Advances in Pediatric Surgery Simulation-Based Training
Abstract
:1. Introduction
1.1. The History of Pediatric Surgery Training
1.2. Current Challenges in Training Pediatric Surgeons
2. Methods
2.1. The Onset of Simulation-Based Training in Pediatric Surgery
2.2. Cadaver and Vivisection
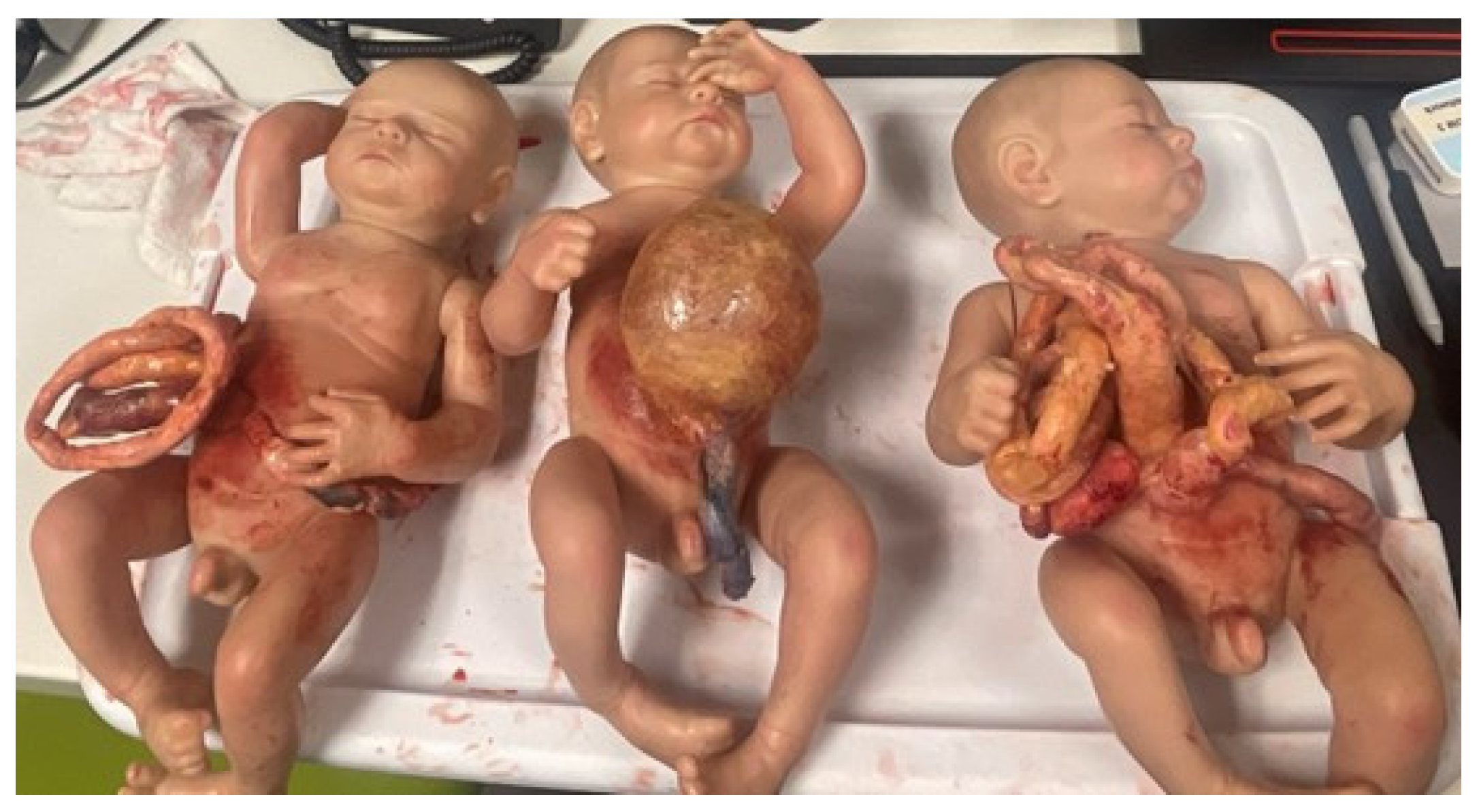
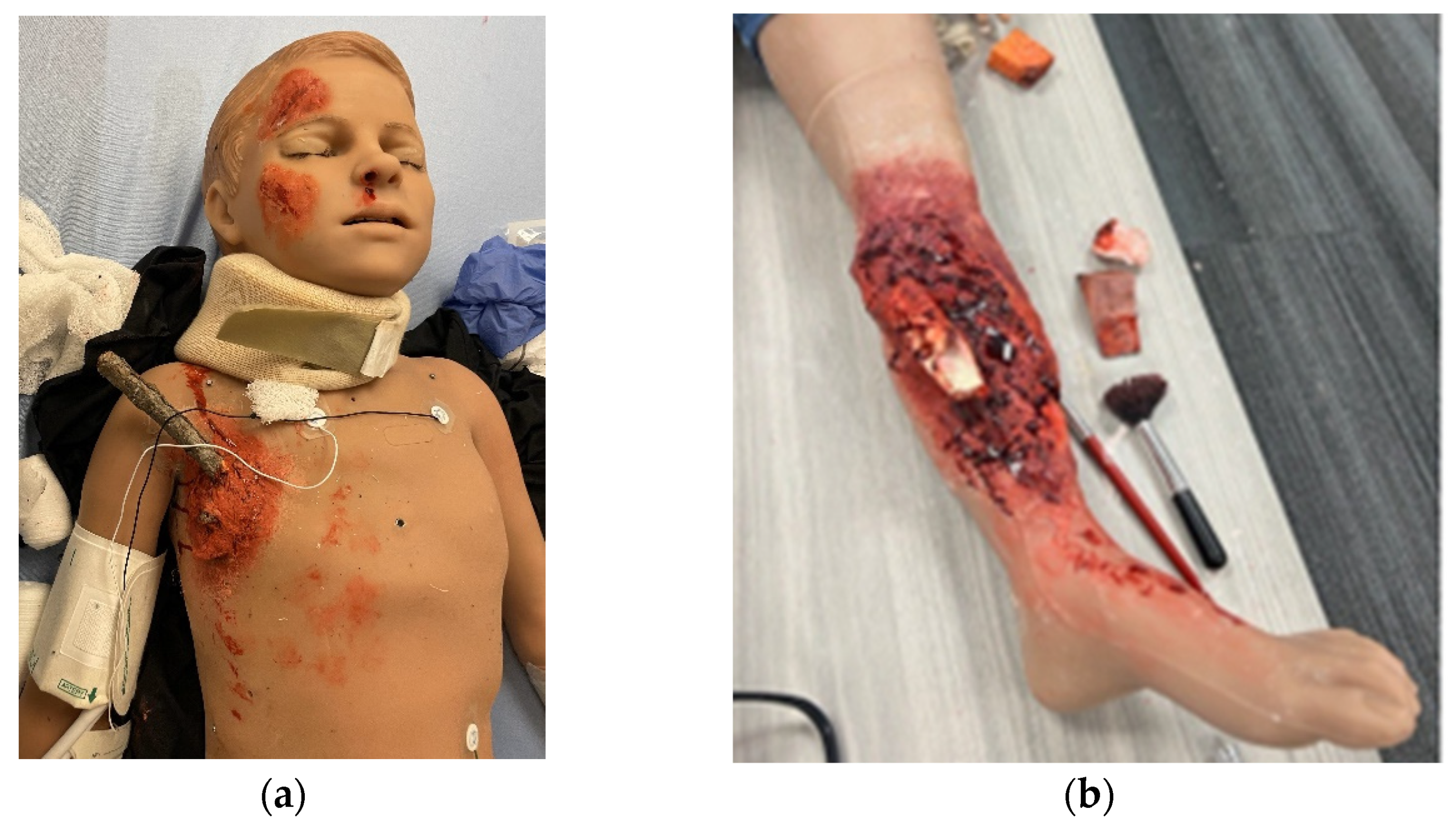
2.3. Simulation Models and Trainers
2.4. Virtual, Augmented, and Mixed Reality
3. The Future Direction of the Simulation-Based Training of Pediatric Surgeons
3.1. Technology Expansion
3.2. The Widespread Implementation of Simulation-Based Curricula
3.3. The Metric-Based Evaluation of Surgical Trainees in Simulation Training
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomaszewski, J.; Casella, D. Pediatric Laparoscopic and Robot-Assisted Laparoscopic Surgery: Technical Considerations. J. Endourol. 2012, 26, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Lalchandani, P.; Dunn, J.C.Y. Global comparison of pediatric surgery workforce and training. J. Pediatr. Surg. 2015, 50, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Wirtzfeld, D. The history of women in surgery. Can. J. Surg. 2009, 52, 317–320. [Google Scholar] [PubMed]
- Majumdar, S.K. History of women in surgery: An overview. Bull. Indian. Inst. Hist. Med. Hyderabad 2000, 30, 59–64. [Google Scholar] [PubMed]
- Bill, A.H. Herbert E. Coe, 1881–1968. J. Pediatr. Surg. 1969, 4, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Koop, C.E. A perspective on the early days of Pediatric Surgery. J. Pediatr. Surg. 1999, 34 (Suppl. S1), 38–45. [Google Scholar] [CrossRef] [PubMed]
- Spitz, L. The history of paediatric surgery in the United Kingdom and the influence of the national health service on its development. J. Pediatr. Surg. 2012, 47, 29–35. [Google Scholar] [CrossRef]
- Ladd, W.E. Progress in the diagnosis and treatment of intussusception. Boston Med. Surg. J. 1913, 168, 542–544. [Google Scholar] [CrossRef]
- Warren, J.M. Surgical Observations with Cases; Ticknor and Fields: Boston, MA, USA, 1867. [Google Scholar]
- Ravitch, M.M.; Sabiston, D.C. Anal ileostomy with preservation of the sphincter: A proposed operation in patients requiring total colectomy for benign lesions. Surg. Gynecol. Obstet. 1947, 84, 1095–1099. [Google Scholar]
