Retinopathy of Prematurity in Eight Portuguese Neonatal Intensive Care Units: Incidence, Risk Factors, and Progression—A Prospective Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Population
2.3. Data Collection
- Pathologies, such as early sepsis (onset in the first 72 h of life), late sepsis (onset after the first 72 h of life), moderate and severe bronchopulmonary dysplasia [21], peri-intraventricular hemorrhage grade ≥ 2 [22], cystic periventricular leukomalacia [22], hemodynamically significant patent ductus arteriosus [23], necrotizing enterocolitis, and phototherapy.
- Treatments, including days of mechanical ventilation (invasive and non-invasive) and maximum fraction of inspired oxygen (FiO2), surfactant use, erythropoietin or darbepoetin, systemic and inhaled corticosteroid, non-steroidal anti-inflammatory, diuretic, red blood cell (RBC), platelets, and plasma transfusions received.
- Metabolic acidosis (considered if pH < 7.2 and bicarbonate < 16 mmol/L) measured on the first day of life at two time points (within the first 2 h and between 2 and 24 h).
- Body weight throughout the first month of life.
- Number of days with hyperglycemia (blood glucose > 125 mg/dL, measured every 8 h using a point-of-care glucometer) in the first three weeks of life.
- Nutritional data, including the day of the start of trophic, nutritious and total enteral nutrition, as well as breast milk feeding.
- Length of hospital stay (in days).
- Biochemical parameters during the first week of life: serum urea (mg/dL), creatinine (mg/dL), and total and direct bilirubin (mg/dL).
2.4. ROP Screening and Ophthalmological Data Collection
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinical Information
3.2. Factors That Were Influencing ROP Development and Progression
3.3. Characteristics of ROP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellström, A.; Smith, L.E.H.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Skrehot, H.C.; Bhatt, A.; Herce, H.; Weng, C.Y. Epidemiology of Retinopathy of Prematurity in the US From 2003 to 2019. JAMA Ophthalmol. 2023, 141, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.H.; Gu, D.F.; Dai, Y.; Chen, Y.H.; Yang, Z.M.; Lu, L.J. The relationship between probiotics and retinopathy of prematurity in preterm infants: A population-based retrospective study in China. Front. Pediatr. 2023, 11, 1055992. [Google Scholar] [CrossRef] [PubMed]
- Banjac, L.; Banjac, G.; Kotur-Stevuljević, J.; Spasojević-Kalimanovska, V.; Gojković, T.; Bogavac-Stanojević, N.; Jelić-Ivanović, Z.; Banjac, G. Pro-oxidants and antioxidants in retinopathy of prematurity. Acta Clin. Croat. 2018, 57, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth Hartnett, M. Discovering Mechanisms in the Changing and Diverse Pathology of Retinopathy of Prematurity: The Weisenfeld Award Lecture. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Advances in understanding and management of retinopathy of prematurity. Surv. Ophthalmol. 2017, 62, 257–276. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Morrison, M.A.; Smith, S.; Yanovitch, T.L.; Young, T.L.; Colaizy, T.; Momany, A.; Dagle, J.; Carlo, W.A.; Clark, E.A.S.; et al. Genetic variants associated with severe retinopathy of prematurity in extremely low birth weight infants. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6194–6203. [Google Scholar] [CrossRef]
- Bizzarro, M.J.; Hussain, N.; Jonsson, B.; Feng, R.; Ment, L.R.; Gruen, J.R.; Zhang, H.; Bhandari, V. Genetic susceptibility to retinopathy of prematurity. Pediatrics 2006, 118, 1858–1863. [Google Scholar] [CrossRef]
- Fevereiro-Martins, M.; Santos, A.C.; Marques-Neves, C.; Guimarães, H.; Bicho, M.; On behalf of the GenE-Rop Study Group. Genetic Modulation of the Erythrocyte Phenotype Associated with Retinopathy of Prematurity—A Multicenter Portuguese Cohort Study. Int. J. Mol. Sci. 2023, 24, 11817. [Google Scholar] [CrossRef]
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch. Ophthalmol. 1984, 102, 1130–1134. [Google Scholar] [CrossRef]
- Quinn, G.E. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Paul Chan, R.V.; Berrocal, A.; Binenbaum, G.; Blair, M.; Peter Campbell, J.; Capone, A.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef] [PubMed]
- Chan-Ling, T.; Gole, G.A.; Quinn, G.E.; Adamson, S.J.; Darlow, B.A. Pathophysiology, screening and treatment of ROP: A multi-disciplinary perspective. Prog. Retin. Eye Res. 2018, 62, 77–119. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Pathophysiology of Retinopathy of Prematurity. Annu. Rev. Vis. Sci. 2023, 9, 39–70. [Google Scholar] [CrossRef] [PubMed]
- Liegl, R.; Hellström, A.; Smith, L.E.H. Retinopathy of prematurity: The need for prevention. Eye Brain 2016, 8, 91–102. [Google Scholar] [CrossRef]
- Ryu, J. New Aspects on the Treatment of Retinopathy of Prematurity: Currently Available Therapies and Emerging Novel Therapeutics. Int. J. Mol. Sci. 2022, 23, 8529. [Google Scholar] [CrossRef]
- Mataftsi, A.; Dimitrakos, S.A.; Adams, G.G.W. Mediators involved in retinopathy of prematurity and emerging therapeutic targets. Early Hum. Dev. 2011, 87, 683–690. [Google Scholar] [CrossRef]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflammation 2017, 14, 165. [Google Scholar] [CrossRef]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef]
- Fevereiro-Martins, M.; Santos, A.C.; Marques-Neves, C.; Guimarães, H.; Bicho, M.; Afonso, C.; Ferreira, J.; Santo, R.E.; Teixeira, F.; Rosa, R.; et al. Complete blood count parameters as biomarkers of retinopathy of prematurity: A Portuguese multicenter study. Graefe Arch. Clin. Exp. Ophthalmol. 2023, 261, 2997–3006. [Google Scholar] [CrossRef]
- Proença, E.; Vasconcellos, G.; Rocha, G.; Carreira, M.L.; Mateus, M.; Santos, I.D.; Sossai, M.D.R.; Morais, B.; Guimarães, I.M.H. Displasia broncopulmonar. Soc. Port. Neonatol. 2009. Available online: https://spp.pt/UserFiles/file/Protocolos/Displasia_Boncopulmonar_RN_2009.pdf (accessed on 12 January 2024).
