Surgical and Endoscopic Intervention for Chronic Pancreatitis in Children: The Kings College Hospital Experience
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility of Relevant Articles
2.3. Outcomes
3. Demographics and Aetiology
4. Review of Therapeutic Interventions
4.1. Endoscopic Ultrasound
4.2. Endoscopic Retrograde Cholangiopancreatography (ERCP)
| Aetiology of Chronic Pancreatitis (n = 59) | Diagnostic Findings | |
|---|---|---|
| Idiopathic | n = 11 (19%) |
|
| Gallstones | n = 22 (37%) | |
| Hereditary | n = 10 (17%) | |
| Pancreatobiliary junction anomalies | n = 8 (14%) | |
| Autoimmune | n = 8 (14%) | |
| No. of ERCPs (n = 126) | ||
| Stent placement | n = 78 (62%) | Indication: |
|
| |
4.3. Surgical Intervention in Children with Chronic Pancreatitis
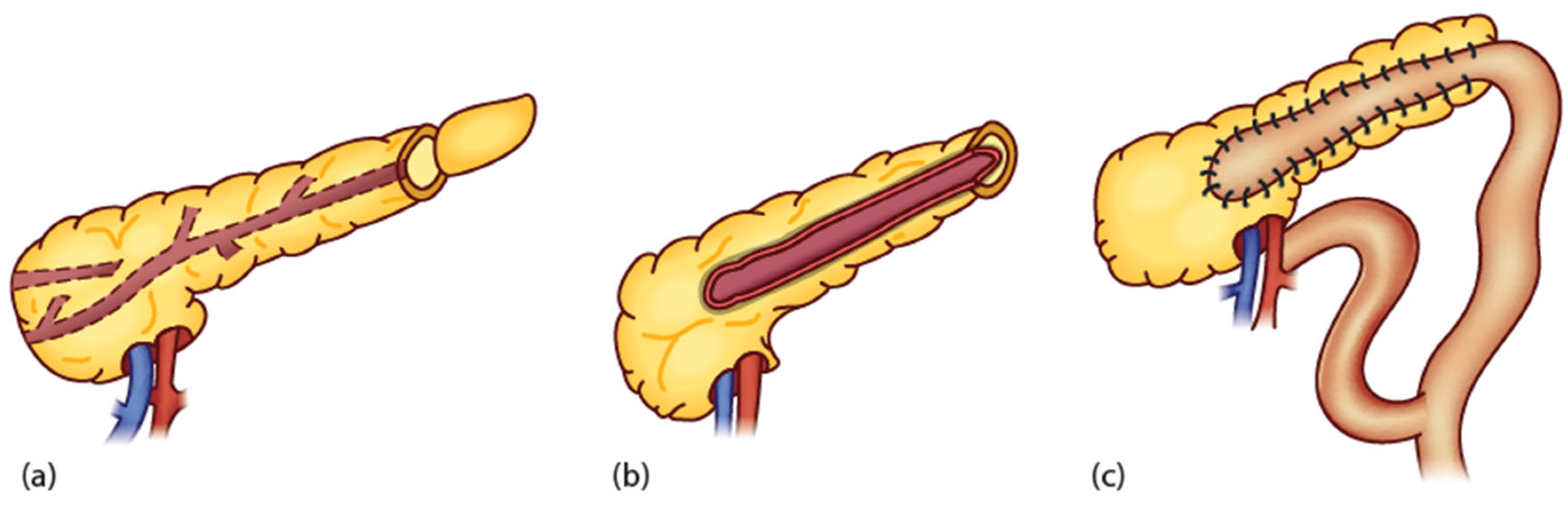
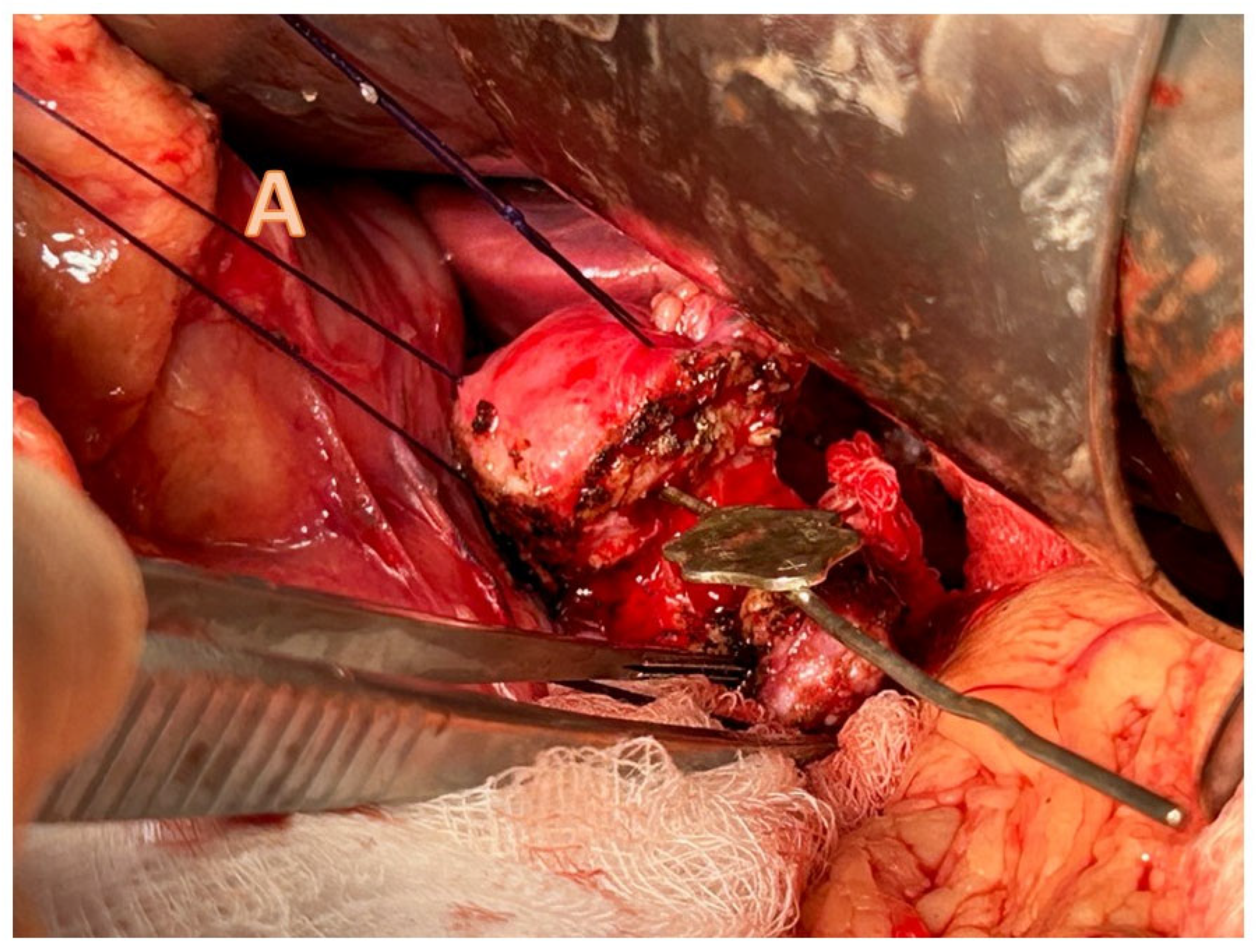
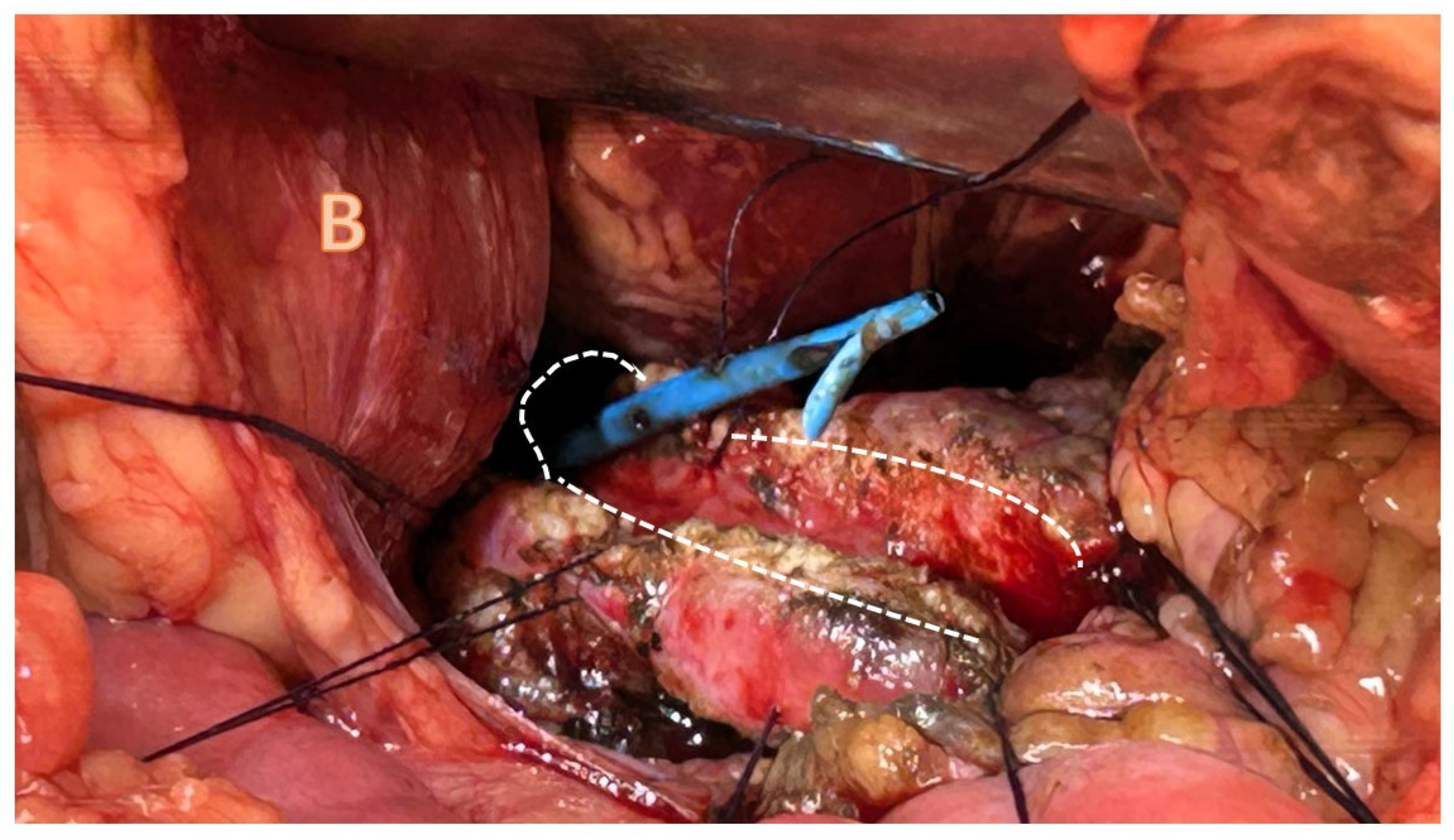
4.3.1. Pancreatojejunostomy
4.3.2. Other Surgical Options
4.3.3. Total Pancreatectomy and Islet Cell Transplant
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CELA3B | Chymotrypsin-like elastase family member 3B |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CPA-1 | Carboxypeptidase A1 |
| CLDN2 | Claudin-2 |
| CTRC | Chymotrypsin C |
| ECM | Extracellular matrix |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| EUS | Endoscopic ultrasound |
| INSPPIRE | International Study Group of Pediatric Pancreatitis: In Search for a CuRE |
| MDT | Multi-disciplinary team |
| MRCP | Magnetic resonance cholangiopancreatography |
| PRSS1 and PRSS2 | Protein serine type 1 and type 2 |
| SPINK1 | Serine protease inhibitor Kazal type 1 |
References
- Kumar, S.; Ooi, C.Y.; Werlin, S.; Abu-El-Haija, M.; Barth, B.; Bellin, M.D.; Durie, P.R.; Fishman, D.S.; Freedman, S.D.; Gariepy, C.; et al. Risk Factors Associated With Pediatric Acute Recurrent and Chronic Pancreatitis: Lessons From INSPPIRE. JAMA Pediatr. 2016, 170, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Boam, T.; Gabriel, M.; Rogoyski, B.G.; Ram, A.D.; Awan, A. Surgical Drainage Procedures for Paediatric Chronic Pancreatitis: A Scoping Review. Pediatr. Surg. Int. 2022, 38, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Spanier, B.W.M.; Bruno, M.J.; Dijkgraaf, M.G.W. Incidence and Mortality of Acute and Chronic Pancreatitis in the Netherlands: A Nationwide Record-Linked Cohort Study for the Years 1995–2005. World J. Gastroenterol. 2013, 19, 3018. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Suzuki, M.; Sakurai, Y.; Nakano, S.; Naritaka, N.; Minowa, K.; Sai, J.K.; Shimizu, T. Genetic Analysis of Japanese Children With Acute Recurrent and Chronic Pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Koziel, D.; Gluszek, S.; Kowalik, A.; Chlopek, M.; Pieciak, L. Genetic Mutations in SPINK1, CFTR, CTRC Genes in Acute Pancreatitis. BMC Gastroenterol. 2015, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Gorry, M.C.; Preston, R.A.; Furey, W.; Sossenheimer, M.J.; Ulrich, C.D.; Martin, S.P.; Gates, L.K.; Amann, S.T.; Toskes, P.P.; et al. Hereditary Pancreatitis Is Caused by a Mutation in the Cationic Trypsinogen Gene. Nat. Genet. 1996, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Gorry, M.C.; Gabbaizedeh, D.; Furey, W.; Gates, J.; Preston, R.A.; Aston, C.E.; Zhang, Y.; Ulrich, C.; Ehrlich, G.D.; Whitcomb, D.C. Mutations in the Cationic Trypsinogen Gene Are Associated with Recurrent Acute and Chronic Pancreatitis. Gastroenterology 1997, 113, 1063–1068. [Google Scholar] [CrossRef]
