Zero-Fluoroscopy Catheter Ablation of Supraventricular Tachycardias in the Pediatric Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Electrophysiology Study
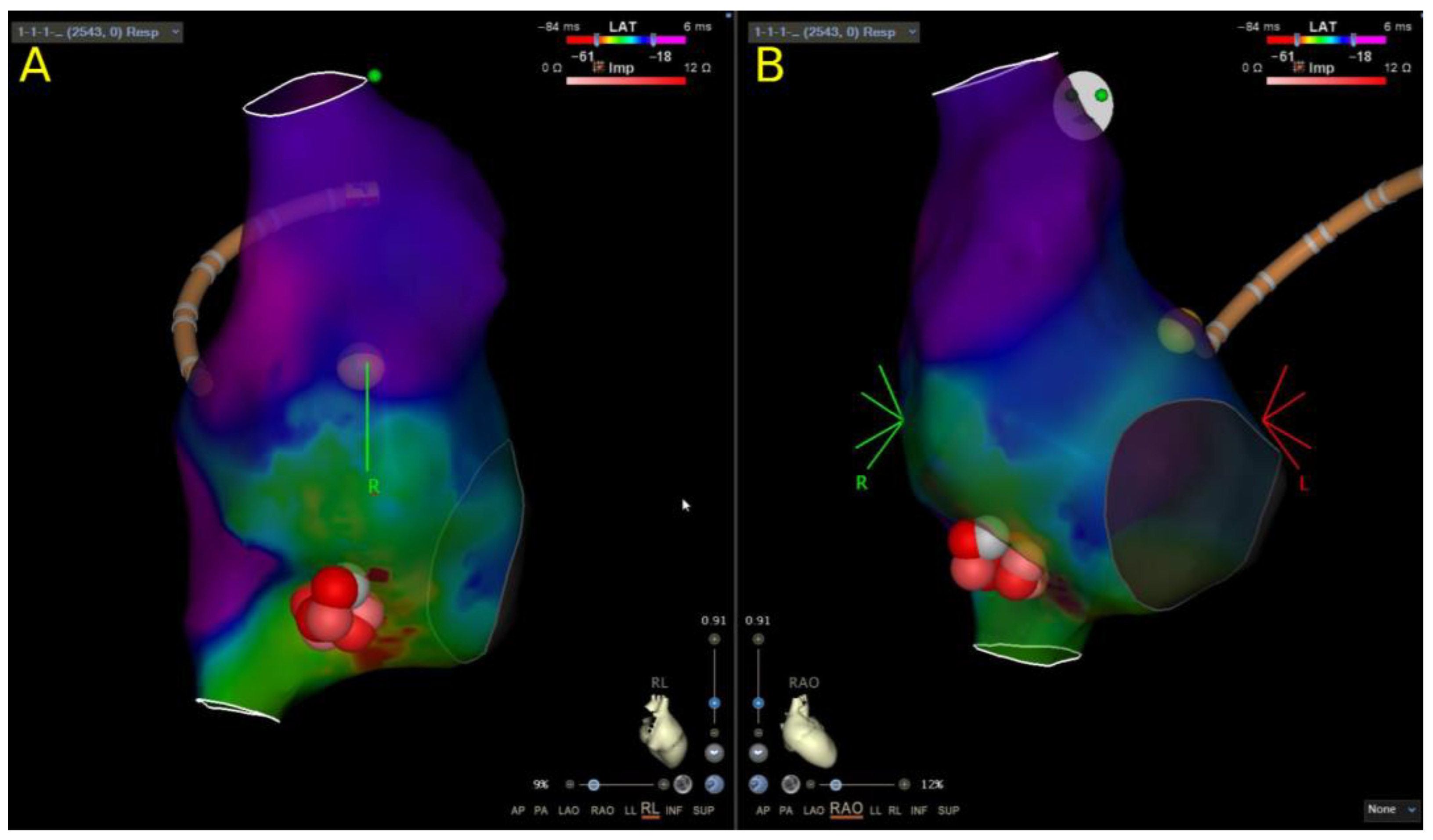
2.3. Left-Sided Access
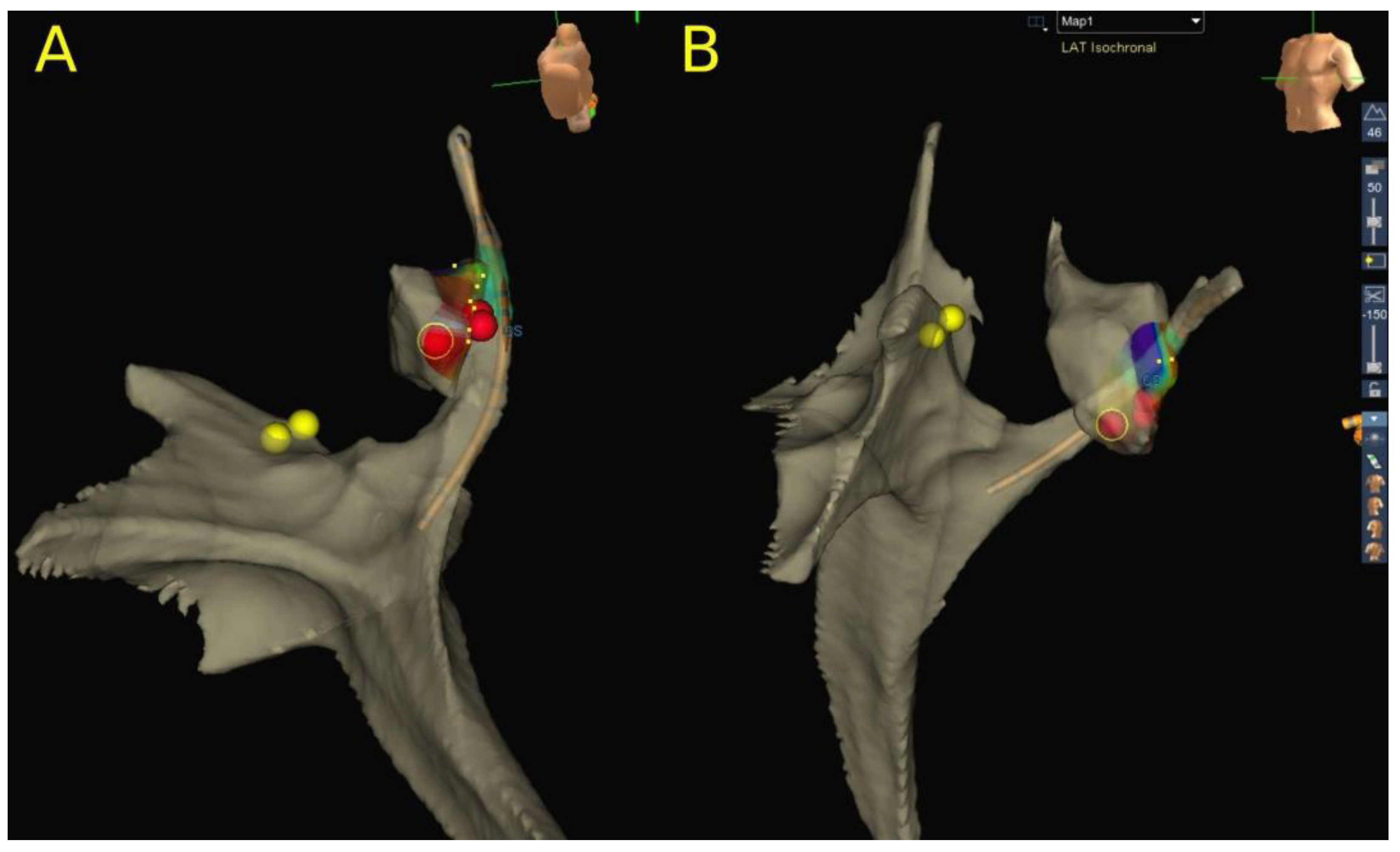
2.4. Mapping and Ablation of AVNRT
2.5. Mapping and Ablation of AVRT
2.6. Ablation of AT
2.7. Definition of Procedural and Follow-Up Parameters
2.8. Statistical Analysis
3. Results
3.1. Procedural Characteristics
3.2. Atrioventricular Nodal Reentry Tachycardia
3.3. Atrioventricular Reentry Tachycardia
3.4. Atrial Tachycardia
3.5. Follow-Up
4. Discussion
4.1. The Role of ICE in Pediatric SVT Ablation Procedures
4.2. Catheter Ablation of AVNRT
4.3. Catheter Ablation of Accessory Pathways
4.4. Catheter Ablation of Atrial Tachycardia
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orejarena, L.A.; Vidaillet, H.; De Stefano, F.; Nordstrom, D.L.; Vierkant, R.A.; Smith, P.N.; Hayes, J.J. Paroxysmal supraventricular tachycardia in the general population. J. Am. Coll. Cardiol. 1998, 31, 150–157. [Google Scholar] [CrossRef]
- Wu, M.-H.; Chen, H.-C.; Kao, F.-Y.; Huang, S.-K. Postnatal cumulative incidence of supraventricular tachycardia in a general pediatric population: A national birth cohort database study. Heart Rhythm 2016, 13, 2070–2075. [Google Scholar] [CrossRef]
- Brugada, J.; Blom, N.; Sarquella-Brugada, G.; Blomstrom-Lundqvist, C.; Deanfield, J.; Janousek, J.; Abrams, D.; Bauersfeld, U.; Brugada, R.; Drago, F.; et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Eurospace 2013, 15, 1337–1382. [Google Scholar] [CrossRef]
- Wagner, L.K.; Eifel, P.J.; Geise, R.A. Potential biological effects following high X-ray dose interventional procedures. J. Vasc. Interv. Radiol. 1994, 5, 71–84. [Google Scholar] [CrossRef]
- Vano, E.; Ubeda, C.; Leyton, F.; Miranda, P.; Gonzalez, L. Staff radiation doses in interventional cardiology: Correlation with patient exposure. Pediatr. Cardiol. 2009, 30, 409–413. [Google Scholar] [CrossRef]
- Venneri, L.; Rossi, F.; Botto, N.; Andreassi, M.G.; Salcone, N.; Emad, A.; Lazzeri, M.; Gori, C.; Vano, E.; Picano, E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: Insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am. Heart J. 2009, 157, 118–124. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Balter, S.; Cowley, M.; Hodgson, J.; Klein, L.W. Occupational hazards of interventional cardiologists: Prevalence of orthopedic health problems in contemporary practice. Catheter. Cardiovasc. Interv. 2004, 63, 407–411. [Google Scholar] [CrossRef]
- ICRP; Khong, P.L.; Ringertz, H.; Donoghue, V.; Frush, D.; Rehani, K.; Appelgate, K.; Sanchez, R. ICRP 121: Radiological protection in pediatric diagnostic and interventional radiology. Ann. ICRP 2013, 42, 1–63. [Google Scholar] [CrossRef]
- Yang, L.; Sun, G.; Chen, X.; Chen, G.; Yang, S.; Guo, P.; Wang, Y.; Wang, D.W. Meta-Analysis of Zero or Near-Zero Fluoroscopy Use During Ablation of Cardiac Arrhythmias. Am. J. Cardiol. 2016, 118, 1511–1518. [Google Scholar] [CrossRef]
