Abstract
Background: The impact of and countermeasures for Ureaplasma spp. in neonates remain controversial. The aim of this study was to evaluate the associated perinatal factors that can predict the likelihood of respiratory tract Ureaplasma spp. colonization and analyze the subsequent clinical course of affected infants, thereby providing the rationale for their diagnosis, treatment, and future study. Methods: This was a retrospective observational study of infants born at a gestational age (GA) of less than 32 weeks. Results: The prevalence of respiratory tract Ureaplasma spp. colonization was 25.8% (75/291), and it increased with a decrease in GA and birth weight (BW). Maternal vaginal Ureaplasma spp. colonization increased the risk of neonatal Ureaplasma spp. colonization, with an OR of 7.8 (95% CI: 3.1, 20.0). Infants with Ureaplasma spp. colonization had a higher white blood cell (WBC) count, normal C-reactive protein (CRP) level, and higher failure rate of weaning from mechanical ventilation (30.7% vs. 17.1%, p = 0.014); they also suffered more from interstitial pneumonia (20.0% vs. 5.6%, p < 0.001) and bronchopulmonary dysplasia (36.0% vs. 13.4%, p < 0.001). Infants receiving anti-Ureaplasma spp. treatment had a lower GA, lower BW, and more severe respiratory syndromes. However, the difference in respiratory manifestation became insignificant after adjusting for GA. Conclusions: GA and maternal vaginal Ureaplasma spp. colonization could be used to predict neonatal respiratory tract Ureaplasma spp. colonization. An elevated WBC count combined with normal CRP is a good marker of Ureaplasma spp. colonization/infection. It is conventional practice to start anti-Ureaplasma spp. treatment when infants present with a deteriorated respiratory condition. This practice warrants further investigation considering GA as a predominant intermediate variable.
1. Introduction
Ureaplasma species (Ureaplasma spp.) are the most common organisms present in the genital tract of pregnant women [1]. The invasion of the amniotic cavity by Ureaplasma spp. can cause chorioamnionitis, fetal inflammatory response syndrome, and preterm birth (PTB) [2], as demonstrated by the isolation of Ureaplasma spp. in placentae and amniotic fluid [3,4,5]. Ureaplasma spp. are also frequently isolated from the respiratory tract of preterm infants through vertical transmission from their mothers [6,7]. Colonization or infection with Ureaplasma spp. has been associated with a series of neonatal morbidity and sequelae, especially chronic lung disease (CLD) and bronchopulmonary dysplasia (BPD) [8]. With the increasing survival of the more immature infants, BPD continues to be one of the most hazardous health-threatening complications and places a substantial burden on families and the public. Numerous studies have shown that Ureaplasma spp. can induce a pro-inflammatory and pro-fibrotic response, thus contributing to interstitial pneumonia and the development of BPD [9,10,11,12]. However, some studies found that positive Ureaplasma spp. screening alone was not associated with BPD [13]. A recent meta-analysis did not draw a conclusion about the role of Ureaplasma spp. in adverse pregnancy and birth outcomes [14]. The heterogeneity of these studies might be attributed to “small study effects” or underlying confounding factors [15,16].
Since the pathogenic effect of Ureaplasma spp. on neonatal respiratory outcomes is controversial, there is still no consensus on which antimicrobial modality should be used to counteract Ureaplasma spp. colonization or infection. As opportunistic pathogens, it is difficult to clearly define their colonization or infection in a newborn infant. Diagnosing Ureaplasma spp. infection remains a challenge. Conventionally, Ureaplasma spp. confirmed either by culture or RT-PCR from tracheal or nasopharyngeal secretions is thought of as an infection [17,18]. Only a few studies have investigated the specific clinical course of Ureaplasma spp. infection [19]. Early identification of infants at the highest risk of Ureaplasma spp. infection would help to initiate appropriate treatments. Herein, we conducted this study with the aim of evaluating relevant perinatal factors that could predict the likelihood of respiratory tract Ureaplasma spp. colonization and analyzing the subsequent clinical course of affected infants; in doing so, we hoped to provide a rationale for their diagnosis, treatment, and future study.