- Wu, D.; Bampton, T.J.; Scott, H.S.; Brown, A.; Kassahn, K.; Drogemuller, C.; De Sousa, S.M.C.; Moore, D.; Ha, T.; Chen, J.W.C.; et al. The Clinical and Genetic Features of Hereditary Pancreatitis in South Australia. Med. J. Aust. 2022, 216, 578–582. [Google Scholar] [CrossRef]
- Witt, H.; Luck, W.; Hennies, H.C.; Claßen, M.; Kage, A.; Laß, U.; Landt, O.; Becker, M. Mutations in the Gene Encoding the Serine Protease Inhibitor, Kazal Type 1 Are Associated with Chronic Pancreatitis. Nat. Genet. 2000, 25, 213–216. [Google Scholar] [CrossRef]
- Chen, J.M.; Mercier, B.; Audrezet, M.P.; Ferec, C. Mutational Analysis of the Human Pancreatic Secretory Trypsin Inhibitor (PSTI) Gene in Hereditary and Sporadic Chronic Pancreatitis. J. Med. Genet. 2000, 37, 67. [Google Scholar] [CrossRef]
- Liu, J.; Lu, S.Y.; Wang, Y.G.; Wei, Z.Y.; Zhang, H.X. SPINK1 Gene Is Significantly Associated with Pancreatitis: A Comprehensive Meta-Analysis. Pancreas 2017, 46, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Sharer, N.; Schwarz, M.; Malone, G.; Howarth, A.; Painter, J.; Super, M.; Braganza, J. Mutations of the Cystic Fibrosis Gene in Patients with Chronic Pancreatitis. N. Engl. J. Med. 1998, 339, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.A.; Friedman, K.J.; Noone, P.G.; Knowles, M.R.; Silverman, L.M.; Jowell, P.S. Relation between Mutations of the Cystic Fibrosis Gene and Idiopathic Pancreatitis. N. Engl. J. Med. 1998, 339, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chang, Y.T.; Wei, S.C.; Tien, Y.W.; Liang, P.C.; Jan, I.S.; Su, Y.N.; Wong, J.M. Spectrum of Mutations and Variants/Haplotypes of CFTR and Genotype-Phenotype Correlation in Idiopathic Chronic Pancreatitis and Controls in Chinese by Complete Analysis. Clin. Genet. 2007, 71, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Witt, H.; Beer, S.; Rosendahl, J.; Chen, J.-M.; Chandak, G.R.; Masamune, A.; Bence, M.; Szmola, R.; Oracz, G.; Macek, M.J.; et al. Variants in CPA1 Are Strongly Associated with Early Onset Chronic Pancreatitis. Nat. Genet. 2013, 45, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Witt, H.; Szmola, R.; Bhatia, E.; Ózsvári, B.; Landt, O.; Schulz, H.U.; Gress, T.M.; Pfützer, R.; Löhr, M.; et al. Chymotrypsin C (CTRC) Variants That Diminish Activity or Secretion Are Associated with Chronic Pancreatitis. Nat. Genet. 2008, 40, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Chen, J.M.; Scotet, V.; Le Maréchal, C.; Férec, C. Association of Rare Chymotrypsinogen C (CTRC) Gene Variations in Patients with Idiopathic Chronic Pancreatitis. Hum. Genet. 2008, 123, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, X.-T.; Weng, X.-L.; Zhou, D.-Z.; Sun, C.; Xia, T.; Hu, L.-H.; Lai, X.-W.; Ye, B.; Liu, M.-Y.; et al. Comprehensive Screening for PRSS1, SPINK1, CFTR, CTRC and CLDN2 Gene Mutations in Chinese Paediatric Patients with Idiopathic Chronic Pancreatitis: A Cohort Study. BMJ Open 2013, 3, e003150. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Shelton, C.A.; Brand, R.E. Genetics and Genetic Testing in Pancreatic Cancer. Gastroenterology 2015, 149, 1252–1264.e4. [Google Scholar] [CrossRef]
- Moore, P.C.; Cortez, J.T.; Chamberlain, C.E.; Alba, D.; Berger, A.C.; Quandt, Z.; Chan, A.; Cheng, M.H.; Bautista, J.L.; Peng, J.; et al. Elastase 3B Mutation Links to Familial Pancreatitis with Diabetes and Pancreatic Adenocarcinoma. J. Clin. Investig. 2019, 129, 4676. [Google Scholar] [CrossRef]
- Borghei, P.; Sokhandon, F.; Shirkhoda, A.; Morgan, D.E. Anomalies, Anatomic Variants, and Sources of Diagnostic Pitfalls in Pancreatic Imaging. Radiology 2013, 266, 28–36. [Google Scholar] [CrossRef]