- Debreceni, D.; Janosi, K.; Vamos, M.; Komocsi, A.; Simor, T.; Kupo, P. Zero and Minimal Fluoroscopic Approaches During Ablation of Supraventricular Tachycardias: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 856145. [Google Scholar] [CrossRef]
- Balli, S.; Kucuk, M. Transcatheter ablation using near-zero fluoroscopy in children with focal atrial tachycardia: A single-centre experience. Cardiol. Young 2020, 30, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Balli, S.; Kucuk, M.; Orhan Bulut, M.; Kemal Yucel, I.; Celebi, A. Transcatheter cryoablation procedures without fluoroscopy in pediatric patients with atrioventricular nodal reentrant tachycardia: A single-center experience. Acta Cardiol. Sin. 2018, 34, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Elkiran, O.; Akdeniz, C.; Karacan, M.; Tuzcu, V. Electroanatomic mapping-guided catheter ablation of atrial tachycardia in children with limited/zero fluoroscopy. Pacing Clin. Electrophysiol. 2019, 42, 453–457. [Google Scholar] [CrossRef]
- Clark, B.C.; Sumihara, K.; McCarter, R.; Berul, C.I.; Moak, J.P. Getting to zero: Impact of electroanatomical mapping on fluoroscopy use in pediatric catheter ablation. J. Interv. Card. Electrophysiol. 2016, 46, 183–189. [Google Scholar] [CrossRef]
- Tseng, W.-C.; Wu, M.-H.; Lu, C.-W.; Wu, K.-L.; Wang, J.-K.; Lin, M.-T.; Chen, C.-A.; Chiu, S.-N. Zero fluoroscopy during ablation of right-sided supraventricular tachycardia substrates in a pediatric population—Initial experience in Taiwan. Acta Cardiol. Sin. 2019, 35, 476–483. [Google Scholar] [CrossRef]
- Koca, S.; Paç, F.A.; Eriş, D.; Zabun, M.M.; Özeke, Ö.; Özcan, F. Electroanatomic mapping-guided pediatric catheter ablation with limited/zero fluoroscopy. Anatol. J. Cardiol. 2018, 20, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mah, D.Y.; Miyake, C.Y.; Sherwin, E.D.; Walsh, A.; Anderson, M.J.; Western, K.; Abrams, D.J.; Alexander, M.E.; Cecchin, F.; Walsh, E.P.; et al. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Eurospace 2014, 16, 277–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jan, M.; Yazici, M.; Prolič Kalinšek, T.; Žižek, D.; Kuhelj, D.; Pernat, A.; Lakič, N. Fluoroless radiofrequency and cryo-ablation of atrioventricular nodal reentry tachycardia in adults and children: A single-center experience. J. Interv. Card. Electrophysiol. 2020, 61, 155–163. [Google Scholar] [CrossRef]
- Scaglione, M.; Ebrille, E.; Caponi, D.; Blandino, A.; Di Donna, P.; Siboldi, A.; Bertero, G.; Anselmino, M.; Raimondo, C.; Sardi, D.; et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing Clin. Electrophysiol. 2013, 36, 1460–1467. [Google Scholar] [CrossRef]
- Kerst, G.; Weig, H.-J.; Weretka, S.; Seizer, P.; Hofbeck, M.; Gawaz, M.; Schreieck, J. Contact force–controlled zero-fluoroscopy catheter ablation of right-sided and left atrial arrhythmia substrates. Heart Rhythm 2012, 9, 709–714. [Google Scholar] [CrossRef]