2. Materials and Methods
2.1. Subjects
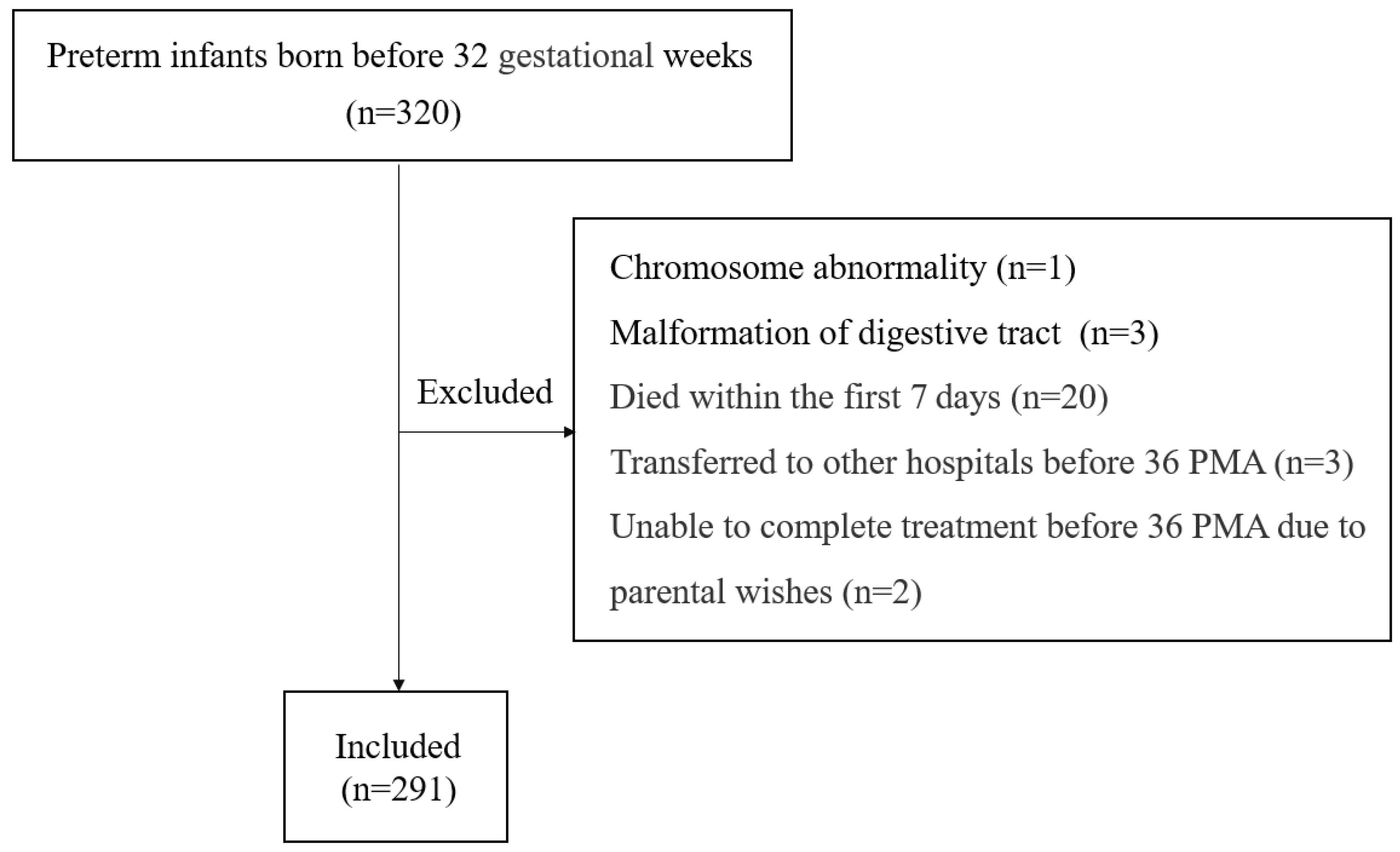
This study included all infants who were born before a gestational age (GA) of 32 weeks and admitted to the neonatal intensive care unit (NICU) at Zhangzhou Affiliated Hospital of Fujian Medical University in China between January 2022 and December 2023. The exclusion criteria were as follows: (1) infants with major congenital malformations; (2) infants who died within the first 7 days after birth; (3) infants who were transferred to other hospitals before 36 weeks postmenstrual age (PMA); and (4) infants who were unable to continue treatment before 36 PMA due to parental wishes.