- Kronfli, R.; Davenport, M. Insights into the Pathophysiology and Classification of Type 4 Choledochal Malformation. J. Pediatr. Surg. 2020, 55, 2642–2646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Deng, Z.H.; Gong, B. Clinical Characteristics and Endoscopic Treatment of Pancreatitis Caused by Pancreaticobiliary Malformation in Chinese Children. J. Dig. Dis. 2022, 23, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Stringer, M.D.; Davison, S.M.; McClean, P.; Rajwal, S.; Puntis JW, L.; Sheridan, M.; Ramsden, W.; Wodley, H. Multidisciplinary Management of Surgical Disorders of the Pancreas in Childhood. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M. Chronic Pancreatitis. In Surgery of the Liver, Bile Ducts and Pancreas in Children; Davenport, M., Heaton, N., Superina, R., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ravindranath, A.; Srivastava, A.; Yachha, S.K.; Poddar, U.; Sarma MSen Saraswat, V.A.; Mohindra, S.; Yadav, R.R.; Kumar, S. Childhood Pancreatic Trauma: Clinical Presentation, Natural History and Outcome. Pancreatology 2020, 20, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chen, W.; Xia, R.; Peng, Y.; Niu, P.; Fan, H. Pancreatic Stellate Cells and the Targeted Therapeutic Strategies in Chronic Pancreatitis. Molecules 2023, 28, 5586. [Google Scholar] [CrossRef] [PubMed]
- Kohoutova, D.; Tringali, A.; Papparella, G.; Perri, V.; Boškoski, I.; Hamanaka, J.; Costamagna, G. Endoscopic Treatment of Chronic Pancreatitis in Pediatric Population: Long-Term Efficacy and Safety. United Eur. Gastroenterol. J. 2019, 7, 270–277. [Google Scholar] [CrossRef]
- Chowdhury, S.D.; Chacko, A.; Ramakrishna, B.S.; Dutta, A.K.; Augustine, J.; Koshy, A.K.; Simon, E.G.; Joseph, A.J. Clinical Profile and Outcome of Chronic Pancreatitis in Children. Indian Pediatr. 2013, 50, 1016–1019. [Google Scholar] [CrossRef]
- Anushree, N.; Lal, S.B.; Rana, S.S.; Saxena, A.; Venkatesh, V.; Sharma, A.K.; Dayal, D.; Verma, S. Morphological and Functional Recovery Following Acute and Acute Recurrent Pancreatitis in Children: A Prospective Sequential 2-Point Evaluation. Pancreatology 2022, 22, 698–705. [Google Scholar] [CrossRef]
- Puttaiah Kadyada, S.; Thapa, B.R.; Kaushal, K.; Walia, R.; Rana, S.V.; Dhaka, N.; Lal, S.B.; Prasad, R.; Das, S.; Thakur, R.; et al. Incomplete Functional and Morphological Recovery after Acute and Acute Recurrent Pancreatitis in Children. J. Gastroenterol. Hepatol. 2019, 34, 293–301. [Google Scholar] [CrossRef]
- Scheers, I.; Ergun, M.; Aouattah, T.; Piessevaux, H.; Borbath, I.; Stephenne, X.; De Magnée, C.; Reding, R.; Sokal, E.; Veyckemans, F.; et al. Diagnostic and Therapeutic Roles of Endoscopic Ultrasound in Pediatric Pancreaticobiliary Disorders. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.G.; Kumar, M.; Cieplik, N.; Thorburn, D.; Johnson, G.J.; Webster, G.J.; Chapman, M.H.; Lindley, K.J.; Pereira, S.P. Paediatric Pancreaticobiliary Endoscopy: A 21-Year Experience from a Tertiary Hepatobiliary Centre and Systematic Literature Review. BMC Pediatr. 2018, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Ávila, F.I.; Duarte-Medrano, G.; Herrera-Mora, D.; Lopez-Arce, G.; Leal-García, M.; Ramírez-Martínez, M.; Ramírez-Luna, M. Endoscopic Ultrasound in Pediatric Patients with Pancreatobiliary Disease. Surg. Laparosc. Endosc. Percutaneous Technol. 2019, 29, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Ragab, K.M.; El-Kassas, M.; Madkour, A.; Okasha, H.H.; Agwa, R.H.; Ghoneem, E.A. Safety and Efficacy of Endoscopic Ultrasound as a Diagnostic andtherapeutic Tool in Pediatric Patients: A Multicenter Study. Ther. Adv. Gastrointest. Endosc. 2022, 15, 26317745221136767. [Google Scholar]