- Thomas, V. Pioneering Research in Surgical Shock and Cardiovascular Surgery: Vivien Thomas and His Work with Alfred Blalock: An Autobiography; University of Pennsylvania Press: Philadelphia, PA, USA, 1985. [Google Scholar]
- Johnson, D.G. Excellence in search of recognition. J. Pediatr. Surg. 1986, 21, 1019–1031. [Google Scholar] [CrossRef]
- Walsh, D. Pediatric surgery: A career resource. Am. J. Surg. 2010, 199, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Ameh, E.A.; Adejuyigbe, O.; Nmadu, P.T. Pediatric surgery in Nigeria. J. Pediatr. Surg. 2006, 41, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Saing, H. Training and delivery of pediatric surgery in Asia. J. Pediatr. Surg. 2000, 35, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Schmedding, A.; Rolle, U.; Czauderna, P. European Pediatric Surgical Training. Eur. J. Pediatr. Surg. 2017, 27, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Beasley, S.W. The challenges facing training in pediatric surgery worldwide. Front. Pediatr. 2013, 1, 24. [Google Scholar] [CrossRef]
- Sawyer, T.; White, M.; Zaveri, P.; Chang, T.; Ades, A.; French, H.; Anderson, J.; Auerbach, M.; Johnston, L.; Kessler, D. Learn, see, practice, prove, do, maintain: An evidence-based pedagogical framework for procedural skill training in medicine. Acad. Med. 2015, 90, 1025–1033. [Google Scholar] [CrossRef]
- Aydin, A.; Raison, N.; Khan, M.S.; Dasgupta, P.; Ahmed, K. Simulation-based training and assessment in urological surgery. Nat. Rev. Urol. 2016, 13, 503–519. [Google Scholar] [CrossRef]
- Morgan, M.; Aydin, A.; Salih, A.; Robati, S.; Ahmed, K. Current status of simulation-based training tools in orthopedic surgery: A systematic review. J. Surg. Educ. 2017, 74, 698–716. [Google Scholar] [CrossRef]
- Musbahi, O.; Aydin, A.; Al Omran, Y.; Skilbeck, C.J.; Ahmed, K. Current status of simulation in otolaryngology: A systematic review. J. Surg. Educ. 2017, 74, 203–215. [Google Scholar] [CrossRef]
- Markel, M.; Lacher, M.; Hall, N.J.; Martynov, I.; Hinojosa, A.S.; de Augustin Asensio, J.C.; Fortmann, C.; Hukkinen, M.; Mutanen, A.; Ford, K.; et al. Training in minimally invasive surgery: Experience of paediatric surgery trainees in Europe. Br. J. Surg. 2023, 110, 1397–1399. [Google Scholar] [CrossRef]
- Torres, A.; Inzunza, M.; Jarry, C.; Serrano, F.; Varas, J.; Zavala, A. Development and Validation of a New Laparoscopic Endotrainer for Neonatal Surgery and Reduced Spaces. Arq. Bras. Cir. Dig. 2021, 33, e1559. [Google Scholar] [CrossRef] [PubMed]