Data were extracted from the electronic medical record system, including demographic information, obstetric complications, and neonatal morbidities.
2.2. Clinical Practice for Ureaplasma spp. Detection
In our NICU, preterm infants were routinely screened for Ureaplasma spp. upon admission. Respiratory tract samples were obtained from the tracheal tubes (tracheal aspirates) or nasopharynx (swabs or sputum). Both nasopharyngeal and tracheal samples could be used for detection, as described previously [20]. Pregnant women who had signs of premature birth selectively underwent examination for Ureaplasma spp. using vaginal swabs. Swabs were infiltrated with saline upon collection. Swabs, aspirates, and sputum were placed in sterile and sealed containers for examination [21]. DNA was extracted using a magnetic bead-based method on an automatic extractor (GeneRotex 96) before being amplified and analyzed on an RT-PCR platform (Abi7500) in accordance with the manufacturer’s instructions. The PCR detection kit was specific for Ureaplasma spp. but could not differentiate between biovars and serovars. We used an existing detection protocol outlined in previous studies [19].
2.3. Clinical Practice for CBC and CRP Detection
All infants had two consecutive complete blood count (CBC) and C-reactive protein (CRP) examinations (in a 24 h interval) after birth.
2.4. The Initial Administration of Antibiotics
All enrolled infants received intravenous penicillin (50,000 units·kg−1) plus cefotaxime (50 mg·kg−1) every 12 h for at least 72 h until the results of cultures were available.
2.5. Diagnostic Criteria
PTB is a heterogeneous syndrome consisting of various phenotypes based on etiologic complexities [22]. In this study, we divided PTB into two subgroups known as “Spontaneous PTB” and “Iatrogenic PTB”.
Spontaneous PTB: preterm birth that follows spontaneous labor or premature rupture of the membranes (PROM) [23].
Iatrogenic PTB: preterm birth initiated by the provider of maternity care in response to maternal illness or signs of fetal compromise, including placental abruption, pre-existing hypertension, pre-eclampsia, antepartum hemorrhage, fetal distress, etc. [24].
Interstitial pneumonia was diagnosed depending on the appearance of chest radiographs, encompassing diffuse streaky interstitial infiltration and coarse reticular infiltration [25]. All infants received a chest radiographic examination within 24 h after birth and at least one more chest radiographic examination before 36 weeks PMA.
BPD was defined as the need for supplemental oxygen or assisted ventilation (including continuous positive airway pressure, intermittent positive pressure ventilation, nasal continuous positive airway pressure, non-invasive positive pressure ventilation, and nasal cannula flow) for ≥3 consecutive days to maintain arterial oxygen saturation in the 90–95% range at 36 weeks PMA [26].
Patent ductus arteriosus (PDA) was diagnosed if echocardiography showed signs of ductus arteriosus shunt. All infants received a bedside echocardiographic examination within 7 days of birth.
2.6. Ethics
This was a retrospective study. Approval for this study was obtained from the Institutional Review Board of Zhangzhou Affiliated Hospital of Fujian Medical University (2022KYZ297); informed consent was waived due to the retrospective nature of the study.
2.7. Statistical Analysis
Statistical analysis was performed using SPSS 26.0 software. The Chi-square test or Fisher’s exact test was used to analyze categorical variables. Continuous data with a normal distribution are described as means ± SDs and were analyzed using a t-test of two independent samples between the two groups. Continuous data that did not conform to a normal distribution are presented as the medians (25% percentiles, 75% percentiles) and were analyzed among groups using the Mann–Whitney U test. Multivariate regression was used to calculate the adjusted advantage ratio and the corresponding 95% confidence interval. A double-tailed p-value of <0.05 was considered statistically significant.