- Taborda, A.; Pereira, A.; Graça, A.; Conceição, C.; Faria, C.; Trindade, C.; Chaves, F.; Pinto, F.; Corrêa, F.; Costa, J.; et al. Revisão do Consenso de Neuro-imagiologia Neonatal—versão maio 2013. Soc. Port. Neonatol. 2013. Available online: https://www.spneonatologia.pt/wp-content/uploads/2016/11/2010-Neuroimagiologia.pdf (accessed on 12 January 2024).
- Salazar, A.; Guedes, A.; Álvares, S.; Soares, P.; Morais, S.; Pires, A.; Tiago, J.; Andrade, H.; Fernandes, E.; Sampaio, M.A.; et al. Consenso nacional abordagem diagnóstica e terapêutica da persistência do canal arterial no recém-nascido pré-termo. Soc. Port. Neonatol. 2010. Available online: https://www.spp.pt/userfiles/file/protocolos/persistencia_canal_arterial_rn_pre_termo_2010.pdf (accessed on 11 January 2024).
- Good, W.V.; Hardy, R.J.; Dobson, V.; Palmer, E.A.; Phelps, D.L.; Quintos, M.; Tung, B. Revised Indications for the Treatment of Retinopathy of Prematurity: Results of the Early Treatment for Retinopathy of Prematurity Randomized Trial. Arch. Ophthalmol. 2003, 121, 1684–1696. [Google Scholar] [CrossRef]
- Leng, Y.; Huang, W.; Ren, G.; Cai, C.; Tan, Q.; Liang, Y.; Yang, W.; Gao, Z. The treatment and risk factors of retinopathy of prematurity in neonatal intensive care units. BMC Ophthalmol. 2018, 18, 301. [Google Scholar] [CrossRef]
- García, H.; Villasis-Keever, M.A.; Zavala-Vargas, G.; Bravo-Ortiz, J.C.; Pérez-Méndez, A.; Escamilla-Núñez, A. Global Prevalence and Severity of Retinopathy of Prematurity over the Last Four Decades (1985–2021): A Systematic Review and Meta-Analysis. Arch. Med. Res. 2024, 55, 102967. [Google Scholar] [CrossRef]
- Almeida, A.C.; Silva, G.A.; Santini, G.; Brízido, M.; Correia, M.; Coelho, C.; Borrego, L.M. Correlation between hyperglycemia and glycated albumin with retinopathy of prematurity. Sci. Rep. 2021, 11, 22321. [Google Scholar] [CrossRef]
- Almeida, R.L.; Costa, R.M.; Bennett, M.; Alfaiate, M.; Basto, L. Retinopathy of prematurity: Eight-year outcomes of a Portuguese neonatal intensive care unit. Nascer Crescer Birth Growth Med. J. 2023, 32, 82–88. [Google Scholar] [CrossRef]
- Figueiredo, R.; Sarmento, T.M.; Garrido, J.; Marques, M.I.N.; Almeida, T.; Carrasquinho, S. Applicability of the ROPScore as a predictive algorithm for early detection of retinopathy of prematurity. Rev. Soc. Port. Oftalmol. 2020, 44. [Google Scholar] [CrossRef]
- Malheiro, L.; Falcão, I.; Neiva, L.; Almeida, A.; Maia, S.; Miranda, V.; Parreira, R.; Menéres, P. Application of the WINROP model in Retinopathy of Prematurity (ROP) screening in a Portuguese cohort of premature infants. Rev. Bras. Oftalmol. 2019, 78, 30–36. [Google Scholar] [CrossRef]
- Yucel, O.E.; Eraydin, B.; Niyaz, L.; Terzi, O. Incidence and risk factors for retinopathy of prematurity in premature, extremely low birth weight and extremely low gestational age infants. BMC Ophthalmol. 2022, 22, 367. [Google Scholar] [CrossRef] [PubMed]
- Hellström, W.; Martinsson, T.; Morsing, E.; Gränse, L.; Ley, D.; Hellström, A. Low fraction of fetal haemoglobin is associated with retinopathy of prematurity in the very preterm infant. Br. J. Ophthalmol. 2022, 106, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS ONE 2019, 14, e0219934. [Google Scholar] [CrossRef] [PubMed]
- Teofili, L.; Papacci, P.; Bartolo, M.; Molisso, A.; Orlando, N.; Pane, L.; Giannantonio, C.; Serrao, F.; Bianchi, M.; Valentini, C.G.; et al. Transfusion-Free Survival Predicts Severe Retinopathy in Preterm Neonates. Front. Pediatr. 2022, 10, 814194. [Google Scholar] [CrossRef]
- Podraza, W. A new approach to neonatal medical management that could transform the prevention of retinopathy of prematurity: Theoretical considerations. Med. Hypotheses 2020, 137, 109541. [Google Scholar] [CrossRef]
- García González, E.; Casanova, M.A.; Samarkanova, D.; Aldecoa-Bilbao, V.; Teresa-Palacio, M.; Farssac Busquets, E.; Figueras-Aloy, J.; Salvia-Roigés, M.; Querol, S. Feasibility of umbilical cord blood as a source of red blood cell transfusion in preterm infants. Blood Transfus. 2021, 19, 510–517. [Google Scholar] [CrossRef]
- Teofili, L.; Papacci, P.; Orlando, N.; Bianchi, M.; Pasciuto, T.; Mozzetta, I.; Palluzzi, F.; Giacò, L.; Giannantonio, C.; Remaschi, G.; et al. BORN study: A multicenter randomized trial investigating cord blood red blood cell transfusions to reduce the severity of retinopathy of prematurity in extremely low gestational age neonates. Trials 2022, 23, 1010. [Google Scholar] [CrossRef]
- Ribeiro, C.; Rocha, G.; Flôr-de-Lima, F.; Ferreras, C.; Moita, R.; Silva, R.; Azevedo, I. Thrombocytopenia requiring platelet transfusions is predictive of retinopathy of prematurity in preterm infants. Minerva Pediatr. 2023. [Google Scholar] [CrossRef]
