Exploring Differences in Dietary Diversity and Micronutrient Adequacy between Costa Rican and Mexican Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Sociodemographic Variables
2.3. Anthropometric Assessment
2.4. Dietary Intake Assessment
2.5. Minimum Dietary Diversity
2.6. Micronutrient Intake Adequacy
2.7. Minimum Dietary Diversity Cut-Off
2.8. Statistical Analyses
3. Results
3.1. General Characteristics of the Study Participants by Country and Area of Residence
3.2. Minimum Dietary Diversity by the General Characteristics of the Study Participants
3.3. Consumption of Food Groups
3.4. Nutrient Probability of Adequacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lytle, L.A. Nutritional Issues for Adolescents. J. Am. Diet. Assoc. 2002, 102, S8–S12. [Google Scholar] [CrossRef]
- Torrico, J.C. Dietary Diversity Score as an Indicator of Micronutrient Intake in Filipino Children and Adolescents. Asia Pac. J. Clin. Nutr. 2021, 30, 696–703. [Google Scholar] [CrossRef]
- Wiafe, M.A.; Apprey, C.; Annan, R.A. Dietary Diversity and Nutritional Status of Adolescents in Rural Ghana. Nutr. Metab. Insights 2023, 16, 117863882311584. [Google Scholar] [CrossRef]
- Worku, M.; Hailemicael, G.; Asegedech, W. Dietary Diversity Score and Associated Factors among High School Adolescent Girls in Gurage Zone, Southwest Ethiopia. World J. Nutr. Health 2017, 5, 41–45. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, S.; Sánchez-Pimienta, T.G.; Batis, C.; Cediel, G.; Marrón-Ponce, J.A. Minimum Dietary Diversity in Mexico: Establishment of Cutoff Point to Predict Micronutrients Adequacy. Eur. J. Clin. Nutr. 2022, 76, 739–745. [Google Scholar] [CrossRef]
- Monge-Rojas, R.; Vargas-Quesada, R.; Gómez, G. Role of Residence Area on Diet Diversity and Micronutrient Intake Adequacy in Urban and Rural Costa Rican Adolescents. Nutrients 2022, 14, 5093. [Google Scholar] [CrossRef]
- Baxter, J.B.; Wasan, Y.; Islam, M.; Cousens, S.; Soofi, S.B.; Ahmed, I.; Sellen, D.W.; Bhutta, Z.A. Dietary Diversity and Social Determinants of Nutrition among Late Adolescent Girls in Rural Pakistan. Matern. Child Nutr. 2022, 18, e13265. [Google Scholar] [CrossRef]
- Caplan, P. Food, Health and Identity; Taylor and Francis: Hoboken, NJ, USA, 2013; ISBN 978-0-203-44379-8. [Google Scholar]
- Long, J.; Vargas, L.A. Food Culture in Mexico; Greenwood Press: Westport, CT, USA, 2005; ISBN 978-1-4294-7451-1. [Google Scholar]
- Janer, Z.; Albala, K. Latino Food Culture; Food Cultures in America/Ken Albala, general ed.; Greenwood Press: Westport, CT, USA, 2008; ISBN 978-0-313-34027-7. [Google Scholar]
- Sonnenfeld, D.A.; Oosterveer, P. Food, Globalization and Sustainability; Taylor & Francis: Abingdon, UK, 2012; ISBN 978-1-280-87435-2. [Google Scholar]
- Verger, E.O.; Le Port, A.; Borderon, A.; Bourbon, G.; Moursi, M.; Savy, M.; Mariotti, F.; Martin-Prevel, Y. Dietary Diversity Indicators and Their Associations with Dietary Adequacy and Health Outcomes: A Systematic Scoping Review. Adv. Nutr. 2021, 12, 1659–1672. [Google Scholar] [CrossRef]
- Nithya, D.J.; Bhavani, R.V. Dietary Diversity and Its Relationship with Nutritional Status among Adolescents and Adults in Rural India. J. Biosoc. Sci. 2018, 50, 397–413. [Google Scholar] [CrossRef]
- Nithya, D.J.; Bhavani, R.V. Factors Which May Limit the Value of Dietary Diversity and Its Association with Nutritional Outcomes in Preschool Children in High Burden Districts of India. Asia Pac. J. Clin. Nutr. 2018, 27, 413–420. [Google Scholar] [CrossRef]
- Ruel, M.T. Operationalizing Dietary Diversity: A Review of Measurement Issues and Research Priorities. J. Nutr. 2003, 133, 3911S–3926S. [Google Scholar] [CrossRef]
