiloff-Smith, A. Audiovisual speech perception in Williams syndrome. Neuropsychologia 2002, 40, 1396–1406. [Google Scholar] [CrossRef]
- D’Souza, D.; D’Souza, H.; Johnson, M.H.; Karmiloff-Smith, A. Audio-visual speech perception in infants and toddlers with Down syndrome, fragile X syndrome, and Williams syndrome. Inf. Behav. Dev. 2016, 44, 249–262. [Google Scholar] [CrossRef]
- Lederman, S.J.; Klatzky, R.L. Relative availability of surface and object properties during early haptic processing. J. Exp. Psychol. Hum. Percept. Perform. 1997, 23, 1680–1707. [Google Scholar] [CrossRef] [PubMed]
- Gentaz, E.; Hatwell, Y. Haptic processing of spatial and material object properties. In Touching for Knowing. Cognitive Psychology of Haptic Manual Perception; Hatwell, Y., Streri, A., Gentaz, E., Eds.; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2003; pp. 123–159. [Google Scholar]
- Di Stefano, N.; Spence, C. Roughness perception: A multisensory/crossmodal perspective. Atten. Percept. Psychophys. 2022, 84, 2087–2114. [Google Scholar] [CrossRef] [PubMed]
- Katz, D. The World of Touch; Krueger, L.E., Translator; Erlbaum: Hillsdale, NJ, USA, 1989; (Original work published in 1925). [Google Scholar]
- Heller, M.A. Texture perception in sighted and blind observers. Percept. Psychophys. 1989, 45, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lederman, S.J. Tactile roughness of grooved surfaces: The touching process and effects of macro- and microsurface structure. Percept. Psychophys. 1974, 16, 385–395. [Google Scholar] [CrossRef]
- Srinivasan, M.A.; LaMotte, R.H. Tactual discrimination of softness: Abilities and mechanisms. In Somesthesis and the Neurobiology of the Somatosensory Cortex; Franzen, O., Johansson, R., Terenius, L., Eds.; Birkhäuser: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1996; pp. 123–136. [Google Scholar]
- Heller, M.A.; Gentaz, E. Psychology of Touch and Blindness; Psychology Press: New York, NY, USA, 2013. [Google Scholar]
- Picard, D. Partial perceptual equivalence between vision and touch for texture information. Acta Psychol. 2006, 121, 227–248. [Google Scholar] [CrossRef]
- Picard, D. Tactual, visual, and cross-modal transfer of texture in 5- and 8-year-old children. Perception 2007, 36, 722–736. [Google Scholar] [CrossRef]
- Garbin, C.P. Visual-haptic perceptual nonequivalence for shape information and its impact upon cross-modal performance. J. Exp. Psychol. Hum. Percept. Perform. 1988, 14, 547–553. [Google Scholar] [CrossRef]
- Garbin, C.P. Visual-touch perceptual equivalence for shape information in children and adults. Percept. Psychophys. 1990, 48, 271–279. [Google Scholar] [CrossRef]
- Hollins, M.; Risner, S.R. Evidence for the duplex theory of tactile texture perception. Percept. Psychophys. 2000, 62, 695–705. [Google Scholar] [CrossRef]
- Lederman, S.J.; Klatzky, R.L. Multisensory texture perception. In The Handbook of Multisensory Processes; Calvert, G.A., Spence, C., Stein, B.E., Eds.; MIT Press: Cambridge, MA, USA, 2004; pp. 107–122. [Google Scholar]
- Okamoto, S.; Nagano, H.; Yamada, Y. Psychophysical dimensions of tactile perception of textures. IEEE Trans. Haptics. 2013, 6, 81–93. [Google Scholar] [CrossRef]
- Raven, J.; Raven, J.C.; Court, J.H. Manual for Raven’s Progressive Matrices and Vocabulary Scales; Oxford Psychologist Press: Oxford, UK, 1998. [Google Scholar]
- Van Herwegen, J.; Farran, E.; Annaz, D. Item and error analysis on Raven’s Coloured Progressive Matrices in Williams Syndrome. Res. Dev. Disabil. 2011, 32, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lederman, S.J.; Klatzky, R.L. Hand movements: A window into haptic object recognition. Cogn. Psychol. 1987, 19, 342–368. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Del Viva, M.; Sandini, G.; Burr, D.C. Young children do not integrate visual and haptic form information. Curr. Biol. 2008, 18, 694–698. [Google Scholar] [CrossRef]