- Scaglione, M.; Ebrille, E.; Caponi, D.; Siboldi, A.; Bertero, G.; Di Donna, P.; Gabbarini, F.; Raimondo, C.; Di Clemente, F.; Ferrato, P.; et al. Zero-fluoroscopy ablation of accessory pathways in children and adolescents: CARTO3 electroanatomic mapping combined with RF and cryoenergy. Pacing Clin. Electrophysiol. 2015, 38, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, A.M.; Smith, P.C.; Timberlake, D.T.; McNinch, N.L.; Smith, G.L.; Lane, J.R.; Clark, J.M. Procedural outcomes of fluoroless catheter ablation outside the traditional catheterization lab. Europace 2017, 19, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Žižek, D.; Antolič, B.; Prolič Kalinšek, T.; Štublar, J.; Kajdič, N.; Jelenc, M.; Jan, M. Intracardiac echocardiography-guided transseptal puncture for fluoroless catheter ablation of left-sided tachycardias. J. Interv. Card. Electrophysiol. 2021, 61, 595–602. [Google Scholar] [CrossRef]
- Jan, M.; Kalinšek, T.P.; Štublar, J.; Jelenc, M.; Pernat, A.; Žižek, D.; Lakič, N. Intra-cardiac ultrasound guided approach for catheter ablation of typical right free wall accessory pathways. BMC Cardiovasc. Disord. 2020, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, R.; Cappato, R.; Curnis, A.; Della Bella, P.; Padeletti, L.; Raviele, A.; Santini, M.; Salerno-Uriarte, J.A. Transseptal catheterization in the electrophysiology laboratory: Data from a multicenter survey spanning 12 years. J. Am. Coll Cardiol. 2006, 47, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, G.; Sinner, M.F.; Lutz, M.; Müller-Nurasyid, M.; Kääb, S.; Reinecke, H. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: A nationwide in-hospital analysis of administrative data for Germany in 2014. Eur. Heart J. 2018, 39, 4020–4029. [Google Scholar] [CrossRef] [PubMed]
- Matoshvili, Z.; Bastani, H.; Bourke, T.; Braunschweig, F.; Drca, N.; Gudmundsson, K.; Insulander, P.; Jemtrén, A.; Kennebäck, G.; Saluveer, O.; et al. Safety of fluoroscopy-guided transseptal approach for ablation of left-sided arrhythmias. Europace 2017, 19, 2023–2026. [Google Scholar] [CrossRef]
- Bayrak, F.; Chierchia, G.B.; Namdar, M.; Yazaki, Y.; Sarkozy, A.; De Asmundis, C.; Muller-Burri, S.A.; Rao, J.; Ricciardi, D.; Sorgente, A.; et al. Added value of transesophageal echocardiography during transseptal puncture performed by inexperienced operators. Europace 2012, 14, 661–665. [Google Scholar] [CrossRef]
- Baykaner, T.; Quadros, K.K.; Thosani, A.; Yasmeh, B.; Mitra, R.; Liu, E.; Belden, W.; Liu, Z.; Costea, A.; Brodt, C.R.; et al. Safety and efficacy of zero fluoroscopy transseptal puncture with different approaches. Pacing Clin. Electrophysiol. 2020, 43, 12–18. [Google Scholar] [CrossRef]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology: Working with the Anatomy Rather Than Fighting It. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef]
- Jingquan, Z.; Deyong, L.; Huimin, C.; Hua, F.; Xuebin, H.; Chenyang, J.; Yan, L.; Xuebin, L.; Min, T.; Zulu, W.; et al. Intracardiac echocardiography Chinese expert consensus. Front. Cardiovasc. Med. 2022, 9, 1012731. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Sumihara, K.; Berul, C.I.; Moak, J.P. Off the pedal: Fluoroless transseptal puncture in pediatric supraventricular tachycardia ablation. Pacing Clin. Electrophysiol. 2017, 40, 1254–1259. [Google Scholar] [CrossRef]