- Mirmiran, P.; Azadbakht, L.; Esmaillzadeh, A.; Azizi, F. Dietary Diversity Score in Adolescents—A Good Indicator of the Nutritional Adequacy of Diets: Tehran Lipid and Glucose Study. Asia Pac. J. Clin. Nutr. 2004, 13, 56–60. [Google Scholar]
- FAO. FHI 360 Minimum Dietary Diversity for Women: A Guide for Measurement; FAO: Rome, Italy, 2016; ISBN 978-92-5-109153-1. [Google Scholar]
- Nguyen, P.H.; Huybregts, L.; Sanghvi, T.G.; Tran, L.M.; Frongillo, E.A.; Menon, P.; Ruel, M.T. Dietary Diversity Predicts the Adequacy of Micronutrient Intake in Pregnant Adolescent Girls and Women in Bangladesh, but Use of the 5-Group Cutoff Poorly Identifies Individuals with Inadequate Intake. J. Nutr. 2018, 148, 790–797. [Google Scholar] [CrossRef]
- Akter, R.; Sugino, H.; Akhter, N.; Brown, C.L.; Thilsted, S.H.; Yagi, N. Micronutrient Adequacy in the Diet of Reproductive-Aged Adolescent Girls and Adult Women in Rural Bangladesh. Nutrients 2021, 13, 337. [Google Scholar] [CrossRef]
- Steyn, N.; Nel, J.; Nantel, G.; Kennedy, G.; Labadarios, D. Food Variety and Dietary Diversity Scores in Children: Are They Good Indicators of Dietary Adequacy? Public Health Nutr. 2006, 9, 644–650. [Google Scholar] [CrossRef]
- Hatløy, A.; Torheim, L.E.; Oshaug, A. Food Variety—A Good Indicator of Nutritional Adequacy of the Diet? A Case Study from an Urban Area in Mali, West Africa. Eur. J. Clin. Nutr. 1998, 52, 891–898. [Google Scholar] [CrossRef]
- Isabirye, N.; Bukenya, J.N.; Nakafeero, M.; Ssekamatte, T.; Guwatudde, D.; Fawzi, W. Dietary Diversity and Associated Factors among Adolescents in Eastern Uganda: A Cross-Sectional Study. BMC Public Health 2020, 20, 534. [Google Scholar] [CrossRef]
- Gómez Delgado, Y.; Velázquez Rodríguez, E.B. Salud y cultura alimentaria en México. RDU 2019, 20, 1–11. [Google Scholar] [CrossRef]
- González-Arce, R. La Alimentación Tradicional Costarricense: Propuestas para su Revitalización, 1st ed.; FAO: San José, Costa Rica, 2012. [Google Scholar]
- Programa Estado de la Nación. Sétimo Informe Estado de La Educación/Seventh State of Education Report; Programa Estado de la Nación: San José, CA, USA, 2019. [Google Scholar]
- Romero-Martínez, M.; Shamah-Levy, T.; Cuevas-Nasu, L.; Méndez Gómez-Humarán, I.; Gaona-Pineda, E.B.; Gómez-Acosta, L.M.; Rivera-Dommarco, J.Á.; Hernández-Ávila, M. Diseño Metodológico de La Encuesta Nacional de Salud y Nutrición de Medio Camino 2016. Salud Publica Mex. 2017, 59, 299. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Censos, Costa Rica. Clasificación de Distritos Según Grado de Urbanización: Metodología; Instituto Nacional de Estadistica y Censos: San José, CA, USA, 2018; ISBN 978-9930-525-25-8. [Google Scholar]
- Bartholomew, D.J.; Steele, F.; Steele, F.; Moustaki, I. Analysis of Multivariate Social Science Data, 2nd ed.; Chapman and Hall/CRC: London, UK; Boca Raton, FL, USA, 2008; ISBN 978-0-429-14567-4. [Google Scholar]
- Preedy, V.R. (Ed.) Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease; Springer: New York, NY, USA, 2012; ISBN 978-1-4419-1787-4. [Google Scholar]
- Lohman, T.G.; Roche, A.F.; Martorell, R. (Eds.) Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988; ISBN 978-0-87322-121-4. [Google Scholar]
- Habicht, J.P. Standardization of quantitative epidemiological methods in the field. Bol. Oficina Sanit. Panam. 1974, 76, 375–384. [Google Scholar]
- World Health Organization. Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. In Methods and Development; de Onis, M., Ed.; WHO Child Growth Standards; WHO Press: Geneva, Switzerland, 2006; ISBN 978-92-4-154693-5. [Google Scholar]
- Escuela de Nutrición, UCR ValorNut UCR: Programa Para El Cálculo de Valor Nutritivo de Los Alimentos. Available online: https://nutricion2.ucr.ac.cr/auth/register (accessed on 1 January 2023).