- Patel, S.; Marshak, J.; Daum, F.; Iqbal, S. The Emerging Role of Endoscopic Ultrasound for Pancreaticobiliary Diseases in the Pediatric Population. World J. Pediatr. 2017, 13, 300–306. [Google Scholar] [CrossRef]
- Roseau, G.; Palazzo, L.; Dumontier, I.; Mougenot, J.F.; Chaussade, S.; Navarro, J.; Couturier, D. Endoscopic Ultrasonography in the Evaluation of Pediatric Digestive Diseases: Preliminary Results. Endoscopy 1998, 30, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Sakamoto, H.; Matsui, U.; Ito, Y.; Maekawa, K.; von Schrenck, T.; Kudo, M. A Novel Perfusion Imaging Technique of the Pancreas: Contrast-Enhanced Harmonic EUS (with Video). Gastrointest. Endosc. 2008, 67, 141–150. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastava, A.; Rai, P.; Yachha, S.K.; Poddar, U. Yield of Endoscopic Ultrasound in Children and Adolescent with Acute Recurrent Pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 461–465. [Google Scholar] [CrossRef]
- Mahajan, R.; Simon, E.G.; Chacko, A.; Reddy, D.V.; Kalyan, P.R.; Joseph, A.J.; Dutta, A.K.; Chowdhury, S.D.; Kurien, R.T. Endoscopic Ultrasonography in Pediatric Patients—Experience from a Tertiary Care Center in India. Indian J. Gastroenterol. 2016, 35, 14–19. [Google Scholar] [CrossRef]
- Gordon, K.; Conway, J.; Evans, J.; Petty, J.; Fortunato, J.E.; Mishra, G. EUS and EUS-Guided Interventions Alter Clinical Management in Children with Digestive Diseases. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 242–246. [Google Scholar] [CrossRef]
- Piester, T.L.; Liu, Q.Y. EUS in Pediatrics: A Multicenter Experience and Review. Front. Pediatr. 2021, 9, 709461. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Torroni, F.; Foschia, F.; De Angelis, P.; Caldaro, T.; Santi, M.R.; Di Abriola, G.F.; Caccamo, R.; Monti, L.; Dall’Oglio, L. Surgery or Endoscopy to Treat Duodenal Duplications in Children. J. Pediatr. Surg. 2011, 46, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Makin, E.; Harrison, P.M.; Patel, S.; Davenport, M. Pancreatic Pseudocysts in Children: Treatment by Endoscopic Cyst Gastrostomy. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Maspons, A.; Othman, M.O. The Therapeutic Use of Endoscopic Ultrasonography in Pediatric Patients Is Safe: A Case Series. Saudi J. Gastroenterol. 2015, 21, 391. [Google Scholar] [PubMed]
- Nabi, Z.; Talukdar, R.; Lakhtakia, S.; Reddy, D.N. Outcomes of Endoscopic Drainage in Children with Pancreatic Fluid Collections: A Systematic Review and Meta-Analysis. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 251. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, J.; Bang, J.Y.; Trevino, J.; Varadarajulu, S. Endoscopic Ultrasound-Guided Drainage of Pancreatic Fluid Collections in Children. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 30–35. [Google Scholar] [CrossRef]
- Jazrawi, S.F.; Barth, B.A.; Sreenarasimhaiah, J. Efficacy of Endoscopic Ultrasound-Guided Drainage of Pancreatic Pseudocysts in a Pediatric Population. Dig. Dis. Sci. 2011, 56, 902–908. [Google Scholar] [CrossRef]
- Dalal, A.; Kamat, N.; Patil, G.; Daftary, R.; Maydeo, A. Usefulness of Endoscopic Ultrasound in Children with Pancreatobiliary and Gastrointestinal Symptoms. Endosc. Int. Open 2022, 10, E192. [Google Scholar] [CrossRef]
- Krishna, R.P.; Lal, R.; Sikora, S.S.; Yachha, S.K.; Pal, L. Unusual Causes of Extrahepatic Biliary Obstruction in Children: A Case Series with Review of Literature. Pediatr. Surg. Int. 2008, 24, 183–190. [Google Scholar] [CrossRef]