3. Results
3.1. Prevalence of Ureaplasma spp. in Preterm Infants Born before 32 Weeks GA
A total of 320 preterm infants born before a GA of 32 weeks were admitted to our NICU during the study period. Four infants had severe deformities. Twenty infants died within the first 7 days after birth. Before 36 weeks of PMA, three infants were transferred to other hospitals, and two infants were unable to continue treatment due to parental wishes. Thus, a final 291 infants were eligible for the analysis (Figure 1). The mean GA was 29.1 ± 1.8 weeks (range: 23 weeks +5 days–31 weeks +6 days), and the mean birth weight (BW) was 1335 g (range: 540–2300 g). Among the study population, 85.9% (250/291) were defined as spontaneous PTBs and 14.1% (41/291) were defined as iatrogenic PTBs. The proportion of in vitro fertilization (IVF) was 8.6% (25/291). All the included infants were tested for Ureaplasma spp., and the overall positive rate was 25.8% (75/291). The rate of positivity was similar in tracheal tube samples (28.8%) and nasopharyngeal samples (23.5%). The rate of positivity reached 35.9% among neonates with GA < 30 weeks and 51.8% among neonates with GA < 28 weeks. With the decrease in GA, the rate of positivity increased (Table 1). The odds ratio (OR) of every one week decrease in GA to Ureaplasma spp. colonization was 1.5 (95% CI: 1.3–1.7, p < 0.001). In addition to GA, the rate of positivity was also higher among infants with lower BW. Ureaplasma spp. was detected in 37.5% of neonates weighing <1250 g and 50.0% of neonates weighing <1000 g.

Figure 1.
Flow diagram of the study.

Table 1.
The prevalence of Ureaplasma spp. colonization in different GA groups.
A total of 164 pregnant women were tested for Ureaplasma spp. before delivery. The prevalence of Ureaplasma spp. colonization in the maternal vaginal tract was 52.4% (i.e., 86 of 164 samples collected before delivery). Among spontaneous PTBs, 79 individuals tested positive. The rate of positivity was higher in spontaneous PTBs than in iatrogenic PTBs (55.6% vs. 31.8%, p = 0.037). There was no difference in maternal Ureaplasma spp. colonization between women who underwent IVF and women who conceived spontaneously (63.2% vs. 51.0%, p = 0.320).
3.2. Perinatal Factors Associated with Neonatal Ureaplasma spp. Colonization
Infants with Ureaplasma spp. colonization were born at an earlier GA and had a lower BW than those without Ureaplasma spp. colonization. A higher proportion of spontaneous PTB, PROM > 18 h, maternal vaginal Ureaplasma spp. colonization and vaginal delivery were observed in mothers who delivered infants colonized by Ureaplasma spp. (Table 2). The rate of each form of conception (IVF versus spontaneous) did not differ significantly between Ureaplasma spp.-positive and -negative groups. After adjusting for GA via logistic regression, maternal vaginal Ureaplasma spp. colonization was still significantly associated with neonatal Ureaplasma spp. colonization (Table 3).

Table 2.
Clinical characteristics of the study population.

Table 3.
Association between perinatal factors and neonatal Ureaplasma spp. colonization.
3.3. Postnatal Clinical Characteristics of Neonatal Ureaplasma spp. Colonization
The consecutive monitoring of blood tests after birth showed that the white blood cell (WBC) count of infants with Ureaplasma spp. colonization was higher than that of infants without Ureaplasma spp. colonization, while the CRP levels did not differ significantly between the two groups. Further comparison between different GA groups showed that an increase in WBC count occurred in subgroups divided by GA (Table 4). In addition to blood index, their clinical manifestation showed that infants with Ureaplasma spp. colonization were more frequently ventilated within 72 h after birth, and the rates of interstitial pneumonia and BPD were higher than that in the Ureaplasma spp.-negative group (Table 2). However, after adjusting for GA via logistic regression, the differences were not significant, indicating that GA might be a prominent confounding variable (Table 3).

Table 4.
Comparison of BW, WBC count, and serum CRP in different GA groups.