- Friedman, D.J.; Pokorney, S.D.; Ghanem, A.; Marcello, S.; Kalsekar, I.; Yadalam, S.; Akar, J.G.; Freeman, J.V.; Goldstein, L.; Khanna, R.; et al. Predictors of cardiac perforation with catheter ablation of atrial fibrillation. JACC Clin. Electrophysiol. 2020, 6, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Luani, B.; Rauwolf, T.; Genz, C.; Schmeißer, A.; Wiemer, M.; Braun-Dullaeus, R.C. Intracardiac echocardiography versus fluoroscopy for endovascular and endocardial catheter navigation during cryo-ablation of the slow pathway in AVNRT patients. Cardiovasc. Ultrasound 2019, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Kupo, P.; Saghy, L.; Bencsik, G.; Kohari, M.; Makai, A.; Vamos, M.; Benak, A.; Miklos, M.; Raileanu, G.; Schvartz, N.; et al. Randomized trial of intracardiac echocardiography-guided slow pathway ablation. J. Interv. Card. Electrophysiol. 2022, 63, 709–714. [Google Scholar] [CrossRef]
- Rajendra, A.; Hunter, T.D.; Morales, G.X.; Zei, P.; Boo, L.M.; Varley, A.; Osorio, J. Steerable sheath visualizable under 3D electroanatomical mapping facilitates paroxysmal atrial fibrillation ablation with minimal fluoroscopy. J. Interv. Card. Electrophysiol. 2023, 66, 381–388. [Google Scholar] [CrossRef]
- Janosi, K.; Debreceni, D.; Janosa, B.; Bocz, B.; Simor, T.; Kupo, P. Visualizable vs. standard, non-visualizable steerable sheath for pulmonary vein isolation procedures: Randomized, single-centre trial. Front. Cardiovasc. Med. 2022, 9, 1033755. [Google Scholar] [CrossRef]
- Collins, K.K.; Schaffer, M.S. Use of cryoablation for treatment of tachyarrhythmias in 2010: Survey of current practices of pediatric electrophysiologists. Pacing Clin. Electrophysiol. 2011, 34, 304–308. [Google Scholar] [CrossRef]
- Krause, U.; Paul, T.; Della Bella, P.; Gulletta, S.; A Gebauer, R.; Paech, C.; Kubus, P.; Janousek, J.; Ferrari, P.; De Filippo, P. Pediatric catheter ablation at the beginning of the 21st century: Results from the european multicenter pediatric catheter ablation registry ‘EUROPA’. Eurospace 2021, 23, 431–440. [Google Scholar] [CrossRef]
- Krause, U.; Backhoff, D.; Klehs, S.; Kriebel, T.; Paul, T.; Schneider, H.E. Catheter ablation of pediatric AV nodal reentrant tachycardia: Results in small children. Clin. Res. Cardiol. 2015, 104, 990–997. [Google Scholar] [CrossRef]
- Avari, J.N.; Jay, K.S.; Rhee, E.K. Experience and results during transition from radiofrequency ablation to cryoablation for treatment of pediatric atrioventricular nodal reentrant tachycardia. Pacing Clin. Electrophysiol. 2008, 31, 454–460. [Google Scholar] [CrossRef]
- Papagiannis, J.; Papadopoulou, K.; Rammos, S.; Katritsis, D. Cryoablation versus radiofrequency ablation for atrioventricular nodal reentrant tachycardia in children: Long-term results. Hell. J. Cardiol. 2010, 51, 122–126. [Google Scholar]