- Taj, M.A.; Leghari, A.; Qureshi, S.; Ghazanfar, S.; Niaz, S.K.; Quraishy, M.S. Endoscopic Retrograde Cholangiopancreatography: A Therapeutic Modality in Children and Adolescents. J. Pak. Med. Assoc. 2012, 62, 98–101. [Google Scholar]
- Felux, J.; Sturm, E.; Busch, A.; Zerabruck, E.; Graepler, F.; Stüker, D.; Manger, A.; Kirschner, H.-J.; Blumenstock, G.; Malek, N.P.; et al. ERCP in Infants, Children and Adolescents Is Feasible and Safe: Results from a Tertiary Care Center. United Eur. Gastroenterol. J. 2017, 5, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.D.R.; Nawarathne, N.M.M.; Samarawickrama, V.T.; Deraniyagala, M.P.; Luxman, W.G.E.; Fernandopulle, A.N.R. Endoscopic Retrograde Cholangiopancreatography in Children: Feasibility, Success, and Safety with Standard Adult Endoscopes and Accessories. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 406. [Google Scholar] [CrossRef] [PubMed]
- Vepakomma, D. Pediatric Pancreatitis: Outcomes and Current Understanding. J. Indian Assoc. Pediatr. Surg. 2020, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Fogel, E.L.; Sherman, S.; McHenry, L.; Watkins, J.L.; Croffie, J.M.; Gupta, S.K.; Fitzgerald, J.F.; Lazzell-Pannell, L.; Schmidt, S.; et al. Diagnostic and Therapeutic Endoscopic Retrograde Cholangiopancreatography in Children: A Large Series Report. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Vitale, D.S.; Abu-El-Haija, M.; Anton, C.G.; Crotty, E.; Li, Y.; Zhang, B.; Trout, A.T. Magnetic Resonance Cholangiopancreatography vs Endoscopy Retrograde Cholangiopancreatography for Detection of Anatomic Variants of the Pancreatic Duct in Children. J. Pediatr. 2022, 244, 120–124. [Google Scholar] [CrossRef]
- Weng, M.; Wang, L.; Weng, H.; Gu, J.; Wang, X. Utility of Endoscopic Retrograde Cholangiopancreatography in Infant Patients with Conservational Endoscopy. Transl. Pediatr. 2021, 10, 2506. [Google Scholar] [CrossRef]
- Barakat, M.T.; Husain, S.Z.; Gugig, R. Safety and Efficacy of Minor Papillotomy in Children and Adolescents with Pancreas Divisum. Pancreatology 2023, 23, 171–175. [Google Scholar] [CrossRef]
- Li, Z.S.; Wang, W.; Liao, Z.; Zou, D.W.; Jin, Z.D.; Chen, J.; Wu, R.P.; Liu, F.; Wang, L.W.; Shi, X.G.; et al. A Long-Term Follow-up Study on Endoscopic Management of Children and Adolescents with Chronic Pancreatitis. Am. J. Gastroenterol. 2010, 105, 1884–1892. [Google Scholar] [CrossRef]
- Ford, K.; Paul, A.; Harrison, P.; Davenport, M. Surgical Success in Chronic Pancreatitis: Sequential Endoscopic Retrograde Cholangiopancreatography and Surgical Longitudinal Pancreatojejunostomy (Puestow Procedure). Eur. J. Pediatr. Surg. 2016, 26, 232–239. [Google Scholar] [CrossRef]
- Prommer, R.; Kienbauer, M.; Kargl, S.; Schöfl, R. Hereditary Pancreatitis in Childhood: Course of Disease and Complications. Wien. Klin. Wochenschr. 2021, 133, 669–673. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Novikov, A.; Weaver, K.; Marfatia, P.; Buscaglia, J.M.; DiMaio, C.J.; Diehl, D.; Gabr, M.M.; Gaidhane, M.; Siddiqui, A.; et al. Fully Covered Self-Expanding Metal Stents for Refractory Pancreatic Duct Strictures in Symptomatic Chronic Pancreatitis, US Experience. Endosc. Int. Open 2019, 7, E1419–E1423. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.T.; Keane, M.G.; Grammatikopoulos, T.; Reffitt, D.; Davenport, M.; Devlin, J.; Harrison, P.; Joshi, D. Endoscopic management of paediatric chronic pancreatitis: Retrospective series from the KCH Liver Institute. Gastrointest. Endosc. 2022, 95, AB428–AB429. [Google Scholar] [CrossRef]