- Asada, N. Tubular immaturity causes erythropoietin-deficiency anemia of prematurity in preterm neonates. Sci. Rep. 2018, 8, 4448. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Estienne, M.; Bessoles, S.; Echchakir, H.; Pederzoli-Ribeil, M.; Chiron, A.; Aldaz-Carroll, L.; Leducq, V.; Zhang, Y.; Souyri, M.; et al. Erythropoietin is a major regulator of thrombopoiesis in thrombopoietin-dependent and -independent contexts. Exp. Hematol. 2020, 88, 15–27. [Google Scholar] [CrossRef]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2020, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Nilsson, A.K.; Wackernagel, D.; Pivodic, A.; Vanpee, M.; Sjöbom, U.; Hellgren, G.; Hallberg, B.; Domellöf, M.; Klevebro, S.; et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.; Mohamed, G.; Othman, M. Retinopathy of prematurity: A study of prevalence and risk factors. Middle East Afr. J. Ophthalmol. 2012, 19, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Fortes Filho, J.B.; Valiatti, F.B.; Eckert, G.U.; Da Costa, M.C.; Silveira, R.C.; Procianoy, R.S. Is being small for gestational age a risk factor for retinopathy of prematurity? A study with 345 very low birth weight preterm infants. J. Pediatr. 2009, 85, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.H.; Ells, A.L.; Blair, M.P.; Shah, P.K.; Harper, C.A.; Martinez-Castellanos, M.A.; Prakalapakorn, S.G.; Denis, E.; Lusobya, R.C.; Greenwald, M.J.; et al. Retinopathy of Prematurity in the 21st Century and the Complex Impact of Supplemental Oxygen. J. Clin. Med. 2023, 12, 1228. [Google Scholar] [CrossRef]
- Mitsiakos, G.; Papageorgiou, A. Incidence and factors predisposing to retinopathy of prematurity in inborn infants less than 32 weeks of gestation. Hippokratia 2016, 20, 121–126. [Google Scholar]
- Lin, Y.W.; Chen, S.N.; Muo, C.H.; Sung, F.C.; Lin, M.H. Risk of Retinopathy of Prematurity in Preterm Births with Respiratory Distress Syndrome: A Population-Based Cohort Study in Taiwan. Int. J. Gen. Med. 2022, 15, 2149–2162. [Google Scholar] [CrossRef]
- de Pediatria, S.P.; Direção-Geral da Saúde. Prescrição de Surfactante Pulmonar na Síndrome de Dificuldade Respiratória do Recém-nascido. Available online: https://www.spp.pt/userfiles/file/evidencias em pediatria/dgs_012_2012_actualizada 08.2014.pdf (accessed on 25 January 2024).
- Dammann, O.; Stansfield, B.K. Neonatal sepsis as a cause of retinopathy of prematurity: An etiological explanation. Prog. Retin. Eye Res. 2024, 98, 101230. [Google Scholar] [CrossRef]
- Wang, X.; Tang, K.; Chen, L.; Cheng, S.; Xu, H. Association between sepsis and retinopathy of prematurity: A systematic review and meta-analysis. BMJ Open 2019, 9, e025440. [Google Scholar] [CrossRef]
- Bonafiglia, E.; Gusson, E.; Longo, R.; Ficial, B.; Tisato, M.G.; Rossignoli, S.; Caltran, G.; Pedrotti, E.; Beghini, R.; Marchini, G. Early and late onset sepsis and retinopathy of prematurity in a cohort of preterm infants. Sci. Rep. 2022, 12, 11675. [Google Scholar] [CrossRef]
- Bas, A.Y.; Demirel, N.; Koc, E.; Ulubas Isik, D.; Murat Hirfanoglu, İ.; Tunc, T. Clinical science Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): A prospective, multicentre study in 69 neonatal intensive care units. Br. J. Ophthalmol. 2018, 102, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.K.; Lee, H.J.; Ko, J.H.; Park, J.H.; Park, J.Y.; Choi, C.W.; Yoon, C.H.; Ahn, S.J.; Park, K.H.; Woo, S.J.; et al. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J. Neuroinflamm. 2014, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shen, W.; Wu, F.; Mao, J.; Liu, L.; Chang, Y.M.; Zhang, R.; Ye, X.Z.; Qiu, Y.P.; Ma, L.; et al. Effect of early initiation of enteral nutrition on short-term clinical outcomes of very premature infants: A national multicenter cohort study in China. Nutrition 2023, 107, 111912. [Google Scholar] [CrossRef] [PubMed]
- Bremner, A.; Yen Chan, L.; Jones, C.; Shah, S.P. Comparison of Weight-Gain-Based Prediction Models for Retinopathy of Prematurity in an Australian Population. J. Ophthalmol. 2023, 2023, 8406287. [Google Scholar] [CrossRef] [PubMed]
- Jensez, A.K.; Ying, G.S.; Huang, J.; Quinn, G.E.; Binenbaum, G. Postnatal Serum Insulin-like Growth Factor I and Retinopathy of Prematurity. Retina 2017, 37, 867–872. [Google Scholar] [CrossRef]
- Hellström, A.; Hård, A.L. Screening and novel therapies for retinopathy of prematurity—A review. Early Hum. Dev. 2019, 138, 104846. [Google Scholar] [CrossRef]
- Langford, K.; Nicoiaides, K.; Miell, J.P. Maternal and fetal insulin-like growth factors and their binding proteins in the second and third trimesters of human pregnancy. Hum. Reprod. 1998, 13, 1389–1393. [Google Scholar] [CrossRef]