- Bökkerink, G.M.J.; Joosten, M.; Leijte, E.; Verhoeven, H.; de Blaauw, I.; Botden, S.M.B.I. Take-Home Laparoscopy Simulators in Pediatric Surgery: Is More Expensive Better? J. Laparoendos. Adv. Surg. Tech. A 2021, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Memon, I. Cadaver dissection is obsolete in medical training! a misinterpreted notion. Med. Princ. Pract. 2018, 27, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Badash, I.; Burtt, K.; Solorzano, C.A.; Carey, J.N. Innovations in surgery simulation: A review of past, current, and future, techniques. Ann. Transl. Med. 2016, 4, 453. [Google Scholar] [CrossRef] [PubMed]
- Gummery, E.; Cobb, K.A.; Mossop, L.H.; Cobb, M.A. Student perceptions of veterinary anatomy practical classes: A longitudinal study. J. Vet. Med. Educ. 2018, 45, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Sarker, S.K. Simulation in surgery: A review. Scott. Med. J. 2011, 56, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Palmisani, F.; Sezen, P.; Haag, E.; Metzelder, M.L.; Krois, W. The “chicken-leg anastomosis”: Low-cost tissue-realistic simulation model for esophageal atresia training in pediatric surgery. Front. Pediatr. 2022, 10, 893639. [Google Scholar] [CrossRef]
- Etlinger, P.; Barroso, C.; Miranda, A.; Pinto, J.M.; Lamas-Pinheiro, R.; Ferreira, H.; Leão, P.; Kovács, T.; Juhász, L.; Szabó, L.S.; et al. Characterization of technical skill progress in a standardized rabbit model for training in laparoscopic duodenal atresia repair. Surg. Endosc. 2022, 36, 2456–2465. [Google Scholar] [CrossRef]
- Sbragia, L.; Oria, M.; Scorletti, F.; Del Mar Romero Lopez, M.; Schmidt, A.F.; Levy, B.; Peiro, J.L. A novel surgical toxicological-free model of diaphragmatic hernia in fetal rats. Pediatr. Res. 2022, 92, 118–124. [Google Scholar] [CrossRef]
- Bergmeister, K.D.; Aman, M.; Kramer, A.; Schenck, T.L.; Riedl, O.; Daeschler, S.C.; Aszmann, O.C.; Bergmeister, H.; Golriz, M.; Mehrabi, A.; et al. Simulating Surgical Skills in Animals: Systematic Review, Costs & Acceptance Analyses. Front. Vet. Sci. 2020, 7, 570852. [Google Scholar] [CrossRef]