Of the 75 infants with Ureaplasma spp. colonization, 50 (66.7%) infants received treatment with erythromycin (35 infants—15 mg·kg−1 every 12 h for 14 days) or azithromycin (15 infants—10 mg·kg−1 for 7 days followed by 5 mg·kg−1 for 7 days). Compared with infants who did not receive drug treatment, infants in the treatment group had lower GA and BW and a higher WBC count (Table 5). Higher rates of failure to respond to non-invasive respiratory support within 72 h, interstitial pneumonia, and BPD were also observed in the treatment group. These results revealed that neonatologists tended to give prescriptions to infants based on GA, BW, WBC count, and the deterioration of the respiratory condition. However, after stratification by GA, the difference in respiratory manifestation became insignificant (Table 6).

Table 5.
Clinical characteristics of infants with and without anti-Ureaplasma spp. treatment.

Table 6.
Clinical characteristics of infants with and without anti-Ureaplasma spp. treatment in different GA groups.
Among the 50 infants who received drug treatment, the rate of BPD did not differ significantly between those who received erythromycin versus azithromycin (erythromycin group: 40.0%; azithromycin group: 53.3%, p = 0.384).
4. Discussion
This study revealed the epidemiological characteristics of Ureaplasma spp. colonization in pregnant women and their offspring with a GA of less than 32 weeks. The overall prevalence of Ureaplasma spp. colonization was 52.4% in the maternal vaginal tract and 25.8% in the neonatal respiratory tract, respectively. Maternal vaginal Ureaplasma spp. colonization was consistently associated with neonatal respiratory tract Ureaplasma spp. colonization. Sobouti B et al. reported a rate of transmission from mothers to their neonates as high as 60–70% [27]. Screening of mothers would help to identify the risk of neonatal infection. If a vaginal test is unfeasible, perinatal factors—such as spontaneous PTB, PROM, and vaginal delivery—could be used for tentative prediction because these factors were also partially associated with neonatal Ureaplasma spp. colonization in the compound GA strata. PTB is a heterogeneous syndrome encompassing various phenotypes based on etiology [22]. It is well-recognized that two of its major clinical etiologies are iatrogenic and spontaneous PTB [28]. There is growing awareness that PTB phenotypes are associated with neonatal outcomes; this has prompted earlier prenatal interventions with the aim of reducing perinatal complications [29]. Spontaneous PTBs account for the majority of PTBs, and their cause has remained imperceptible. In many cases, spontaneous PTBs are thought to be caused by infection or inflammatory processes [30]. Among the pathogenic bacteria associated with chorioamnionitis, Ureaplasma spp. are the most prevalent organisms isolated from placental membranes and amniotic fluid [31,32,33]. Gerber S et al. tested Ureaplasma spp. in transabdominal amniotic fluid among asymptomatic women at 15–17 weeks gestation and found that Ureaplasma spp.-positive women were at greater risk of subsequent preterm labor and delivery [34]. Some scholars have suggested placing Ureaplasma spp. within the context of chorioamnionitis diagnosis. Prior to intrauterine infection, lower genital tract Ureaplasma colonization is suspected [35,36]. Its consequences might depend on the virulence, bacterial load, duration, and host immune response [37]. Previous studies have demonstrated that colonization of Ureaplasma spp. in the female vagina could activate the production of cytokines, prostaglandins, uterine contractions, and dilatation of the cervix, causing spontaneous PTB with an intact membrane or PROM [38,39]. PROM has historically been classed as spontaneous PTB [28]. Maternal Ureaplasma spp. infection mainly occurs in pregnancies after PROM, which might increase the likelihood of vertical transmission to the fetus or neonate [36]. Viscardi RM et al. demonstrated that PROM > 72 h is a good predictor for Ureaplasma spp. lower airway tract colonization in preterm infants [18]. A shorter duration of PROM (PROM >18 h) was found to be significant in our study. This result indicated the necessity of targeted monitoring and intervention in the early stages of PROM.