- Pivodic, A.; Hård, A.L.; Löfqvist, C.; Smith, L.E.H.; Wu, C.; Bründer, M.C.; Lagrèze, W.A.; Stahl, A.; Holmström, G.; Albertsson-Wikland, K.; et al. Individual Risk Prediction for Sight-Threatening Retinopathy of Prematurity Using Birth Characteristics. JAMA Ophthalmol. 2020, 138, 21–29. [Google Scholar] [CrossRef]
- Löfqvist, C.; Hansen-Pupp, I.; Andersson, E.; Holm, K.; Smith, L.E.H.; Ley, D.; Hellström, A. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch. Ophthalmol. 2009, 127, 622–627. [Google Scholar] [CrossRef]
- Choi, J.H.; Löfqvist, C.; Hellström, A.; Heo, H. Efficacy of the Screening Algorithm WINROP in a Korean Population of Preterm Infants. JAMA Ophthalmol. 2013, 131, 62–66. [Google Scholar] [CrossRef]
- Chaves-Samaniego, M.J.; Gómez Cabrera, C.; Chaves-Samaniego, M.C.; Escudero Gómez, J.; García Campos, J.M.; Muñoz Hoyos, A.; García Serrano, J.L. Multicenter validation study of the WINROP algorithm as a method for detecting retinopathy of prematurity. J. Matern. Neonatal Med. 2020, 33, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Braimah, I.Z.; Enweronu-Laryea, C.; Sackey, A.H.; Kenu, E.; Agyabeng, K.; Ofori-Adjei, I.O.D.B.; Beyuo, V.; Oku, A.; Essuman, V.A. Incidence and risk factors of retinopathy of prematurity in Korle-Bu Teaching Hospital: A baseline prospective study. BMJ Open 2020, 10, e035341. [Google Scholar] [CrossRef] [PubMed]
- Wikstrand, M.H.; Hrd, A.L.; Niklasson, A.; Smith, L.; Löfqvist, C.; Hellström, A. Maternal and neonatal factors associated with poor early weight gain and later retinopathy of prematurity. Acta Paediatr. Int. J. Paediatr. 2011, 100, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Jordan, B.K.; Scottoline, B.; Ostmo, S.R.; Coyner, A.S.; Singh, P.; Kalpathy-Cramer, J.; Erdogmus, D.; Chan, R.V.P.; Chiang, M.F.; et al. Oxygenation Fluctuations Associated with Severe Retinopathy of Prematurity Insights from a Multimodal Deep Learning Approach. Ophthalmol. Sci. 2024, 4, 100417. [Google Scholar] [CrossRef]
- Erdal, H.; Demirtas, M.S.; Kılıcbay, F.; Tunc, G. Evaluation of Oxidative Stress Levels and Dynamic Thiol-disulfide Balance in Patients with Retinopathy of Prematurity. Curr. Eye Res. 2023, 48, 1026–1033. [Google Scholar] [CrossRef]
- Stark, A.; Dammann, C.; Nielsen, H.C.; Volpe, M.A.V. A pathogenic relationship of bronchopulmonary dysplasia and retinopathy of prematurity? A review of angiogenic mediators in both diseases. Front. Pediatr. 2018, 6, 125. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharjee, I.; Heis, F.; Sears, J.E.; Aly, H. Blood urea nitrogen, a marker for severe retinopathy of prematurity? HHS Public Access. J. Perinatol. 2023, 43, 830–832. [Google Scholar] [CrossRef]
- Hinds, T.D.; Stec, D.E. Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension 2018, 72, 788–795. [Google Scholar] [CrossRef]
- Au, S.C.L.; Tang, S.M.; Rong, S.S.; Chen, L.J.; Yam, J.C.S. Association between hyperglycemia and retinopathy of prematurity: A systemic review and meta-analysis. Sci. Rep. 2015, 5, 9091. [Google Scholar] [CrossRef]
- Masoumi, Z.; Familari, M.; Källén, K.; Ranstam, J.; Olofsson, P.; Hansson, S.R. Fetal hemoglobin in umbilical cord blood in preeclamptic and normotensive pregnancies: A cross-sectional comparative study. PLoS ONE 2017, 12, e0176697. [Google Scholar] [CrossRef]
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 2017, 41, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Port, A.D.; Chan, R.V.P.; Ostmo, S.; Choi, D.; Chiang, M.F. Risk factors for retinopathy of prematurity: Insights from outlier infants. Graefe Arch. Clin. Exp. Ophthalmol. 2014, 252, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Cipriani, S.; Noli, S.; Franchi, M.; Corrao, G.; Parazzini, F.; Somigliana, E. The changing impact of assisted reproductive techniques on preterm birth during the period 2007–2020 in Lombardy, Northern Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 278, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Dabir, S.; Mohankumar, A.; Srivatsa, D.V.; Munusamy, S.; Berendschot, T.T.J.M.; Rajan, M.; Azad, R. Retinopathy of prematurity in preterm infants born following assisted conception versus spontaneously conceived pregnancies—A 2-year retrospective observational study from an urban tertiary eye care referral center in South India. Indian J. Ophthalmol. 2023, 71, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.T.; Levine, D.A.; Hutchinson, A.K.; Rao, P.; Hubbard, G.B. Clinical Features and Outcomes of Infants with Retinopathy of Prematurity Who Fail Anti-VEGF Therapy. Retina 2021, 41, 2269–2277. [Google Scholar] [CrossRef]
- Henriques, G.; Brito, C.; Teixeira, S. Consenso Clínico Retinopatia da Prematuridade. Soc. Port. Pediatr. 2014. Available online: https://www.spneonatologia.pt/wp-content/uploads/2016/11/2014-ROP.pdf (accessed on 2 January 2024).