- Santangeli, P.; Proietti, R.; Di Biase, L.; Bai, R.; Natale, A. Cryoablation versus radiofrequency ablation of atrioventricular nodal reentrant tachycardia. J. Interv. Card. Electrophysiol. 2014, 39, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hanninen, M.; Yeung-Lai-Wah, N.; Massel, D.; Gula, L.J.; Skanes, A.C.; Yee, R.; Klein, G.J.; Manlucu, J.; Leong-Sit, P. Cryoablation versus RF ablation for AVNRT: A meta-analysis and systematic review. J. Cardiovasc. Electrophysiol. 2013, 24, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Gist, K.; Tigges, C.; Smith, G.; Clark, J. learning curve for zero-fluoroscopy catheter ablation of AVNRT: Early versus late experience. Pacing Clin. Electrophysiol. 2011, 34, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.Y.; Ratnasamy, C.; Sokoloski, M.; Young, M.-L. Low Recurrence rate in treating atrioventricular nodal reentrant tachycardia with triple freeze-thaw cycles. Pacing Clin. Electrophysiol. 2013, 36, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, L.L.L.; Leal, M.; Hollis, Z.; Tanega, J.; Alberte, C. Cryoablation for AVNRT: Importance of ablation endpoint criteria. J. Cardiovasc. Electrophysiol. 2012, 23, 729–734. [Google Scholar] [CrossRef]
- Bearl, D.W.; Mill, L.; Kugler, J.D.; Prusmack, J.L.; Erickson, C.C. Visualization of Atrioventricular Nodal Reentry Tachycardia Slow Pathways Using Voltage Mapping for Pediatric Catheter Ablation. Congenit. Heart Dis. 2015, 10, E172–E179. [Google Scholar] [CrossRef]
- Drago, F.; Battipaglia, I.; Russo, M.S.; Remoli, R.; Pazzano, V.; Grifoni, G.; Allegretti, G.; Silvetti, M.S. Voltage gradient mapping and electrophysiologically guided cryoablation in children with AVNRT. Europace 2018, 20, 665–672. [Google Scholar] [CrossRef]
- Eryazici, P.L.S.; Razminia, M.; D’silva, O.; Chavez, J.R.; Ciftci, F.D.; Turner, M.; Wang, T.; Zheutlin, T.A.; Kehoe, R.F. Time-limited cryomapping during tachycardia: Improved long-term outcomes for cryoablation of AVNRT. J. Interv. Card. Electrophysiol. 2016, 47, 125–131. [Google Scholar] [CrossRef]
- Kafalı, H.C.; Özgür, S.; Şahin, G.T.; Akay, E.; Güzeltaş, A.; Ergül, Y. Cryoablation with an 8-mm tip catheter for typical AVNRT in children: A single center 5-year experience. J. Interv. Card. Electrophysiol. 2021, 62, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Karacan, M.; Çelik, N.; Akdeniz, C.; Tuzcu, V. Long-term outcomes following cryoablation of atrioventricular nodal reentrant tachycardia in children. Pacing Clin. Electrophysiol. 2018, 41, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.M.; Jorgensen, N.W.; Radbill, A.E.; Bradley, D.J.; Silva, J.N.; Tsao, S.; Kanter, R.J.; Tanel, R.E.; Trivedi, B.; Young, M.-L.; et al. What have we learned in the last 20 years? A comparison of a modern era pediatric and congenital catheter ablation registry to previous pediatric ablation registries. Heart Rhythm 2019, 16, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Van Hare, G.F.; Javitz, H.; Carmelli, D.; Saul, J.P.; Tanel, R.E.; Fischbach, P.S.; Kanter, R.J.; Schaffer, M.; Dunnigan, A.; Colan, S.; et al. Pediatric Electrophysiology Society. Prospective assessment after pediatric cardiac ablation: Demographics, medical profiles, and initial outcomes. J. Cardiovasc. Electrophysiol. 2004, 15, 759–770. [Google Scholar] [CrossRef]