- Klatzky, R.L.; Lederman, S.J. Toward a computational model of constraint-driven exploration and haptic object identification. Perception 1993, 22, 597–621. [Google Scholar] [CrossRef]
- Klatzky, R.L.; Lederman, S.J.; Hamilton, C.; Grindley, M.; Swendsen, R.H. Feeling textures through a probe: Effects of probe and surface geometry and exploratory factors. Percept. Psychophys 2003, 65, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Uljarević, M.; Labuschagne, I.; Bobin, R.; Atkinson, A.; Hocking, D.R. Brief report: The impact of sensory hypersensitivity and intolerance of uncertainty on anxiety in Williams syndrome. J. Autism. Dev. Disord. 2018, 48, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Janes, E.; Riby, D.M.; Rodgers, J. Exploring the prevalence and phenomenology of repetitive behaviours and abnormal sensory processing in children with Williams Syndrome. J. Intellect. Disabil. Res. 2014, 58, 746–757. [Google Scholar] [CrossRef]
- Wuang, Y.-P.; Tsai, H.-Y. Sensorimotor and visual perceptual functioning in school-aged children with Williams syndrome. J. Intellect. Disabil. Res. 2017, 61, 348–362. [Google Scholar] [CrossRef]
- Farran, E.K.; Purser, H.R.; Jarrold, C.; Thomas, M.S.; Scerif, G.; Stojanovik, V.; Van Herwegen, J. Cross-sectional and longitudinal assessment of cognitive development in Williams syndrome. Dev. Sci. 2023, e13421. [Google Scholar] [CrossRef]
- Rose, S.A.; Feldman, J.F.; Jankowski, J.J.; Rossem, R.V. A cognitive cascade in infancy: Pathways from prematurity to later mental development. Intelligence 2008, 36, 367–378. [Google Scholar] [CrossRef]
- Rose, S.A.; Feldman, J.F.; Jankowski, J.J.; Van Rossem, R. Pathways from prematurity and infant abilities to later cognition. Child Dev. 2005, 76, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Giannopulu, I.; Cusin, F.; Escolano, S.; Dellatolas, G. Cognitive associations of bimanual haptico-visual recognition in preschoolers. Child Neuropsychol. 2008, 14, 227–236. [Google Scholar] [CrossRef] [PubMed]
| WS N = 23 | TD-CA N = 23 | TD-MA N = 23 | |
|---|---|---|---|
| Chronological Age; Mean (SD) | 21.9 (9.7) | 21.8 (9.8) | 6.3 (1.2) |
| % Females | 43.5 | 47.8 | 47.8 |
| % Right-handed | 87 | 91 | 91 |
| RPCM Score; Mean (SD) | 17.7 (a) (7.9) | _ (b) | 18 (c) (7.6) |
| Tasks | TD-CA | TD-MA | WS |
|---|---|---|---|
| Visual | 95.4 (5.1) | 93.8 (6.5) | 92.7 (10.6) |
| Haptic | 84.5 (13.3) | 87.8 (10.1) | 87.5 (11.9) |
| Visuo-haptic | 84.2 (12.5) | 93.2 (8) | 86.7 (13.5) |
| Tasks | TD-CA | TD-MA | WS |
|---|---|---|---|
| Visual | 91.8 (8.9) | 85.9 (19.7) | 76.6 (29) |
| Haptic | 66.3 (13.3) | 47.8 (25.5) | 38 (27.6) |
| Visuo-haptic | 68.5 (16.8) | 43.5 (25.5) | 26.6 (22.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheam, C.; Barisnikov, K.; Gentaz, E.; Lejeune, F. Multisensory Texture Perception in Individuals with Williams Syndrome. Children 2023, 10, 1494. https://doi.org/10.3390/children10091494
Cheam C, Barisnikov K, Gentaz E, Lejeune F. Multisensory Texture Perception in Individuals with Williams Syndrome. Children. 2023; 10(9):1494. https://doi.org/10.3390/children10091494
Chicago/Turabian StyleCheam, Caroline, Koviljka Barisnikov, Edouard Gentaz, and Fleur Lejeune. 2023. "Multisensory Texture Perception in Individuals with Williams Syndrome" Children 10, no. 9: 1494. https://doi.org/10.3390/children10091494
APA StyleCheam, C., Barisnikov, K., Gentaz, E., & Lejeune, F. (2023). Multisensory Texture Perception in Individuals with Williams Syndrome. Children, 10(9), 1494. https://doi.org/10.3390/children10091494