- Ramírez-Silva, I.; Barragán-Vázquez, S.; Rodríguez-Ramírez, S.; Rivera-Dommarco, J.; Mejía-Rodríguez, F.; Barquera-Cervera, S.; Tolentino-Mayo, M.; Villalpando-Hernández, S.; Ancira-Moreno, M. Base de Alimentos de México (BAM): Compilación de la Composición de los Alimentos Frecuentemente Consumidos en el País; Base de Alimentos de México (BAM): Cuernavaca, Mexico, 2021. [Google Scholar]
- Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes. Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000; p. 9956. ISBN 978-0-309-07183-3. [Google Scholar]
- Carriquiry, A.L. Estimation of Usual Intake Distributions of Nutrients and Foods. J. Nutr. 2003, 133, 601S–608S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; p. 11537. ISBN 978-0-309-15742-1. [Google Scholar]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; ISBN 978-92-4-154612-6.
- Tarabla, H. Epidemiología Diagnóstica, 1st ed.; Ediciones UNL: Santa Fe, Argentina, 2020; ISBN 978-987-749-209-5. [Google Scholar]
- StataCorp Stata Statistical Software; Release 14; StataCorp: College Station, TX, USA, 2015.
- IBM Corp. IBM SPSS Statistics for Windows (Version 27.0); IBM Corp.: New York, NY, USA, 2020. [Google Scholar]
- Chalermsri, C.; Rahman, S.M.; Ekström, E.-C.; Muangpaisan, W.; Aekplakorn, W.; Satheannopakao, W.; Ziaei, S. Socio-Demographic Characteristics Associated with the Dietary Diversity of Thai Community-Dwelling Older People: Results from the National Health Examination Survey. BMC Public Health 2022, 22, 377. [Google Scholar] [CrossRef] [PubMed]
- Workicho, A.; Belachew, T.; Argaw, A.; Roba, A.; Ghosh, S.; Kershaw, M.; Lachat, C.; Kolsteren, P. Maternal Nutritional Status Mediates the Association between Maternal Age and Birth Outcomes. Matern. Child Nutr. 2020, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jordan, I.; Röhlig, A.; Glas, M.G.; Waswa, L.M.; Mugisha, J.; Krawinkel, M.B.; Nuppenau, E.-A. Dietary Diversity of Women across Agricultural Seasons in the Kapchorwa District, Uganda: Results from a Cohort Study. Foods 2022, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, K.; Tan, S.; Zheng, Y.; Zhao, A.; Wang, P.; Zhang, Y. Dietary Diversity Scores: An Indicator of Micronutrient Inadequacy Instead of Obesity for Chinese Children. BMC Public Health 2017, 17, 440. [Google Scholar] [CrossRef] [PubMed]
- FAO. El Sistema Alimentario en México: Oportunidades para el Campo Mexicano en la Agenda 2030 de Desarrollo Sostenible; FAO: Ciudad de México, Mexico, 2019; ISBN 978-92-5-131229-2. [Google Scholar]
- Vaquiro, N.F. Pobreza, desigualdad y perfil sociodemográfico de los hogares rurales y agropecuarios en la región sur de México. EntreDiversidades Rev. De Cienc. Soc. Y Humanidades 2021, 8, 36–63. [Google Scholar] [CrossRef]
- Aboites, M.; Verduzco, G.; López-Barbosa, L. La Alimentación en el México Rural. In Tierra, Derechos Humanos y Desarrollo: Supuestos y Visiones Desde África y América; Ediciones Egregius: Sevilla, Spain, 2020; pp. 239–272. [Google Scholar]
- Fuentes, E. Agricultura familiar y seguridad alimentaria en el México Rural. Estud. Soc. 2021, 31. [Google Scholar] [CrossRef]
- Valenciano Salazar, J.A.; Pagani Centeno, L.; Álvarez Madrigal, N. Seguridad y Soberanía Alimentaria En Costa Rica; Editorial UNA: Heredia, CR, USA, 2020; ISBN 978-9930-588-01-7. [Google Scholar]
- Aburto, T.C.; Batis, C.; Pedroza-Tobías, A.; Pedraza, L.S.; Ramírez-Silva, I.; Rivera, J.A. Dietary Intake of the Mexican Population: Comparing Food Group Contribution to Recommendations, 2012–2016. Salud Publica Mex. 2022, 64, 267–279. [Google Scholar] [CrossRef]
- Monge-Rojas, R.; Vargas-Quesada, R.; Chinnock, A.; Colón-Ramos, U. Changes in Dietary Intake of Major Nutrients and Food Sources among Costa Rican Adolescents in the Last 20 Years. J. Nutr. 2020, 150, 2405–2411. [Google Scholar] [CrossRef]
- Martorell, R.; Ascencio, M.; Tacsan, L.; Alfaro, T.; Young, M.F.; Addo, O.Y.; Dary, O.; Flores-Ayala, R. Effectiveness Evaluation of the Food Fortification Program of Costa Rica: Impact on Anemia Prevalence and Hemoglobin Concentrations in Women and Children. Am. J. Clin. Nutr. 2015, 101, 210–217. [Google Scholar] [CrossRef]
- Global Fortification Data Exchange Dashboard: Country Fortification–Mexico. 2022. Available online: https://fortificationdata.org/country-fortification-dashboard/?alpha3_code=MEX&lang=en (accessed on 1 January 2023).