- Philip Saul, J.; Kanter, R.J.; Writing Committee; Abrams, D.; Asirvatham, S.; Bar-Cohen, Y.; Blaufox, A.D.; Cannon, B.; Clark, J.; Dick, M.; et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: Developed in partnership with the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American Academy of Pediatrics (AAP), the American Heart Association (AHA), and the Association for European Pediatric and Congenital Cardiology (AEPC). Heart Rhythm 2016, 13, e251–e289. [Google Scholar]
- Papagiannis, J.; Beissel, D.J.; Krause, U.; Cabrera, M.; Telishevska, M.; Seslar, S.; Johnsrude, C.; Anderson, C.; Tisma-Dupanovic, S.; Connelly, D.; et al. Pediatric and Congenital Electrophysiology Society. Atrioventricular Nodal Reentrant Tachycardia in Patients with Congenital Heart Disease: Outcome after Catheter Ablation. Circ. Arrhythm. Electrophysiol. 2017, 10, e004869. [Google Scholar] [CrossRef]
- Jackman, W.M.; Wang, X.Z.; Friday, K.J.; Roman, C.A.; Moulton, K.P.; Beckman, K.J.; McClelland, J.H.; Twidale, N.; Hazlitt, H.A.; Prior, M.I.; et al. Catheter ablation of accessory atrioventricular pathways (Wolff–Parkinson–White Syndrome) by radiofrequency current. N. Engl. J. Med. 1991, 324, 1605–1611. [Google Scholar] [CrossRef]
- Ceresnak, S.R.; Dubin, A.M.; Kim, J.J.; Valdes, S.O.; Fishberger, S.B.; Shetty, I.; Zimmerman, F.; Tanel, R.E.; Epstein, M.R.; Motonaga, K.S.; et al. Success rates in pediatric WPW ablation are improved with 3-Dimensional mapping systems compared with fluoroscopy alone: A multicenter study. J. Cardiovasc. Electrophysiol. 2015, 26, 412–416. [Google Scholar] [CrossRef]
- Casella, M.; Russo, A.D.; Pelargonio, G.; Del Greco, M.; Zingarini, G.; Piacenti, M.; Di Cori, A.; Casula, V.; Marini, M.; Pizzamiglio, F.; et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: The NO-PARTY multicentre randomized trial. Europace 2016, 18, 1565–1572. [Google Scholar] [CrossRef]
- Yu, X.; Dong, Z.; Gao, L.; Lin, L.; Cui, L.; Shao, W.; Yu, W.; Zhen, Z.; Yuan, Y. Transseptal Approach versus Transaortic Approach for Catheter Ablation of Left-Sided Accessory Pathways in Children. Front. Pediatr. 2022, 10, 888029. [Google Scholar] [CrossRef]
- Telishevska, M.; Faelchle, J.; Buiatti, A.; Busch, S.; Reents, T.; Bourier, F.; Semmler, V.; Kaess, B.; Horndasch, M.; Kornmayer, M.; et al. Irrigated-tip catheters for radiofrequency ablation of right-sided accessory pathways in adolescents. Pacing Clin. Electrophysiol. 2017, 40, 1167–1172. [Google Scholar] [CrossRef]
- Schaffer, M.S.; Silka, M.J.; Ross, B.A.; Kugler, J.D. Inadvertent atrioventricular block during radiofrequency catheter ablation. Results of the Pediatric Radiofrequency Ablation Registry. Pediatric Electrophysiology Society. Circulation 1996, 94, 3214–3220. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Jackman, W.M.; Nakagawa, H.; Sun, Y.; Xu, Q.; Beckman, K.J.; Lockwood, D.; Scherlag, B.J.; Lazzara, R.; Po, S.S.; et al. Risk of coronary artery injury with radiofrequency ablation and cryoablation of epicardial posteroseptal accessory pathways within the coronary venous system. Circ. Arrhythmia Electrophysiol. 2014, 7, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Alazard, M.; Lacotte, J.; Horvilleur, J.; Ait-Said, M.; Salerno, F.; Manenti, V.; Piechaud, J.