- Gause, C.D.; Hsiung, G.; Schwab, B.; Clifton, M.; Harmon, C.M.; Barsness, K.A. Advances in Pediatric Surgical Education: A Critical Appraisal of Two Consecutive Minimally Invasive Pediatric Surgery Training Courses. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Escolino, M.; Draghici, I.; Cerulo, M.; Farina, A.; De Pascale, T.; Cozzolino, S.; Settimi, A. Training Models in Pediatric Minimally Invasive Surgery: Rabbit Model versus Porcine Model: A Comparative Study. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Skertich, N.J.; Schimpke, S.W.; Lee, T.; Wiegmann, A.L.; Pillai, S.; Rossini, C.; Madonna, M.B.; Shah, A.N. Pediatric Surgery Simulation-Based Training for the General Surgery Resident. J. Surg. Res. 2021, 258, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.J.; Chacon, C.S.; Haghighi-Osgouei, R.; Houghton, N.; Bello, F.; Clarke, S.A. Development and validation of a novel 3D-printed simulation model for open oesophageal atresia and tracheo-oesophageal fistula repair. Pediatr. Surg. Int. 2022, 38, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Reino-Pires, P.; Lopez, M. Validation of a Low-Cost Do-It-Yourself Model for Neonatal Thoracoscopic Congenital Diaphragmatic Hernia Repair. J. Surg. Educ. 2018, 75, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.; de Blaauw, I.; Botden, S.M.B.I. Validated simulation models in pediatric surgery: A review. J. Pediatr. Surg. 2022, 57, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J.; Bird, J.M.; Smart, P.A.; Wilson, M.R.; Vine, S.J. A Framework for the Testing and Validation of Simulated Environments in Experimentation and Training. Front. Psychol. 2020, 11, 605. [Google Scholar] [CrossRef]
- Van Nortwick, S.S.; Lendvay, T.S.; Jensen, A.R.; Wright, A.S.; Horvath, K.D.; Kim, S. Methodologies for establishing validity in surgical simulation studies. Surgery 2010, 147, 622–630. [Google Scholar] [CrossRef]
- Cho, A.; Basson, S.; Tsang, T. Outcomes of a structured training programme for paediatric laparoscopic inguinal hernia repair. J. Pediatr. Surg. 2013, 48, 404–407. [Google Scholar] [CrossRef]
- Yokoyama, S.; Mizunuma, K.; Kurashima, Y.; Watanabe, Y.; Mizota, T.; Poudel, S.; Kikuchi, T.; Kawai, F.; Shichinohe, T.; Hirano, S. Evaluation methods and impact of simulation-based training in pediatric surgery: A systematic review. Pediatr. Surg. Int. 2019, 35, 1085–1094. [Google Scholar] [CrossRef]
- Patel, E.A.; Aydın, A.; Desai, A.; Dasgupta, P.; Ahmed, K. Current status of simulation-based training in pediatric surgery: A systematic review. J. Pediatr. Surg. 2019, 54, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.P.; Weiner, D. Screen-based simulation and virtual reality for pediatric emergency medicine. Clin. Pediatr. Emerg. Med. 2016, 17, 224–230. [Google Scholar] [CrossRef]