- Global Fortification Data Exchange Dashboard: Country Fortification–Costa Rica. 2017. Available online: https://fortificationdata.org/country-fortification-dashboard/?alpha3_code=CRI&lang=en (accessed on 1 January 2023).
- Guzmán-Soria, E.; De La Garza Carranza, M.T.; García Salazar, J.A.; Rebollar Rebollar, S.; Hernández Martínez, J. Análisis Económico Del Mercado de Frijol Grano En México. Agron. Mesoam. 2019, 30, 131–146. [Google Scholar] [CrossRef]
- Barco Leme, A.C.; Fisberg, R.M.; Veroneze de Mello, A.; Sales, C.H.; Ferrari, G.; Haines, J.; Rigotti, A.; Gómez, G.; Kovalskys, I.; Cortés Sanabria, L.Y.; et al. Food Sources of Shortfall Nutrients among Latin Americans: Results from the Latin American Study of Health and Nutrition (ELANS). Int. J. Environ. Res. Public Health 2021, 18, 4967. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Hofmeyr, G.J.; Cormick, G.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Betrán, A.P. Current Calcium Fortification Experiences: A Review. Ann. N. Y. Acad. Sci. 2021, 1484, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011; p. 13050. ISBN 978-0-309-16394-1. [Google Scholar]
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium Deficiency Worldwide: Prevalence of Inadequate Intakes and Associated Health Outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28. [Google Scholar] [CrossRef]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Narayana Press: Copenhagen, Denmark, 2014; Volume 5, ISBN 978-92-893-2670-4. [Google Scholar]
- Singh, N. Pulses: An Overview. J. Food Sci. Technol. 2017, 54, 853–857. [Google Scholar] [CrossRef]
- Ramakrishnan, U. Nutritional Anemias; CRC Series in Modern Nutrition; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-0-8493-8569-8. [Google Scholar]