- Binenbaum, G.; Bell, E.F.; Donohue, P.; Quinn, G.; Shaffer, J.; Tomlinson, L.A.; Ying, G.S.; Donoh, P.; Nguyen, A.; Duros, T.B.; et al. Development of Modified Screening Criteria for Retinopathy of Prematurity: Primary Results From the Postnatal Growth and Retinopathy of Prematurity Study. JAMA Ophthalmol. 2018, 136, 1034–1040. [Google Scholar] [CrossRef]
- Parmar, U.P.S.; Surico, P.; Singh, R.; Romano, F.; Salati, C.; Spadea, L.; Musa, M.; Gagliano, C.; Mori, T.; Zeppieri, M. Artificial Intelligence (AI) for Early Diagnosis of Retinal Diseases. Medicina 2024, 60, 527. [Google Scholar] [CrossRef]
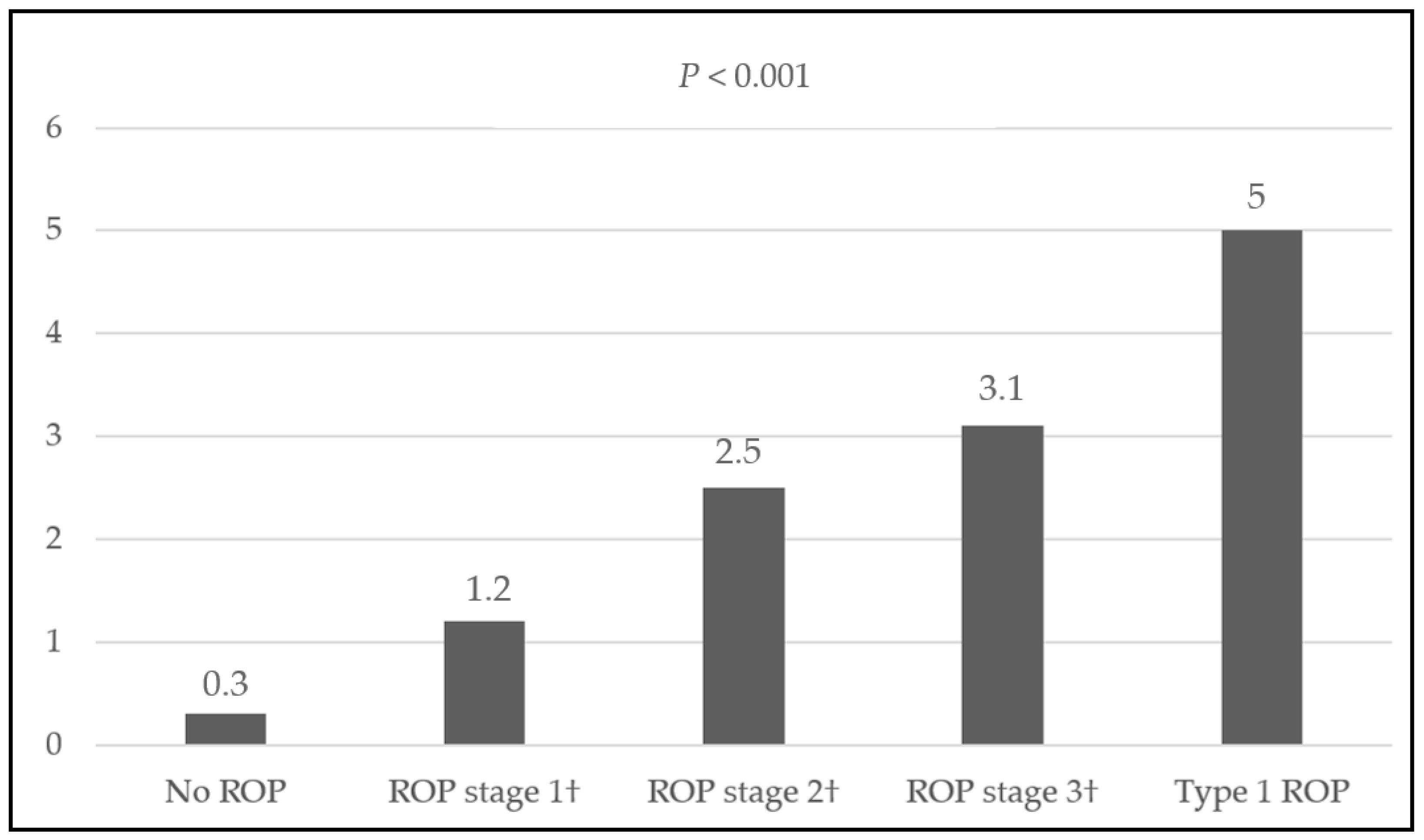

| (a) | (b) | |||||||
|---|---|---|---|---|---|---|---|---|
| Demographic and Clinical Characteristics | No ROP (n = 283) n (%) or Median (Q1–Q3) | ROP (n = 172) n (%) or Median (Q1–Q3) | P | P * | ROP Stages 1, 2, and 3 † (n = 151) n (%) or Median (Q1–Q3) | Type 1 ROP (n = 21) n (%) or Median (Q1–Q3) | P | P * |
| BIRTH | ||||||||
| Eutocic birth | 76 (27.0%) | 52 (30.2%) | 0.454 § | 0.562 | 49 (32.5%) | 3 (14.3%) | 0.127 § | 0.027 |
| Gestational age (weeks) | 30.4 (29.1–31.5) | 28.0 (26.4–29.3) | <0.001 # | NA | 28.3 (26.6–29.6) | 26.1 (25.1–27.2) | <0.001 # | 0.013 |
| ≤28 | 58 (20.5%) | 112 (65.1%) | <0.001 § | NA | 91 (60.3%) | 21 (100%) | 0.002 § | NA |
| 29–31 | 160 (56.5%) | 50 (29.1%) | 50 (33.1%) | 0 (0%) | ||||
| ≥32 | 65 (23.0%) | 10 (5.8%) | 10 (6.6%) | 0 (0%) | ||||
| Birth weight (g) | ||||||||
| ≤1000 | 54 (19.1%) | 95 (55.2%) | <0.001 § | 0.055 | 76 (50.3%) | 19 (90.5%) | 0.002 § | 0.798 |
| 1001–1499 | 171 (60.4%) | 71 (41.3%) | 69 (45.7%) | 2 (9.5%) | ||||
| ≥1500 | 58 (20.5%) | 6 (3.5%) | 6 (4.0%) | 0 (0%) | ||||
| SGA | 81 (29.2%) | 45 (26.9%) | 0.603 § | 0.027 | 39 (26.5%) | 6 (30.0%) | 0.790 § | 0.128 |
| Gender | ||||||||
| Female | 136 (48.1%) | 94 (54.7%) | 0.177 § | 0.185 | 84 (55.6%) | 10 (47.6%) | 0.495 § | 0.487 |
| Male | 147 (51.9%) | 78 (45.3%) | 67 (44.4%) | 11 (52.4%) | ||||
| Resuscitation with endotracheal intubation | 39 (13.9%) | 80 (46.8%) | 0.001 § | 0.099 | 69 (46.0%) | 11 (52.4%) | 0.645 § | 0.006 |
| Oxygen | 191 (68.5%) | 148 (86.5%) | < 0.001 § | 0.337 | 131 (87.3%) | 17 (81.0%) | 0.491 § | 0.120 |
| Maximum FiO2 (%) | 30.00 (27.25–40.00) | 42.50 (30.00–67.50) | <0.001 # | 0.028 | 42.50 (30.00–72.50) | 45.00 (30.00–52.50) | 0.382 # | 0.064 |
| Apgar score 5th min < 7 | 14 (4.9%) | 26 (15.2%) | <0.001 § | 0.155 | 21 (14.0%) | 5 (23.8%) | 0.325 § | 0.699 |
| POSTNATAL | ||||||||
| Metabolic acidosis (first 2 h of life) | 2 (0.9%) | 8 (5.7%) | 0.008 § | 0.165 | 7 (5.6%) | 1 (6.3%) | 1.000 § | 0.752 |
| Metabolic acidosis 1st day (between 2 and 24 h) | 1 (0.6%) | 3 (2.4%) | 0.324 § | 0.098 | 3 (2.8%) | 0 (0.0%) | 1.000 § | 0.999 |
| Co-morbidities | ||||||||
| Bronchopulmonary dysplasia moderate/ severe | 28 (10.0%) | 64 (37.4%) | <0.001 § | 0.741 | 47 (31.3%) | 17 (81.0%) | <0.001 § | 0.053 |
| Peri-intraventricular hemorrhage grade ≥ 2 | 20 (7.1%) | 37 (21.8%) | <0.001 § | 0.384 | 31 (20.8%) | 6 (28.6%) | 0.407 § | 0.713 |
| Cystic periventricular leukomalacia | 4 (1.4%) | 9 (5.3%) | 0.022 § | 0.767 | 7 (4.7%) | 2 (10.0%) | 0.289 § | 0.993 |