- Agarwal, J.; Reddy, D.N.; Talukdar, R.; Lakhtakia, S.; Ramchandani, M.; Tandan, M.; Gupta, R.; Pratap, N.; Rao, G.V. ERCP in the management of pancreatic diseases in children. Gastrointest. Endosc. 2014, 79, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.K.J. Caudal Pancreatico-Jejunostomy for Chronic Relapsing Pancreatitis. Ann. Surg. 1954, 140, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Puestow, C.B.; Gillesby, W.J. Retrograde Surgical Drainage of Pancreas for Chronic Relapsing Pancreatitis. AMA Arch. Surg. 1958, 76, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Partington, P.F.; Rochelle, R.E. Modified Puestow Procedure for Retrograde Drainage of the Pancreatic Duct. Ann. Surg. 1960, 152, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Frey, C.F.; Smith, G.J. Description and Rationale of a New Operation for Chronic Pancreatitis. Pancreas 1987, 2, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Rollins, M.D.; Meyers, R.L. Frey Procedure for Surgical Management of Chronic Pancreatitis in Children. J. Pediatr. Surg. 2004, 39, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.S.; Pelayo, J.C.; Cortes, R.A.; Grethel, E.J.; Wagner, A.J.; Lee, H.; Harrison, M.R.; Farmer, D.L.; Nobuhara, K.K. Surgical Treatment of Childhood Recurrent Pancreatitis. J. Pediatr. Surg. 2007, 42, 1203–1207. [Google Scholar] [CrossRef]
- Ray, S.; Sanyal, S.; Ghatak, S.; Khamrui, S.; Biswas, J.; Saha, S.; Mandal, T.S.; Chattopadhyay, G. Frey Procedure for Chronic Pancreatitis in Children: A Single Center Experience. J. Pediatr. Surg. 2015, 50, 1850–1853. [Google Scholar] [CrossRef]
- Ray, S.; Ansari, Z.; Kumar, D.; Jana, K.; Khamrui, S. Short- and Long-Term Outcome of Surgery for Chronic Pancreatitis in Children: A Single Surgeon Experience. Pediatr. Surg. Int. 2020, 36, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.; Lopoo, J.; Superina, R.A. Longitudinal Pancreaticojejunostomy and Selective Biliary Diversion for Chronic Pancreatitis in Children. J. Pediatr. Surg. 2006, 41, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Kargl, S.; Kienbauer, M.; Duba, H.C.; Schöfl, R.; Pumberger, W. Therapeutic Step-up Strategy for Management of Hereditary Pancreatitis in Children. J. Pediatr. Surg. 2015, 50, 511–514. [Google Scholar] [CrossRef]
- Laje, P.; Adzick, N.S. Modified Puestow Procedure for the Management of Chronic Pancreatitis in Children. J. Pediatr. Surg. 2013, 48, 2271–2275. [Google Scholar] [CrossRef] [PubMed]
- Neblett, W.W.; O’Neill, J.A. Surgical Management of Recurrent Pancreatitis in Children With Pancreas Divisum. Ann. Surg. 2000, 231, 899. [Google Scholar] [CrossRef]
- Jalleh, R.P.; Williamson, R.C. Pancreatic Exocrine and Endocrine Function after Operations for Chronic Pancreatitis. Ann. Surg. 1992, 216, 656–662. [Google Scholar] [CrossRef]
- Warshaw, A.L.; Popp, J.W.J.; Schapiro, R.H. Long-Term Patency, Pancreatic Function, and Pain Relief after Lateral Pancreaticojejunostomy for Chronic Pancreatitis. Gastroenterology 1980, 79, 289–293. [Google Scholar] [CrossRef]
- Hutchins, R.R.; Hart, R.S.; Pacifico, M.; Bradley, N.J.; Williamson, R.C.N. Long-Term Results of Distal Pancreatectomy for Chronic Pancreatitis in 90 Patients. Ann. Surg. 2002, 236, 612–618. [Google Scholar] [CrossRef]
- Jones, R.E.; Zagory, J.A.; Tatum, M.; Tsui, W.S.; Murphy, J. A Retrospective Analysis of Pancreas Operations in Children. Transl. Gastroenterol. Hepatol. 2021, 6, 39. [Google Scholar] [CrossRef]
- Zhang, J.S.; Li, L.; Liu, S.L.; Hou, W.Y.; Diao, M.; Zhang, J.; Li, S.L.; Ming, A.X.; Liu, Y.; Wang, H.B.; et al. Laparoscopic Pancreaticojejunostomy for Pancreatic Ductal Dilatation in Children. J. Pediatr. Surg. 2012, 47, 2349–2352. [Google Scholar] [CrossRef]