- Laskay, N.M.B.; George, J.A.; Knowlin, L.; Chang, T.P.; Johnston, J.M.; Godzik, J. Optimizing Surgical Performance Using Preoperative Virtual Reality Planning: A Systematic Review. World J. Surg. 2023, 47, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Narayanan, M.D.K.; Umana, G.E.; Montemurro, N.; Chaurasia, B.; Deora, H. Virtual Reality in Neurosurgery: Beyond Neurosurgical Planning. Int. J. Environ. Res. Public Health 2022, 19, 1719. [Google Scholar] [CrossRef]
- Hasan, L.K.; Haratian, A.; Kim, M.; Bolia, I.K.; Weber, A.E.; Petrigliano, F.A. Virtual Reality in Orthopedic Surgery Training. Adv. Med. Educ. Pract. 2021, 12, 1295–1301. [Google Scholar] [CrossRef]
- Xia, J.; Samman, N.; Yeung, R.W.; Shen, S.G.; Wang, D.; Ip, H.H.; Tideman, H. Three-dimensional virtual reality surgical planning and simulation workbench for orthognathic surgery. Int. J. Adult Orthod. Orthognath. Surg. 2000, 15, 265–282. [Google Scholar]
- Reinschluessel, A.V.; Muender, T.; Salzmann, D.; Döring, T.; Malaka, R.; Weyhe, D. Virtual Reality for Surgical Planning—Evaluation Based on Two Liver Tumor Resections. Front. Surg. 2022, 9, 821060. [Google Scholar] [CrossRef]
- Pelizzo, G.; Costanzo, S.; Roveri, M.; Lanfranchi, G.; Vertemati, M.; Milani, P.; Zuccotti, G.; Cassin, S.; Panfili, S.; Rizzetto, F.; et al. Developing Virtual Reality Head Mounted Display (HMD) Set-Up for Thoracoscopic Surgery of Complex Congenital Lung MalFormations in Children. Children 2022, 9, 50. [Google Scholar] [CrossRef]
- Mclaughlin, C.; Zagory, J.A.; Fenlon, M.; Park, C.; Lane, C.J.; Meeker, D.; Burd, R.S.; Henri, R.; Upperman, J.S.; Jensen, A.R. Timing of Mortality in Pediatric Trauma Patients: A National Trauma Databank Analysis. J. Pediatr. Surg. 2019, 53, 344–351. [Google Scholar] [CrossRef]
- SIMBODIES. Available online: https://simbodies.com/ (accessed on 20 September 2023).
- SIMULAB. TraumaCHild Pediatric Surgical Simulator. Available online: https://simulab.com/products/traumachild-pediatric-surgical-simulator (accessed on 20 September 2023).
- Operative Experience, Inc. Available online: https://operativeexperience.com/tcs-plus-pro/ (accessed on 20 September 2023).