- Bovell-Benjamin, A.C.; Viteri, F.E.; Allen, L.H. Iron Absorption from Ferrous Bisglycinate and Ferric Trisglycinate in Whole Maize Is Regulated by Iron Status. Am. J. Clin. Nutr. 2000, 71, 1503–1509. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Censos, Costa Rica. Estimación de Población y Vivienda 2022. 2022. Available online: https://inec.cr/poblacion-total (accessed on 1 January 2023).
- Willett, W. Nutritional Epidemiology. In Monographs in Epidemiology and Biostatistics, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2013; ISBN 978-0-19-975403-8. [Google Scholar]
- Albazee, E.; Almahmoud, L. Folic Acid Deficiency and Neural Tube Defects: The Awareness Dilemma. J. Glob. Neurosurg. 2022, 2. [Google Scholar] [CrossRef]
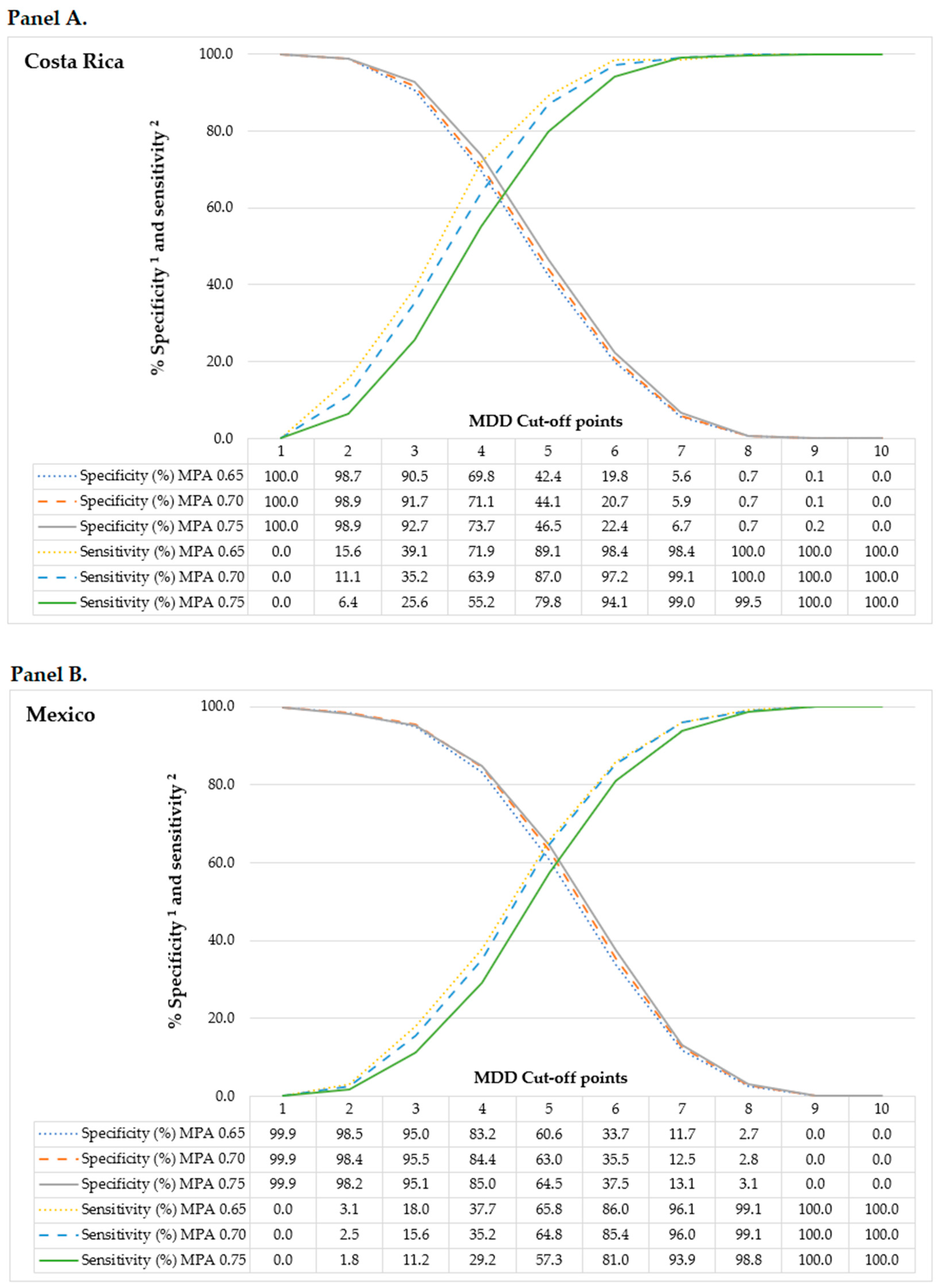
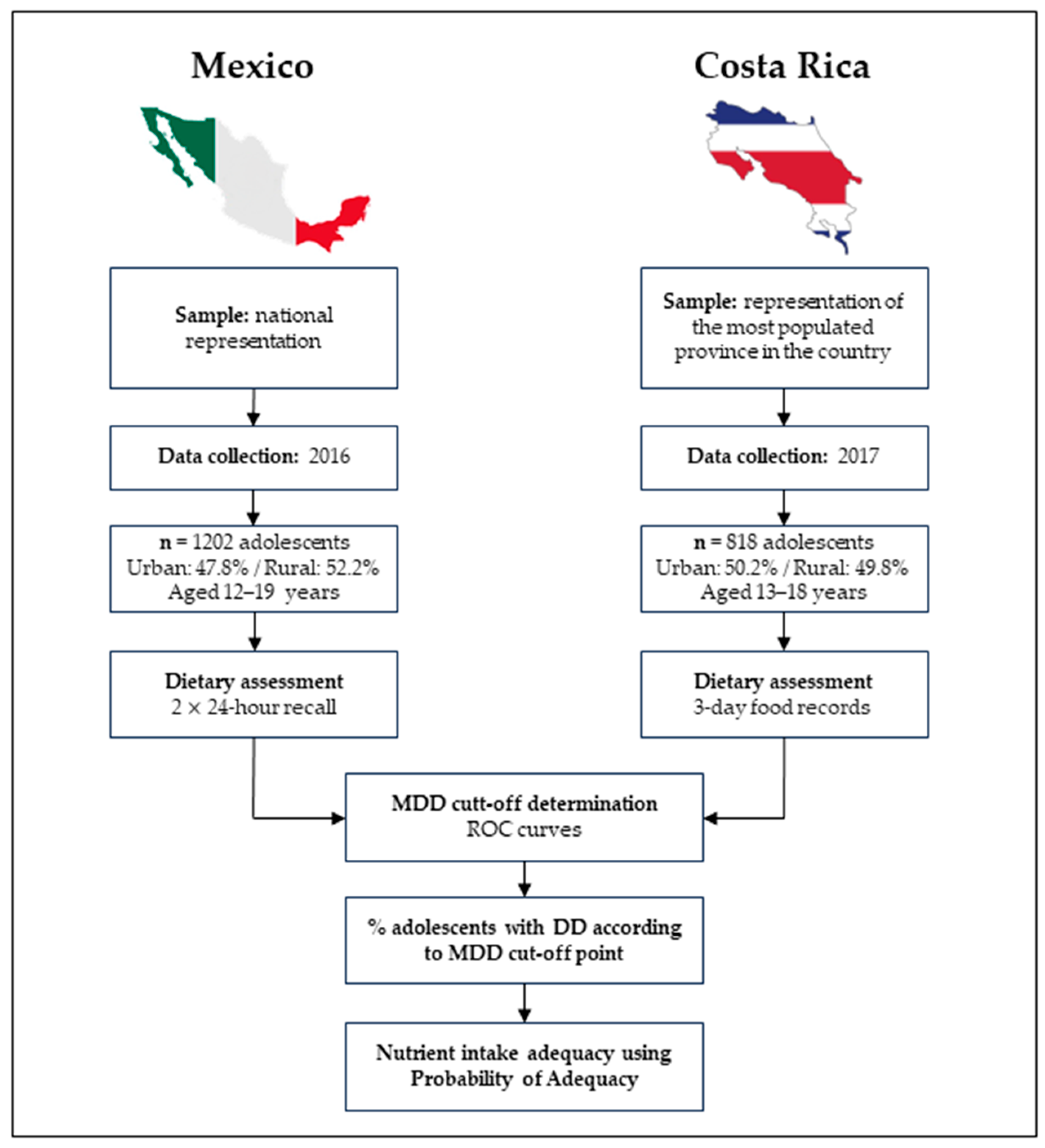


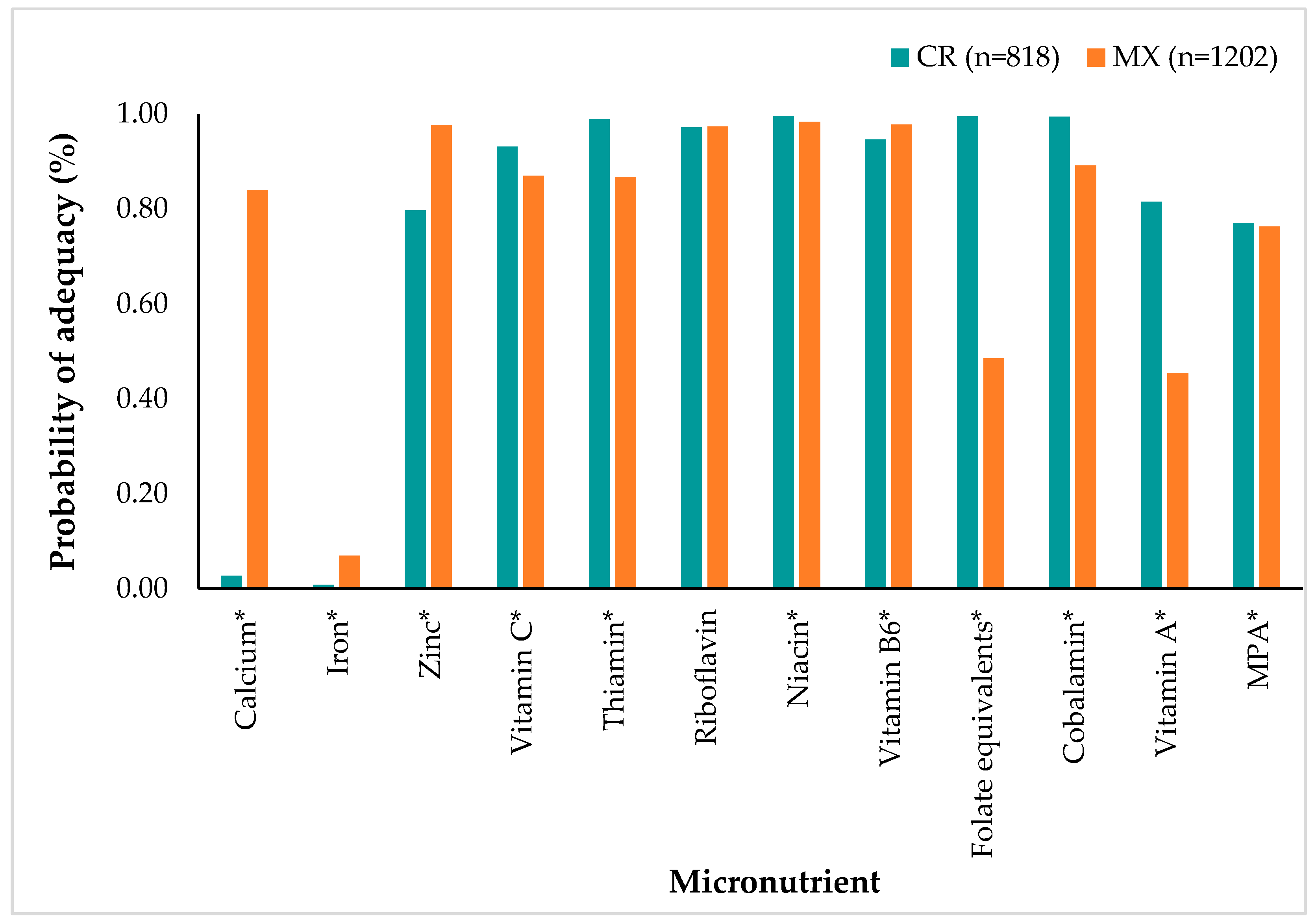
| Characteristics | Costa Rica | Mexico | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall 1 (n = 818) | Area of Residence | Overall 1 (n = 1202) | Area of Residence | |||||
| Urban (n = 411) | Rural (n = 407) | p-Value 2 | Urban (n = 574) | Rural (n = 628) | p-Value 2 | |||
| Age (y) | 15.0 ± 1.7 | 14.9 ± 1.7 | 15.1 ± 1.7 | 0.208 | 15.1 ± 2.3 | 15.2 ± 2.3 | 15.0 ± 2.2 | 0.078 |
| Sex | ||||||||
| Girls | 520 (63.6) | 259 (63.0) a | 261 (64.1) a | 0.741 | 594 (49.4) | 300 (52.3) a | 308 (49.0) a | 0.265 |
| Boys | 298 (36.4) | 152 (37.0) a | 146 (35.9) a | 608 (50.6) | 274 (47.7) a | 320 (51.0) a | ||
| Socioeconomic status | ||||||||
| Low | 263 (32.2) | 87 (21.2) a | 179 (43.2) b | <0.0001 | 406 (33.8) | 115 (20.0) b | 291 (46.3) a | <0.0001 |