In this study, we found that there was a strong inverse association between neonatal Ureaplasma spp. colonization and GA. As GA decreased to less than 28 weeks, the prevalence of Ureaplasma spp. reached 51.5%. Sung et al. reported a higher rate (65%) as GA decreased to less than 26 weeks [7]. We further determined that for every week of decline in GA, the risk of Ureaplasma spp. colonization increased. A similar association was observed between Ureaplasma spp. colonization and BW. These results were consistent with previous studies [21]. Ozdemir et al. reported a positive Ureaplasma spp. detection rate of 33.0% among neonates weighing < 1250 g [40]. A similar rate (37.5%) was reported in our study, and we found a higher rate (50.0%) among infants weighing < 1000 g. Since GA and BW have a strong linear correlation, including GA in logistic regression is customary in observational studies. After incorporating candidate factors, the risk of Ureaplasma spp. respiratory tract colonization in infants can be simply identified antenatally via GA and maternal vaginal Ureaplasma spp. colonization.
The WBC count, shortly after birth, of infants colonized by Ureaplasma spp. was found to be significantly elevated in our study. A similar result has been found in previous studies [17,41]. It is well known that elevated WBC count is a hematologic change indicative of systemic inflammatory response syndrome (SIRS) [42]. Local inflammation characterized by an increase in chemotactic activity and neutrophil count was also found in the tracheobronchial aspirate of patients from the Ureaplasma spp.-colonized group, confirming that Ureaplasma spp. can trigger an inflammatory response [43]. However, an elevated WBC count is often seen in early-onset sepsis (EOS). Another thoroughly investigated laboratory marker used to diagnose neonatal sepsis is CRP [44]. We found that although the WBC count increased in the Ureaplasma spp. group, CRP did not increase simultaneously, which was consistent with the findings revealed by Meadows JT et al. [45]. A combination of increased WBC count and negative CRP might be helpful in distinguishing Ureaplasma spp. infection from bacterial EOS, since CRP always appears increased upon consecutive monitoring of the latter [46]. Regarding short-term neonatal outcomes, several studies have found that infants with Ureaplasma spp. respiratory colonization had a higher incidence of respiratory distress syndrome (RDS), interstitial pneumonia, and BPD [20,47,48]. In our study, in infants with Ureaplasma spp. colonization, we observed a higher rate of failure of weaning from mechanical ventilation within 72 h of birth, interstitial pneumonia, and BPD. However, these results must be interpreted with caution since after adjusting for GA, we failed to detect any differences. A reasonable explanation for this is that the association of Ureaplasma spp. with neonatal respiratory outcomes might be overestimated thanks to the weighted contribution of GA. Therefore, in the future, the real effects of Ureaplasma spp. on preterm outcomes must be analyzed alongside a more detailed GA stratification.
A growing number of scholars have observed that the detection of Ureaplasma spp. in the respiratory tract is not solely indicative of real infection, which places the treatment in a dilemma. As there are no guidelines for clinicians to follow, the decision is always made based on personal empirical judgment, resulting in variations in practice across NICUs. Generally speaking, treatments are informed by a suspected Ureaplasma spp. infection rather than colonization. In our NICU, infants who received treatment had a cluster of characteristics including lower GA, lower BW, higher WBC count, more dependence on mechanical ventilation after 72 h of life, chest X-ray images indicative of interstitial pneumonia, and a higher risk for BPD, suggesting that clinicians largely treat infants who require intensive respiratory support and suffer with poor respiratory conditions. Theilen U et al. also found an increasing trend in interstitial changes (as found via radiology) and a prolonged course of ventilation in infants with Ureaplasma spp. in their tracheal secretion [48]. Infants who underwent prolonged ventilation were at high risk of BPD [49] and, therefore, became potential receivers of anti-Ureaplasma spp. treatment. However, their poor respiratory conditions might be attributed to their more immature GA. It is urgent to investigate specific biomarkers that can guide clinical practice in the first few hours of life. Based on the retrospective nature of