| Necrotizing enterocolitis | 11 (3.9%) | 15 (9.0%) | 0.035 § | 0.831 | 14 (9.6%) | 1 (5.0%) | 1.000 § | 0.071 |
| Early sepsis | 26 (30.2%) | 25 (22.5%) | 0.252 § | 0.926 | 19 (20.7%) | 6 (31.6%) | 0.366 § | 0.048 |
| Late sepsis | 60 (69.8%) | 86 (77.5%) | 0.252 § | 0.926 | 73 (79.3%) | 13 (68.4%) | 0.366 § | 0.048 |
| Hemodynamically significant patent ductus arteriosus | 20 (7.1%) | 43 (25.1%) | <0.001 § | 0.095 | 31 (20.7%) | 12 (57.1%) | 0.001 § | 0.829 |
| Hyperbilirubinemia with phototherapy | 237 (84.0%) | 162 (95.9%) | <0.001 § | 0.275 | 141 (95.3%) | 21 (100.0%) | 0.598 § | 0.999 |
| Number of days with hyperglycemia (in the first 21 days) | 0.00 (0.00–2.00) | 2.00 (0.00–5.00) | <0.001 # | 0.485 | 2.00 (0.00–4.00) | 5.00 (3.00–7.00) | 0.001 # | 0.565 |
| Treatments | ||||||||
| RBC transfusions | 50 (17.7%) | 114 (66.3%) | <0.001 § | NA | 94 (62.3%) | 20 (95.2%) | 0.002 § | NA |
| Platelet transfusions | 9 (3.2%) | 37 (21.5%) | <0.001 § | 0.008 | 29 (19.2%) | 8 (38.1%) | 0.084 § | 0.332 |
| Days of invasive and non-invasive mechanical ventilation | 3.00 (1.00–11.00) | 32.00 (7.00–51.00) | <0.001 # | 0.217 | 26.00 (6.00–48.00) | 54.00 (39.50–78.00) | <0.001 # | 0.481 |
| Days of invasive mechanical ventilation | 0.00 (0.00–1.00) | 0.00 (3.00–17.75) | <0.001 # | 0.096 | 2.00 (0.00–15.00) | 27.00 (12.50–40.50) | <0.001 # | 0.541 |
| Surfactant | 92 (33.6%) | 127 (74.3%) | <0.001 § | 0.015 | 108 (72.0%) | 19 (90.5%) | 0.107 § | 0.793 |
| Erythropoietin or darbepoetin | 9 (3.2%) | 13 (7.6%) | 0.043 § | 0.959 | 11 (7.4%) | 2 (9.5%) | 0.665 § | 0.719 |
| Systemic corticosteroid | 14 (5.0%) | 44 (25.7%) | <0.001 § | 0.891 | 31 (20.7%) | 13 (61.9%) | <0.001 § | 0.133 |
| Inhaled corticosteroid | 15 (6.0%) | 37 (25.2%) | < 0.001 § | 0.242 | 29 (22.3%) | 8 (47.1%) | 0.038 § | 0.239 |
| Bronchodilator | 16 (5.7%) | 29 (16.9%) | <0.001 § | 0.595 | 24 (15.9%) | 5 (23.8%) | 0.358 § | 0.872 |
| Non-steroidal anti-inflammatory | 13 (4.6%) | 32 (18.7%) | <0.001 § | 0.319 | 24 (16.0%) | 8 (38.1%) | 0.031 § | 0.944 |
| Diuretics | 50 (17.7%) | 100 (58.1%) | <0.001 § | 0.157 | 83 (55.0%) | 17 (81.0%) | 0.032 § | 0.792 |
| Weight increase | ||||||||
| Mean daily weight increase up to the 10th day | 0.000 (−6.083–5.575) | −2.900 (−7.944–1.273) | <0.001 # | 0.072 | −3.300 (−8.136–0.782) | −1.500 (−6.841–2.875) | 0.325 ¥ | 0.089 |
| Mean daily weight increase from the 11th to the 20th day | 22.753 (16.861–30.000) | 15.000 (10.417–21.700) | <0.001 # | 0.912 | 15.929 (12.000–22.000) | 9.778 (6.075–14.750) | 0.002 ¥ | 0.426 |
| Mean daily weight increase from the 21st to the 30th day | 28.700 (21.438–35.667) | 20.909 (13.300–28.800) | <0.001 # | 0.913 | 21.214 (15.000–29.706) | 12.000 (5.000–21.650) | <0.001 ¥ | 0.253 |
| Nutrition | ||||||||
| Day of the start of trophic enteral nutrition | 2.00 (2.00–3.00) | 3.00 (2.50–6.00) | <0.001 # | 0.228 | 2.00 (2.00–3.00) | 3.00 (2.50–6.00) | <0.001 # | 0.032 |
| Day of the start of nutritious enteral nutrition | 4.00 (3.00–6.00) | 9.50 (5.25–19.25) | <0.001 # | 0.681 | 5.00 (4.00–7.00) | 9.50 (5.25–19.25) | <0.001 # | 0.022 |