| Middle | 325 (39.7) | 158 (38.4) a | 167 (41.2) a | 433 (36.0) | 195 (34.0) a | 238 (37.9) a | ||
| High | 230 (28.1) | 166 (40.4) a | 64 (15.7) b | 363 (30.2) | 264 (46.0) b | 99 (15.8) a | ||
| Nutritional status | ||||||||
| Non-overweight | 551 (67.4) | 279 (67.9) a | 272 (66.8) a | 0.748 | 757 (63.0) | 333 (58.0) b | 424 (67.5) a | <0.0001 |
| Overweight/obesity | 267 (32.6) | 132 (32.1) a | 135 (33.2) a | 445 (37.0) | 241 (42.0) b | 204 (32.5) a | ||
| Energy intake (kcal) | 1873 ± 763 | 1852 ± 769 | 1896 ± 757 | 0.459 | 1913 ± 898 | 1939 ± 949 | 1888 ± 849 | 0.707 |
| Characteristic | Costa Rica (n = 818) | CR Participants with a Diverse Diet (MDD ≥ 4) | Mexico (n = 1202) | MX Participants with a Diverse Diet (MDD ≥ 5 ) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | p-Value 1 | n (%) | p-Value 1 | Mean | SD | p-Value 1 | n (%) | p-Value 1 | |
| Overall | 4.17 | 1.43 | 544 (66.5) | 4.68 | 1.48 | 668 (55.6) | <0.0001 2 | |||
| Sex | ||||||||||
| Girls | 4.18 | 1.43 | 0.679 | 349 (67.1) | 0.624 | 4.62 | 1.38 | 0.177 | 337 (56.7) | 0.424 |
| Boys | 4.14 | 1.44 | 195 (65.4) | 4.74 | 1.58 | 337 (54.4) | ||||
| Area of residence | ||||||||||
| Urban | 4.00 | 1.42 | 0.001 | 251 (61.1) | 0.001 | 4.70 | 1.51 | 0.632 | 323 (56.3) | 0.642 |
| Rural | 4.33 | 1.43 | 293 (72.0) | 4.66 | 1.46 | 345 (54.9) | ||||
| Socioeconomic status | ||||||||||
| Low | 4.28 | 1.44 | 0.303 | 183 (69.6) | 0.420 | 4.50 a | 1.37 | 0.010 | 211 (52.0) | 0.194 |
| Middle | 4.11 | 1.39 | 213 (65.5) | 4.79 b | 1.50 | 247 (57.0) | ||||
| High | 4.11 | 1.48 | 148 (64.4) | 4.74 a,b | 1.56 | 210 (57.9) | ||||
| Nutritional status | ||||||||||