-F.; Garot, J.; Bonnet, D.; Maltret, A. Preventing the risk of coronary injury in posteroseptal accessory pathway ablation in children: Different strategies and advantages of fluoroscopy integrated 3D-mapping system (CARTO-UNIVU™). J. Interv. Card. Electrophysiol. 2018, 52, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.E.; Kriebel, T.; Gravenhorst, V.D.; Paul, T. Incidence of coronary artery injury immediately after catheter ablation for supraventricular tachycardias in infants and children. Heart Rhythm 2009, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Atienza, F.; Eidelman, G.; Ávila, P.; Pelliza, M.; Castellanos, E.; Loughlin, G.; Datino, T.; Torrecilla, E.G.; Almendral, J.; et al. Safety and efficacy of cryoablation vs. radiofrequency ablation of septal accessory pathways: Systematic review of the literature and meta-analyses. Europace 2018, 20, 1334–1342. [Google Scholar] [CrossRef]
- Kang, K.T.; Etheridge, S.P.; Kantoch, M.J.; Tisma-Dupanovic, S.; Bradley, D.J.; Balaji, S.; Hamilton, R.M.; Singh, A.K.; Cannon, B.C.; Schaffer, M.S.; et al. Current management of focal atrial tachycardia in children: A multicenter experience. Circ. Arrhythm. Electrophysiol. 2014, 7, 664–670. [Google Scholar] [CrossRef]

| Number of Patients | 171 |
|---|---|
| Female gender (Number (%)) | 68 (39.8%) |
| Age (Years) (Mean ± SD) | 12.5 ± 3.8 |
| <10 years | 46 (26.9%) |
| ≥10 years | 125 (73.1%) |
| Weight (kg) (Mean ± SD) | 49.5 ± 18.3 |
| Height (cm) (Mean ± SD) | 157.4 ± 20.6 |
| BMI (kg/m2) (Mean ± SD) | 19.2 ± 3.6 |
| Prior heart surgery (Number (%)) | 5 (2.9%) |
| Congenital abnormality (Number (%)) | 9 (5.3%) |
| CIED (Number (%)) | 0 (0%) |
| Tachycardia-induced cardiomyopathy (Number (%)) | 3 (1.8%) |
| Antiarrhythmic drugs (Number (%)) | 35 (20.5%) |
| Beta blockers | 19 (11.1%) |
| Amiodarone | 1 (0.6%) |
| Propafenone | 19 (11.1%) |
| Patients with multiple arrhythmias (Number (%)) | 4 (2.3%) |
| Number of all arrhythmias | 175 |
| AVNRT (Number (%)) | 79 (45.1%) |
| AVRT (Number (%)) | 80 (45.7%) |
| Right AP | 9 (11.3%) |
| Left AP | 41 (51.3%) |
| Septal AP | 17 (21.3%) |
| Posteroseptal AP | 13 (16.3%) |
| AT (Number (%)) | 14 (8.0%) |
| AF (Number (%)) | 2 (1.1%) |
| Procedural Data | All Procedures | AVNRT | AVRT | AVRT Right AP | AVRT Left AP | AVRT Septal AP | AVRT Posteroseptal AP | AT | AF |
|---|---|---|---|---|---|---|---|---|---|
| Number of procedures | 201 | 86 (42.8%) | 95 (47.3%) | 13 (13.7%) | 42 (44.2%) | 25 (26.3%) | 15 (15.8%) | 18 (9%) | 2 (1.0%) |
| Procedures per patient (Mean) | 1.18 | 1.09 | 1.19 | 1.44 | 1.02 | 1.47 | 1.15 | 1.29 | 1.00 |
| Procedure time (min) (Mean ± SD) | 98.5 ± 55.0 | 83.8 ± 51.0 | 105.1 ± 54.9 | 142.7 ± 66.7 | 89.2 ± 42.3 | 96.4 ± 47.0 | 131.7 ± 66.5 | 150.6 ± 60.1 | 102.5 ± 3.5 |
| Transseptal punctures (Number (%)) | 51 (25.6%) | 0 (0%) | 47 (49.5%) | 0 (0%) | 42 (100.0%) | 1 (4.0%) | 4 (26.7%) | 4 (22.2%) | 0 (0%) |
| Complications (Number (%)) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Major | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Minor | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Procedural success (Number (%)) | 200 (99.5%) | 86 (100%) | 94 (98.9%) | 12 (92.3%) | 42 (100.0%) | 25 (100.0%) | 15 (100.0%) | 18 (100.0%) | 2 (100%) |