| Day of the start of total enteral nutrition | 10.00 (7.00–14.75) | 26.00 (16.50–46.50) | <0.001 # | 0.126 | 13.00 (9.00–20.00) | 26.00 (16.50–46.50) | 0.028 # | 0.932 |
| Breast milk | 158 (82.7%) | 123 (78.3%) | 0.340 § | 0.745 | 110 (79.7%) | 13 (68.4%) | 0.251 § | 0.731 |
| Biochemical parameters | ||||||||
| Urea (mg/dL) | 49.0 (33.5–64.5) | 61.6 (42.0–76.3) | 0.001 # | 0.087 | 58.5 (41.0–74.8) | 65.0 (49.7–93.7) | 0.112 # | 0.956 |
| Creatinine (mg/dL) | 0.7 (0.6–0.8) | 0.8 (0.6–0.9) | <0.001 # | 0.731 | 0.7 (0.6–0.8) | 0.9 (0.8–1.0) | 0.005 # | 0.092 |
| Total bilirubin (mg/dL) | 7.3 (5.8–8.7) | 6.5 (5.3–7.5) | 0.001 # | 0.825 | 6.6 (5.4–7.6) | 5.6 (4.4–7.1) | 0.087 # | 0.913 |
| Direct bilirubin | 0.5 (0.4–0.6) | 0.7 (0.4–0.8) | 0.007 # | 0.277 | 0.7 (0.5–0.8) | 0.4 (0.4–0.8) | 0.241 # | 0.095 |
| Days of hospitalization | 41.7 (29.4–53.2) | 72.8 (53.2–94.5) | <0.001 # | <0.001 | 68.6 (50.8–88.6) | 104.0 (88.9–128.1) | <0.001 # | 0.082 |
| (a) | (b) | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Data | No ROP (n = 283) Mean ± SD | ROP (n = 172) Mean ± SD | P | P * | ROP Stages 1, 2 and 3 † (n = 151) Mean ± SD | Type 1 ROP (n = 21) Mean ± SD | P | P * |
| Oxygen | ||||||||
| Maximum FiO2 1st week | 26.886 ± 43.155 | 31.345 ± 16.841 | <0.001 # | 0.936 | 30.412 ± 17.027 | 38.347 ± 13.809 | 0.001 # | 0.431 |
| Maximum FiO2 2nd week | 21.167 ± 5.110 | 28.512 ± 12.547 | <0.001 # | 0.045 | 27.025 ± 10.478 | 39.364 ± 19.706 | 0.001 # | 0.132 |
| Maximum FiO2 3rd week | 24.158 ± 6.103 | 34.464 ± 17.579 | <0.001 # | 0.444 | 31.953 ± 16.014 | 46.537 ± 20.061 | <0.001 # | 0.250 |
| Blood transfusions | ||||||||
| Number of RBC transfusions | 0.29 ± 0.835 | 2.19 ± 2.445 | <0.001 # | NA | 1.80 ± 2.126 | 4.95 ± 2.837 | <0.001 # | NA |
| Number of platelet transfusions | 0.05 ± 0.316 | 0.41 ± 1.025 | <0.001 # | 0.049 | 0.34 ± 0.857 | 0.86 ± 1.797 | 0.048 # | 0.213 |
| Number of plasma transfusions | 0.01 ± 0.133 | 0.11 ± 0.452 | <0.001 # | 0.929 | 0.11 ± 0.470 | 0.10 ± 0.301 | 0.748 # | 0.146 |
| (a) | (b) | |||||||
|---|---|---|---|---|---|---|---|---|
| Demographic and Clinical Characteristics | No ROP (n = 283) n (%) or Median (Q1–Q3) | ROP (n = 172) n (%) or Median (Q1–Q3) | P | P * | ROP stages 1, 2, and 3 † (n = 151) n (%) or Median (Q1–Q3) | Type 1 ROP (n = 21) n (%) or Median (Q1–Q3) | P | P * |
| Maternal age | ||||||||
| <18 years | 1 (0.4%) | 2 (1.2%) | 0.534 § | 0.598 | 2 (1.3%) | 0 (0%) | 0.749 § | 0.607 |
| 18–35 years | 206 (72.8%) | 121 (70.3%) | 105 (69.5%) | 16 (76.2%) | ||||
| >35 years | 76 (26.9%) | 49 (28.5%) | 44 (29.1%) | 5 (23.8%) | ||||
| Non-Portuguese family ancestry | 66 (23.6%) | 50 (29.4%) | 0.183 § | 0.160 | 48 (32.2%) | 2 (9.5%) | 0.039 § | 0.045 |
| Level of education | ||||||||
| Primary education | 57 (21.0%) | 40 (24.8%) | 0.516 § | 0.234 | 33 (23.4%) | 7 (35.0%) | NA | 0.034 |
| Lower secondary education | 8 (3.0%) | 9 (5.6%) | 8 (5.7%) | 1 (5.0%) | ||||
| Upper secondary education | 86 (31.7%) | 50 (31.1%) | 40 (28.4%) | 10 (50.0%) | ||||
| Post-secondary non-tertiary education | 2 (0.7%) | 1 (0.6%) | 1 (0.7%) | 0 (0.0%) | ||||
| Tertiary education (any stage) | 118 (43.5%) | 61 (37.9%) | 59 (41.8%) | 2 (10.0%) | ||||