| Non-overweight | 4.15 | 1.43 | 0.680 | 367 (66.6) | 0.929 | 4.70 | 1.48 | 0.545 | 427 (56.4) | 0.449 |
| Overweight/obesity | 4.19 | 1.43 | 177 (66.3) | 4.65 | 1.49 | 241 (54.2) | ||||
| Food Group (g/d/1000 kcal) 1 | Costa Rica (n = 818) | Mexico (n = 1202) | ||||
|---|---|---|---|---|---|---|
| Girls (n = 520) | Boys (n = 298) | p-Value 2 | Girls (n = 608) | Boys (n = 594) | p-Value 2 | |
| Starchy staples | 204.2 ± 84.0 | 214.2 ± 81.6 | 0.037 | 133.2 ± 88.5 | 133.5 ± 89.8 | 0.957 |
| Pulses | 41.2 ± 52.8 | 58.8 ± 60.4 | <0.0001 | 10.7 ± 19.6 | 12.2 ± 22.9 | 0.515 |
| Flesh foods | 47.0 ± 47.5 | 45.1 ± 43.6 | 1.000 | 42.8 ± 56.7 | 45.3 ± 54.9 | 0.125 |
| Eggs | 9.5 ± 21.1 | 11.5 ± 21.6 | 0.063 | 19.9 ± 34.7 | 22.1 ± 41.3 | 0.319 |
| Milk and milk products | 77.1 ± 115.9 | 72.7 ± 107.6 | 0.475 | 76.7 ± 114.4 | 82.7 ± 116.1 | 0.222 |
| Nuts and seeds | 0.9 ± 6.8 | 0.7 ± 4.8 | 0.807 | 0.4 ± 4.0 | 0.2 ± 1.8 | 0.148 |
| Other fruits | 45.3 ± 83.2 | 34.1 ± 89.4 | 0.001 | 67.1 ± 102.5 | 58.1 ± 104.7 | 0.022 |
| Other vegetables | 27.6 ± 45.7 | 22.6 ± 50.5 | 0.021 | 46.2 ± 79.5 | 38.7 ± 67.2 | 0.041 |
| Other vitamin A-rich F&V | 7.7 ± 31.9 | 5.5 ± 36.4 | 0.086 | 30.5 ± 91.8 | 27.7 ± 76.1 | 0.046 |
| Dark green leafy vegetables | 3.3 ± 20.3 | 2.2 ± 15.3 | 0.270 | 1.1 ± 7.5 | 1.5 ± 9.9 | 0.650 |
| Food Group (g/d/1000 kcal) 1 | Costa Rica (n = 818) | Mexico (n = 1202) | ||||
|---|---|---|---|---|---|---|
| Urban (n = 411) | Rural (n = 407) | p-Value 2 | Urban (n = 574) | Rural (n = 628) | p-Value 2 | |
| Starchy staples | 199.1 ± 90.0 | 216.7 ± 74.9 | <0.0001 | 121.3 ± 84.9 | 144.4 ± 91.4 | <0.0001 |
| Pulses | 41.4 ± 58.5 | 54.0 ± 53.3 | <0.0001 | 9.8 ± 21.4 | 12.9 ± 21.0 | <0.0001 |