this study and the greater immaturity of the treated group at baseline, we cannot draw a conclusion on the benefit of an antimicrobial regimen. The predominant drugs used in treatment for neonates with Ureaplasma spp. infection are erythromycin and azithromycin. Previous randomized trials have found that erythromycin could not eliminate Ureaplasma spp. from the airways and failed to reduce the incidence of chronic lung disease [50,51]. Viscardi RM et al. found that a 3-day azithromycin regimen (20 mg·kg−1 every 24 h for 3 days) effectively eradicated respiratory tract Ureaplasma colonization but failed to decrease the risk of BPD [52]. However, in recent studies, Chen X et al. found that a 2-week effective azithromycin treatment (10 mg·kg−1 for 7 days followed by 5 mg·kg−1 for 7 days) in Ureaplasma spp.-positive VLBW infants were associated with a reduced risk of BPD [19], and Ballard HO et al. pointed out that early treatment of Ureaplasma-colonized/infected infants with azithromycin might decrease the occurrence of BPD and death [53]. After all, most scholars consider azithromycin the superior choice for anti-Ureaplasma spp. treatment. Recently, azithromycin therapy (a 10-day course of intravenous azithromycin 20 mg·kg−1 for 3 days, followed by 10 mg·kg−1 for 7 days) for CLD secondary to prematurity in the AZTEC study failed to induce a decline in moderate or severe CLD [54]. However, in the AZTEC trial, azithromycin was administrated prophylactically, regardless of Ureaplasma spp. colonization. Infants with respiratory tract Ureaplasma spp. colonization should be targeted; in doing so, we will accrue more evidence to support clinical management. In addition to azithromycin and erythromycin, Motomura K et al. found that clarithromycin could reduce adverse pregnancy and neonatal outcomes induced by Ureaplasma spp. [55]. This regimen should be assessed for efficacy and safety in future RCTs.
This is a retrospective study containing intact data on Ureaplasma spp. detection and the basic demographic information of pregnant women and their offspring, which facilitate reliable analysis of the perinatal epidemiological characteristics of Ureaplasma spp. The results herein provide a basis for further investigation of optimal biomarkers and treatment indications when GA is considered as an intermediate or confounding variable. However, this study has some limitations: Firstly, we did not distinguish between biovars or serotypes of Ureaplasma spp. Several studies have found that Ureaplasma. parvum is more pathogenic in respiratory disease [20]. Evidence of the differences in virulence between serovars is scarce [7]. Secondly, in the absence of relevant data, we did not analyze coinfections with other pathogens or complications, such as bacterial vaginitis and chorioamnionitis. Thirdly, since our sample size was relatively small, our categorization of PTB did not allow us to differentiate between more detailed groups according to an updated taxonomy [22].
5. Conclusions
GA and maternal vaginal Ureaplasma spp. colonization could be used to predict neonatal respiratory tract Ureaplasma spp. colonization. An elevated WBC count combined with normal CRP is a good marker of Ureaplasma spp. colonization/infection. It is conventional practice to start anti-Ureaplasma spp. treatment when infants present with a deteriorated respiratory condition. This practice warrants further investigation considering GA as a predominant intermediate variable.
Author Contributions
Conceptualization, Z.Z. and L.X.; writing—original draft preparation, Z.Z.; writing—review and editing, W.C.; methodology, J.W.; formal analysis, J.W.; data curation, W.C.; investigation, L.X.; supervision, L.X.; funding acquisition, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Startup Fund for Scientific Research of Fujian Medical University (no. 2021QH1257). The funders were not involved in the study design, data collection, analysis, interpretation, or manuscript preparation.
Institutional Review Board Statement
The study was approved by the Institutional Ethical Committee of the Zhangzhou Hospital (no. 2022KYZ297, approval date 6 December 2022). All the patient-related information was anonymized. The Hospital Institution authorized the administrative permission to the study team to access the data for research purposes on the nature of public health.
Informed Consent Statement
Patient informed consent was waived from the Ethical Committee of the Zhangzhou Hospital due to the retrospective nature of the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request, due to privacy.