| Behavioral habits | ||||||||
| Tobacco | 44 (16.2%) | 30 (18.2%) | 0.601 § | 0.179 | 26 (18.1%) | 4 (19.0%) | 1.000 § | 0.492 |
| Alcohol | 6 (2.2%) | 3 (1.8%) | 1.000 § | 0.678 | 3 (2.1%) | 0 (0.0%) | 1.000 § | 0.999 |
| Illicit drugs | 2 (0.7%) | 3 (1.9%) | 0.368 § | 0.511 | 2 (1.4%) | 1 (5.3%) | 0.314 § | 0.139 |
| Obstetric history | ||||||||
| Number of previous births | ||||||||
| 0 | 176 (62.4%) | 105 (61.0%) | 0.958 § | 0.963 | 98 (64.9%) | 7 (33.3%) | 0.021 § | 0.442 |
| 1–3 | 98 (34.8%) | 62 (36,0%) | 49 (32.5%) | 13 (61.9%) | ||||
| ≥4 | 8 (2.8%) | 5 (2.9%) | 4 (2.6%) | 1 (4.8%) | ||||
| Pregnancy data | ||||||||
| Assisted reproduction techniques | 34 (14.6%) | 23 (14.5%) | 1.000 § | 0.843 | 22 (15.6%) | 1 (5.6%) | 0.475 § | 0.013 |
| Multiple births | 97 (34.3%) | 39 (22.8%) | 0.011 § | 0.164 | 35 (23.3%) | 4 (19.0%) | 0.787 § | 0.227 |
| Pathologies in pregnancy | ||||||||
| Chronic arterial hypertension | 18 (6.4%) | 25 (14.6%) | 0.005 § | 0.137 | 19 (12.6%) | 6 (30.0%) | 0.084 § | 0.049 |
| Pregnancy-induced hypertension | 105 (37.1%) | 44 (25.6%) | 0.012 § | 0.722 | 39 (25.8%) | 5 (23.8%) | 1.000 § | 0.261 |
| Chronic hypertension with preeclampsia | 12 (4.2%) | 11 (6.4%) | 0.312 § | 0.561 | 8 (5.3%) | 3 (14.3%) | 0.136 § | 0.168 |
| Diabetes | 39 (13.8%) | 22 (12.8%) | 0.799 § | 0.948 | 18 (12.0%) | 4 (19.0%) | 0.483 § | 0.336 |
| Chorioamnionitis | 29 (10.3%) | 25 (14.7%) | 0.179 § | 0.872 | 23 (15.4%) | 2 (9.5%) | 0.743 § | 0.573 |
| Eyes (%) | Outcome | |
|---|---|---|
| ROP stage 1 | 172 (18.9%) | Spontaneous regression on follow-up |
| ROP stage 2 | 92 (10.0%) | 2 eyes treated with anti-VEGF |
| ROP stage 3 | 66 (7.4%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fevereiro-Martins, M.; Santos, A.C.; Marques-Neves, C.; Bicho, M.; Guimarães, H.; on behalf of the GenE-ROP Study Group. Retinopathy of Prematurity in Eight Portuguese Neonatal Intensive Care Units: Incidence, Risk Factors, and Progression—A Prospective Multicenter Study. Children 2024, 11, 1154. https://doi.org/10.3390/children11101154
Fevereiro-Martins M, Santos AC, Marques-Neves C, Bicho M, Guimarães H, on behalf of the GenE-ROP Study Group. Retinopathy of Prematurity in Eight Portuguese Neonatal Intensive Care Units: Incidence, Risk Factors, and Progression—A Prospective Multicenter Study. Children. 2024; 11(10):1154. https://doi.org/10.3390/children11101154
Chicago/Turabian StyleFevereiro-Martins, Mariza, Ana Carolina Santos, Carlos Marques-Neves, Manuel Bicho, Hercília Guimarães, and on behalf of the GenE-ROP Study Group. 2024. "Retinopathy of Prematurity in Eight Portuguese Neonatal Intensive Care Units: Incidence, Risk Factors, and Progression—A Prospective Multicenter Study" Children 11, no. 10: 1154. https://doi.org/10.3390/children11101154
APA StyleFevereiro-Martins, M., Santos, A. C., Marques-Neves, C., Bicho, M., Guimarães, H., & on behalf of the GenE-ROP Study Group. (2024). Retinopathy of Prematurity in Eight Portuguese Neonatal Intensive Care Units: Incidence, Risk Factors, and Progression—A Prospective Multicenter Study. Children, 11(10), 1154. https://doi.org/10.3390/children11101154