- Arora, A.; Agarwal, P.; Bagdi, R.; Ramasundaram, M.; Sankar Narayanan, M. Laparoscopic Puestow Procedure for Chronic Pancreatitis in Children. J. Indian Assoc. Pediatr. Surg. 2020, 25, 55. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Petrosyan, M.; Kane, T.D. Lateral Pancreaticojejunostomy for Chronic Pancreatitis and Pancreatic Ductal Dilation in Children. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 1397–1402. [Google Scholar] [CrossRef]
- Balduzzi, A.; Zwart, M.J.W.; Kempeneers, R.M.A.; Boermeester, M.A.; Busch, O.R.; Besselink, M.G. Robotic Lateral Pancreaticojejunostomy for Chronic Pancreatitis. J. Vis. Exp. 2019, 154, e60301. [Google Scholar]
- Kirks, R.C.; Lorimer, P.D.; Fruscione, M.; Cochran, A.; Baker, E.H.; Iannitti, D.A.; Vrochides, D.; Martinie, J.B. Robotic Longitudinal Pancreaticojejunostomy for Chronic Pancreatitis: Comparison of Clinical Outcomes and Cost to the Open Approach. Int. J. Med. Robot. 2017, 13, e1832. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Furbetta, N.; Gianardi, D.; Guadagni, S.; Di Franco, G.; Bianchini, M.; Palmeri, M.; Masoni, C.; Di Candio, G.; Cuschieri, A. Use of Barbed Suture without Fashioning the ‘Classical’ Wirsung-Jejunostomy in a Modified End-to-Side Robotic Pancreatojejunostomy. Surg. Endosc. 2021, 35, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.S.; Rattner, D.W.; Warshaw, A.L. Failure of Symptomatic Relief After Pancreaticojejunal Decompression for Chronic Pancreatitis: Strategies for Salvage. Arch. Surg. 1994, 129, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Chromik, A.M.; Seelig, M.H.; Saewe, B.; Müller, C.A.; Weyhe, D.; Felderbauer, P.; Mittelkötter, U.; Tannapfel, A.; Schmidt-Choudhury, A.; Uhl, W. Tailored Resective Pancreatic Surgery for Pediatric Patients with Chronic Pancreatitis. J. Pediatr. Surg. 2008, 43, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Snajdauf, J.; Rygl, M.; Petru, O.; Nahlovsky, J.; Frybova, B.; Durilova, M.; Mixa, V.; Keil, R.; Kyncl, M.; Kodet, R.; et al. Indications and Outcomes of Duodenum-Preserving Resection of the Pancreatic Head in Children. Pediatr. Surg. Int. 2019, 35, 449–455. [Google Scholar] [CrossRef]
- Bellin, M.D.; Carlson, A.M.; Kobayashi, T.; Gruessner, A.C.; Hering, B.J.; Moran, A.; Sutherland, D.E.R. Outcome after Pancreatectomy and Islet Autotransplantation in a Pediatric Population. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 37–44. [Google Scholar] [CrossRef]
- Bellin, M.D.; Freeman, M.L.; Schwarzenberg, S.J.; Dunn, T.B.; Beilman, G.J.; Vickers, S.M.; Chinnakotla, S.; Balamurugan, A.N.; Hering, B.J.; Radosevich, D.M.; et al. Quality of Life Improves for Pediatric Patients After Total Pancreatectomy and Islet Autotransplant for Chronic Pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 793–799. [Google Scholar] [CrossRef]
- Wilson, G.C.; Sutton, J.M.; Salehi, M.; Schmulewitz, N.; Smith, M.T.; Kucera, S.; Choe, K.A.; Brunner, J.E.; Abbott, D.E.; Sussman, J.J.; et al. Surgical Outcomes after Total Pancreatectomy and Islet Cell Autotransplantation in Pediatric Patients. Surgery 2013, 154, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Forlenza, G.P.; Majumder, K.; Berger, M.; Freeman, M.L.; Beilman, G.J.; Dunn, T.B.; Pruett, T.L.; Murati, M.; Wilhelm, J.J.; et al. Total Pancreatectomy with Islet Autotransplantation Resolves Pain in Young Children with Severe Chronic Pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Yachha, S.K.; Chetri, K.; Saraswat, V.A.; Baijal, S.S.; Sikora, S.S.; Lal, R.; Srivastava, A. Management of Childhood Pancreatic Disorders: A Multidisciplinary Approach. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 206–212. [Google Scholar] [PubMed]