- Harrington, C.M.; Kavanagh, D.O.; Quinlan, J.F.; Ryan, D.; Dicker, P.; O’Keeffe, D.; Traynor, O.; Tierney, S. Development and evaluation of a trauma decision-making simulator in Oculus virtual reality. Am. J. Surg. 2018, 215, 42–47. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality. PS Net Patient Safety Network. Algorithm-Based Decision Support System Guides Trauma Staff during Initial Treatment, Leading to Fewer Medical Errors. Available online: https://psnet.ahrq.gov/innovation/algorithm-based-decision-support-system-guides-trauma-staff-during-initial-treatment (accessed on 20 September 2023).
- Satapathy, P.; Hermis, A.H.; Rustagi, S.; Pradhan, K.B.; Padhi, B.K.; Sah, R. Artificial intelligence in surgical education and training: Opportunities, challenges, and ethical considerations—Correspondence. Int. J. Surg. 2023, 109, 1543–1544. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.E.; Abu-Ghname, A.D.; Davis, M.J.; Ali, K.; Winocour, S. Role of Simulation and Artificial Intelligence in Plastic Surgery Training. Plast. Reconst. Surg. 2020, 146, 390e–391e. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Anvar, A.; Chen, A.; Gill, I.; Hung, A.J. How the use of the artificial intelligence could improve surgical skills in urology: State of the art and future perspectives. Curr. Opin. Urol. 2021, 31, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Ledwos, N.; Mirchi, N.; Yilmaz, R.; Winkler-Schwartz, A.; Sawni, A.; Fazlollahi, A.M.; Bissonnette, V.; Bajunaid, K.; Sabbagh, A.J.; Del Maestro, R.F. Assessment of learning curves on a simulated neurosurgical task using metrics selected by artificial intelligence. J. Neurosurg. 2022, 137, 1160–1171. [Google Scholar] [CrossRef]
- Park, J.J.; Tiefenbach, J.; Demetriades, A.K. The role of artificial intelligence in surgical simulation. Front. Med. Technol. 2022, 4, 1076755. [Google Scholar] [CrossRef]
- Singapogu, R.; Burg, T.; Burg, K.J.; Smith, D.E.; Eckenrode, A.H. A perspective on the role and utility of haptic feedback in laparoscopic skills training. Crit. Rev. Biomed. Eng. 2014, 42, 293–318. [Google Scholar] [CrossRef]
- Pena, G.; Altree, M.; Field, J.; Sainsbury, D.; Babidge, W.; Hewett, P.; Maddern, G. Nontechnical skills training for the operating room: A prospective study using simulation and didactic workshop. Surgery 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Matlow, A.G.; Baker, G.R.; Flintoft, V.; Cochrane, D.; Coffey, M.; Cohen, E.; Cronin, C.M.; Damignani, R.; Dubé, R.; Galbraith, R.; et al. Adverse events among children in Canadian hospitals: The Canadian paediatric adverse events study. Can. Med. Assoc. J. 2012, 184, E709–E718. [Google Scholar] [CrossRef]