| Energy source used (RFA/CRA) (Number (%)) | |||||||||
| RFA | 160 (79.6%) | 56 (65.1%) | 85 (89.5%) | 13 (100.0%) | 42 (100.0%) | 16 (64.0%) | 14 (93.3%) | 17 (94.4%) | 2 (100.0%) |
| RFA failure | 2 (1.3%) | 0 (0%) | 2 (2.4%) | 1 (7.7%) | 0 (0%) | 1 (6.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CRA | 45 (22.4%) | 32 (37.2%) | 12 (12.6%) | 0 (0%) | 0 (0%) | 10 (40.0%) | 2 (13.3%) | 1 (5.6%) | 0 (0%) |
| CRA failure | 3 (6.6%) | 2 (6.3%) | 1 (8.3%) | / | / | 0 (0%) | 1 (50.0%) | 0 (0%) | / |
| Both used | 4 (2.0%) | 2 (2.3%) | 2 (2.1%) | 0 (0%) | 0 (0%) | 1 (4.0%) | 1 (6.7%) | 0 (0%) | 0 (0%) |
| Number of RFA lesions (Mean ± SD) | 11.6 ± 10.5 | 13.2 ± 13.1 | 11.1 ± 10.8 | 20.0 ± 17.0 | 8.95 ± 6.0 | 6.0 ± 3.9 | 8.7 ± 7.9 | 13.4 ± 6.4 | 38.0 |
| RFA time (sec) (Mean ± SD) | 374.2 ± 338.8 | 528.1 ± 468.8 | 339.7 ± 330.1 | 625.0 ± 502.0 | 275.1 ± 166.8 | 154.3 ± 101.4 | 261.3 ± 212.6 | 395.3 ± 129.0 | 935 |
| Number of CRA lesions (Mean ± SD) | 3.7 ± 2.4 | 4.0 ± 2.8 | 3.1 ± 0.9 | 8.0 ± 3.0 | / | 3.1 ± 1.1 | 3.0 | / | / |
| CRA time (sec) (Mean ± SD) | 881.4 ± 571.1 | 945.3 ± 673.7 | 746.7 ± 222.7 | 1920.0 ± 679 | / | 754.3 ± 256.6 | 720.0 | / | / |
| Follow-Up | After Successful First Procedure (per Patient) | After All Procedures (per Patient) |
|---|---|---|
| Follow-Up (days) (Mean ± SD) | 488.4 ± 409.5 | 459.7 ± 391.7 |
| Antiarrhythmic drugs (Number (%)) | 7 (4.1%) | 5 (2.9%) |
| Beta blockers | 7 (4.1%) | 5 (2.9%) |
| Amiodarone | 0 (0.0%) | 0 (0.0%) |
| Propafenone | 0 (0.0%) | 0 (0.0%) |
| Long-term success (Number (%)) | ||
| All arrhythmias | 144 (84.2%) | 168 (98.2%) |
| AVNRT | 71 (89.9%) | 78 (98.7%) |
| AVRT | 65 (81.3%) | 78 (97.5%) |
| AVRT right AP | 6 (66.7%) | 9 (100.0%) |
| AVRT left AP | 40 (97.6%) | 41 (100.0%) |
| AVRT septal AP | 9 (52.9%) | 16 (94.1%) |
| AVRT posteroseptal AP | 10 (76.9%) | 12 (92.3%) |
| AT | 10 (71.4%) | 14 (100.0%) |
| AF | 2 (100.0%) | 2 (100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topalović, M.; Jan, M.; Kalinšek, T.P.; Žižek, D.; Štublar, J.; Rus, R.; Kuhelj, D. Zero-Fluoroscopy Catheter Ablation of Supraventricular Tachycardias in the Pediatric Population. Children 2023, 10, 1513. https://doi.org/10.3390/children10091513
Topalović M, Jan M, Kalinšek TP, Žižek D, Štublar J, Rus R, Kuhelj D. Zero-Fluoroscopy Catheter Ablation of Supraventricular Tachycardias in the Pediatric Population. Children. 2023; 10(9):1513. https://doi.org/10.3390/children10091513
Chicago/Turabian StyleTopalović, Mirko, Matevž Jan, Tine Prolič Kalinšek, David Žižek, Jernej Štublar, Rina Rus, and Dimitrij Kuhelj. 2023. "Zero-Fluoroscopy Catheter Ablation of Supraventricular Tachycardias in the Pediatric Population" Children 10, no. 9: 1513. https://doi.org/10.3390/children10091513
APA StyleTopalović, M., Jan, M., Kalinšek, T. P., Žižek, D., Štublar, J., Rus, R., & Kuhelj, D. (2023). Zero-Fluoroscopy Catheter Ablation of Supraventricular Tachycardias in the Pediatric Population. Children, 10(9), 1513. https://doi.org/10.3390/children10091513