- Wilson, G.C.; Sutton, J.M.; Salehi, M.; Schmulewitz, N.; Smith, M.T.; Kucera, S.; Choe, K.A.; Brunner, J.E.; Abbott, D.E.; Sussman, J.J.; et al. Patient and disease characteristics associated with the presence of diabetes mellitus in adults with chronic pancreatitis in the United States. Am. J. Gastroenterol. 2017, 112, 1457–1465. [Google Scholar]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic Pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Hao, L.; Zeng, X.P.; Xin, L.; Wang, D.; Pan, J.; Bi, Y.W.; Ji, J.-T.; Du, T.-T.; Lin, J.-H.; Zhang, D.; et al. Incidence of and risk factors for pancreatic cancer in chronic pancreatitis: A cohort of 1656 patients. Dig. Liver Dis. 2017, 49, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, Y.; Tan, C.; Ke, N.; Du, B.; Liu, X. Risk of pancreatic cancer in patients undergoing surgery for chronic pancreatitis. BMC Surg. 2019, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated Recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]


| Gene | Mechanism | Geography | References |
|---|---|---|---|
| PRSS1 and PRSS2 Encoding Protein Serine type 1 and type 2 | Production of an unstable cationic trypsinogen that exhibits reduced activity, autolysis, and autoactivation | N. America Germany Australia | Whitcomb et al. (1996) [6] Gorry et al. (1997) [7] Kumar et al. (2016) [1] Wu et al. (2022) [8] |
| SPINK1 (p.N34S) Encoding Serine Protease Inhibitor Kazal type 1 | Mutations reduce the inhibition of unregulated and prematurely activated trypsin, causing cellular damage within the parenchyma | China, Korea—IVS 3 + 2 T > C Europe—p.N34S N. America—p.N34S | Witt et al. 2000 [9] Chen et al. 2000 [10] Kumar et al. (2016) [1] Liu et al. (2017) [11] |
| CFTR Encoding Cystic Fibrosis Transmembrane Conductance Regulator | ↓ ductal fluid and ↓ bicarbonate secretion, leading to ↓ intraluminal pH, ↓ washout of the digestive enzymes, and more viscous protein-rich ductal fluid | Europe N. America China/Taiwan | Sharer et al. (1998) [12] Cohn et al. (1998) [13] Chang et al. (2007) [14] |
| CPA1 Encoding Carboxypeptidase A1 | Misfolding CPA1 phenotype protein resulting in pancreatic endoplasmic reticulum (ER) stress | Germany | Witt et al. (2013) [15] |
| CTRC Encoding Chymotrypsin C | Impaired trypsin degradation | Europe China Asia | Rosendahl et al. (2008) [16] Masson et al. (2008) [17] Wang et al. (2013) [18] Koziel 2015 [5] |
| CLDN2 Encoding Claudin 2 | Atypical localization of CLDN2 protein leading to alterations in pancreatic ductal fluid composition and/or imbalance in calcium homeostasis | Europe N. America | Whitcomb et al. (2015) [19] |
| CELA3B Encoding chymotrypsin like elastase 3B | Uncontrolled proteolysis of trypsin due to upregulation of CELA3B | N. America | Moore et al. (2019) [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeropoulos, R.M.; Joshi, D.; Aldeiri, B.; Davenport, M. Surgical and Endoscopic Intervention for Chronic Pancreatitis in Children: The Kings College Hospital Experience. Children 2024, 11, 74. https://doi.org/10.3390/children11010074
Jeropoulos RM, Joshi D, Aldeiri B, Davenport M. Surgical and Endoscopic Intervention for Chronic Pancreatitis in Children: The Kings College Hospital Experience. Children. 2024; 11(1):74. https://doi.org/10.3390/children11010074
Chicago/Turabian StyleJeropoulos, Renos M., Deepak Joshi, Bashar Aldeiri, and Mark Davenport. 2024. "Surgical and Endoscopic Intervention for Chronic Pancreatitis in Children: The Kings College Hospital Experience" Children 11, no. 1: 74. https://doi.org/10.3390/children11010074