- Gawande, A.A.; Zinner, M.J.; Studdert, D.M.; Brennan, T.A. Analysis of errors reported by surgeons at three teaching hospitals. Surgery 2003, 133, 614–621. [Google Scholar] [CrossRef]
- Breaud, J.; Talon, I.; Fourcade, L.; Podevin, G.; Rod, J.; Audry, G.; Dohin, B.; Lecompte, J.-F.; Bensaid, R.; Rampal, V.; et al. The national pediatric surgery simulation program in France: A tool to develop resident training in pediatric surgery. J. Pediatr. Surg. 2019, 54, 582–586. [Google Scholar] [CrossRef]
- Breaud, J.; Azzie, G. Development and assessment of a simulation-based curriculum in pediatric surgical education: Conventional wisdom and lessons learned from the national training program in France. Semin. Pediatr. Surg. 2020, 29, 150902. [Google Scholar] [CrossRef] [PubMed]
- Simulation Enhanced Learning Course for Paediatric Surgical Trainees AKA Bootcamp Virtual. British Association of Paediatric Surgeons. Available online: https://www.baps.org.uk/events/simulation-enhanced-learning-course-for-paediatric-surgical-trainees-aka-bootcamp-virtual/ (accessed on 20 September 2023).
- Cairo, S.B.; Harmon, C.M.; Rothstein, D.H. Minimally invasive surgical exposure among US and Canadian pediatric surgery trainees, 2004–2016. J. Surg. Res. 2018, 231, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bailez, M.M.; Maricic, M.A.; Falcioni, A.G.; Yang, H.C.; Martinez, P.S. Development of a simulation minimally invasive surgery (MIS) training program in a curricula of pediatric surgery: A replicable experience. J. Pediatr. Surg. 2023, 3, 100052. [Google Scholar] [CrossRef]
- Chirdan, L.B.; Ameh, E.A.; Abantanga, F.A.; Sidler, D.; Elhalaby, E.A. Challenges of training and delivery of pediatric surgical services in Africa. J. Pediatr. Surg. 2010, 43, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.W. Developing pediatric surgery in low- and middle- income countries: An evaluation of contemporary education and care delivery models. Semin. Pediatr. Surg. 2016, 25, 43–50. [Google Scholar] [CrossRef] [PubMed]
- PRB. 2022 World Population Date Sheet. Available online: https://www.prb.org/wp-content/uploads/2022/09/2022-World-Population-Data-Sheet-Booklet.pdf (accessed on 18 December 2023).