| Flesh foods | 48.8 ± 46.9 | 43.7 ± 45.2 | 0.111 | 47.3 ± 56.0 | 41.0 ± 55.6 | 0.002 |
| Eggs | 9.6 ± 22.4 | 10.8 ± 20.2 | 0.039 | 20.9 ± 40.1 | 21.1 ± 36.2 | 0.913 |
| Milk and milk products | 84.1 ± 122.5 | 66.9 ± 101.7 | 0.006 | 89.1 ± 120.2 | 71.0 ± 110.0 | 0.005 |
| Nuts and seeds | 0.8 ± 5.5 | 0.9 ± 6.7 | 0.461 | 0.3 ± 2.5 | 0.3 ± 3.6 | 0.871 |
| Other fruits | 42.5 ± 95.2 | 40.0 ± 74.9 | 0.865 | 64.3 ± 109.4 | 61.1 ± 98.1 | 0.767 |
| Other vegetables | 21.9 ± 51.3 | 29.7 ± 43.2 | <0.0001 | 43.4 ± 72.0 | 41.7 ± 75.4 | 0.164 |
| Other vitamin A-rich F&V | 8.4 ± 40.2 | 5.4 ± 25.2 | 0.405 | 36.8 ± 116.6 | 22.1 ± 33.4 | 0.008 |
| Dark green leafy vegetables | 1.4 ± 10.7 | 4.5 ± 24.0 | 0.015 | 1.1 ± 5.2 | 1.5 ± 11.1 | 0.072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monge-Rojas, R.; Vargas-Quesada, R.; Marrón-Ponce, J.A.; Sánchez-Pimienta, T.G.; Batis, C.; Rodríguez-Ramírez, S. Exploring Differences in Dietary Diversity and Micronutrient Adequacy between Costa Rican and Mexican Adolescents. Children 2024, 11, 64. https://doi.org/10.3390/children11010064
Monge-Rojas R, Vargas-Quesada R, Marrón-Ponce JA, Sánchez-Pimienta TG, Batis C, Rodríguez-Ramírez S. Exploring Differences in Dietary Diversity and Micronutrient Adequacy between Costa Rican and Mexican Adolescents. Children. 2024; 11(1):64. https://doi.org/10.3390/children11010064
Chicago/Turabian StyleMonge-Rojas, Rafael, Rulamán Vargas-Quesada, Joaquín Alejandro Marrón-Ponce, Tania G. Sánchez-Pimienta, Carolina Batis, and Sonia Rodríguez-Ramírez. 2024. "Exploring Differences in Dietary Diversity and Micronutrient Adequacy between Costa Rican and Mexican Adolescents" Children 11, no. 1: 64. https://doi.org/10.3390/children11010064