- Bandyopadhyay, S.; Lakhoo, K. Emerging Optimism in Paediatric Surgery in Africa. Afr. J. Paediatr. Surg. 2023, 20, 252–253. [Google Scholar] [CrossRef]
- Hayton, R.A.; Donley, D.K.; Fekadu, A.; Woods, B.K.; Graybill, C.K.; Fitzgerald, T.N. Surgical volunteerism as a collaborative teaching activity can benefit surgical residents in low-middle income countries. Int. Surg. J. 2017, 48, 34–37. [Google Scholar] [CrossRef]
- Reed, C.R.; Commander, S.J.; Sekabira, J.; Kisa, P.; Kakembo, N.; Wesonga, A.; Langer, M.; Villanova, G.A.; Ozgediz, D.; Fitzgerald, T.N. Comparison of Ugandan and North American Pediatric Surgery Fellows’ Operative Experience: Opportunities for Global Training Exchange. J. Surg. Educ. 2020, 77, 606–614. [Google Scholar] [CrossRef]
- Baird, R.; Poenaru, D.; Ganey, M.; Hansen, E.; Emil, S. Partnership in fellowship: Comparative analysis of pediatric surgical training and evaluation of a fellow exchange between Canada and Kenya. J. Pediatr. Surg. 2016, 51, 1704–1710. [Google Scholar] [CrossRef]
- Jooma, U.; Numanoglu, A.; Cox, S. Paediatric surgery training in South Africa: Trainees’ perspectives. Pediatr. Surg. Int. 2020, 36, 1489–1494. [Google Scholar] [CrossRef]
- Traynor, M.D., Jr.; Owino, J.; Rivera, M.; Parker, R.K.; White, R.E.; Steffes, B.C.; Chikoya, L.; Matsumoto, J.M.; Moir, C.R. Surgical Simulation in East, Central, and Southern Africa: A Multinational Survey. J. Surg. Educ. 2021, 78, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Regehr, G.; Reznick, R.; Macrae, H.; Murnaghan, J.; Hutchison, C.; Brown, M. Objective structured assessment of technical skill (OSATS) for surgical residents. Br. J. Surg. 1997, 84, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hance, J.; Aggarwal, R.; Stanbridge, R.; Blauth, C.; Munz, Y.; Darzi, A.; Pepper, J. Objective assessment of technical skills in cardiac surgery. Eur. J. Cardiothorac. Surg. 2005, 28, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.R.; Grantcharov, T.; Schouenborg, L.; Ottosen, C.; Soerensen, J.L.; Ottesen, B. Objective assessment of surgical competence in gynaecological laparoscopy: Development and validation of a procedure-specific rating scale. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, E.; Kolkman, W.; Wolterbeek, R.; Trimbos, B.; Jansen, F.W. Value of an objective assessment tool in the operating room. Can. J. Surg. 2011, 54, 116–122. [Google Scholar] [CrossRef]
- Munz, Y.; Moorthy, K.; Bann, S.; Shah, J.; Ivanova, S.; Darzi, S.A. Ceiling effect in technical skills of surgical residents. Am. J. Surg. 2004, 188, 294–300. [Google Scholar] [CrossRef]
- Strandbygaard, J.; Scheele, F.; Sørensen, J.L. Twelve tips for assessing surgical performance and use of technical assessment scales. Med. Teach. 2017, 39, 32–37. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knowlin, L.T.; Laskay, N.M.B.; Jules, N.P.; Godzik, J.; Chang, T.P.; Spurrier, R.G. Advances in Pediatric Surgery Simulation-Based Training. Children 2024, 11, 34. https://doi.org/10.3390/children11010034
Knowlin LT, Laskay NMB, Jules NP, Godzik J, Chang TP, Spurrier RG. Advances in Pediatric Surgery Simulation-Based Training. Children. 2024; 11(1):34. https://doi.org/10.3390/children11010034
Chicago/Turabian StyleKnowlin, Laquanda T., Nicholas M. B. Laskay, Nehemie P. Jules, Jakub Godzik, Todd P. Chang, and Ryan G. Spurrier. 2024. "Advances in Pediatric Surgery Simulation-Based Training" Children 11, no. 1: 34. https://doi.org/10.3390/children11010034
APA StyleKnowlin, L. T., Laskay, N. M. B., Jules, N. P., Godzik, J., Chang, T. P., & Spurrier, R. G. (2024). Advances in Pediatric Surgery Simulation-Based Training. Children, 11(1), 34. https://doi.org/10.3390/children11010034