Acknowledgments
The authors thank all the colleagues for their help in the implementation process of the study at the Department of Neonatology, Zhangzhou Hospital Affiliated with Fujian Medical University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| NICU | Neonatal intensive care unit |
| PMA | Postmenstrual age |
| PTB | Preterm birth |
| CLD | Chronic lung disease |
| BPD | Bronchopulmonary dysplasia |
| PDA | Patent ductus arteriosus |
| UU | Ureaplasma spp. |
| PROM | Premature rupture of fetal membrane |
| IVF | in vitro fertilization |
| GA | Gestational age |
| BW | Birth weight |
| CBC | Complete blood count |
| WBC | White blood count |
| RDS | Respiratory distress syndrome |
| CRP | C-reactive protein |
| EOS | Early-onset sepsis |
| SIRS | systemic inflammatory response syndrome |
| VLBW | Very low birth weight |
References
- Matasariu, D.R.; Ursache, A.; Agache, A.; Mandici, C.E.; Boiculese, V.L.; Bujor, I.E.; Rudisteanu, D.; Dumitrascu, I.; Schaas, C.M. Genital infection with Ureaplasma urealyticum and its effect on pregnancy. Exp. Ther. Med. 2022, 23, 89. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Suga, S.; Sugimi, S.; Kurata, N.; Yamashita, H.; Yasuhi, I. Vaginal Ureaplasma urealyticum or Mycoplasma hominis and preterm delivery in women with threatened preterm labor. J. Matern. Fetal Neonatal Med. 2022, 35, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.L.; Kallapur, S.G.; Gisslen, T.; Lambers, D.S.; Chougnet, C.A.; Stephenson, S.A.; Jobe, A.H.; Knox, C.L. Placental Infection with Ureaplasma species Is Associated with Histologic Chorioamnionitis and Adverse Outcomes in Moderately Preterm and Late-Preterm Infants. J. Infect. Dis. 2016, 213, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Romero, R.; Park, J.Y.; Hong, J.S.; Yoon, B.H. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. J. Perinat. Med. 2019, 47, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Lim, J.-H.; Shim, S.-S.; Hong, J.-S.; Shim, J.-Y.; Jun, J.K. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labo. Am. J. Obstet. Gynecol. 2003, 189, 919–924. [Google Scholar] [CrossRef]
- Viscardi, R.M. Ureaplasma species: Role in neonatal morbidities and outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F87–F92. [Google Scholar] [CrossRef]
- Sung, T.J.; Xiao, L.; Duffy, L.; Waites, K.B.; Chesko, K.L.; Viscardi, R.M. Frequency of ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr. Infect. Dis. J. 2011, 30, 379–383. [Google Scholar] [CrossRef]
- Viscardi, R.M.; Hasday, J.D. Role of Ureaplasma species in neonatal chronic lung disease: Epidemiologic and experimental evidence. Pediatr. Res. 2009, 65 Pt 2, 84r–90r. [Google Scholar] [CrossRef]
- Schelonka, R.L.; Katz, B.; Waites, K.B.; Benjamin, D.K., Jr. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr. Infect. Dis. J. 2005, 24, 1033–1039. [Google Scholar] [CrossRef]
- Viscardi, R.; Manimtim, W.; He, J.R.; Hasday, J.D.; Sun, C.C.; Joyce, B.; Pierce, R.A. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr. Dev. Pathol. 2006, 9, 143–151. [Google Scholar] [CrossRef]
- Honma, Y.; Yada, Y.; Takahashi, N.; Momoi, M.Y.; Nakamura, Y. Certain type of chronic lung disease of newborns is associated with Ureaplasma urealyticum infection in utero. Pediatr. Int. 2007, 49, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Xiao, L.; Paralanov, V.; Viscardi, R.M.; Glass, J.I. Molecular methods for the detection of Mycoplasma and ureaplasma infections in humans: A paper from the 2011 William Beaumont Hospital Symposium on molecular pathology. J. Mol. Diagn. 2012, 14, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.; Gradzka-Luczewska, A.; Szymankiewicz-Breborowicz, M.; Kawczynska-Leda, N.; Henrich, B.; Waaga-Gasser, A.M.; Speer, C.P. Perinatal Ureaplasma Exposure Is Associated With Increased Risk of Late Onset Sepsis and Imbalanced Inflammation in Preterm Infants and May Add to Lung Injury. Front. Cell Infect. Microbiol. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Jonduo, M.E.; Vallely, L.M.; Wand, H.; Sweeney, E.L.; Egli-Gany, D.; Kaldor, J.; Vallely, A.J.; Low, N. Adverse pregnancy and birth outcomes associated with Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum: A systematic review and meta-analysis. BMJ Open 2022, 12, e062990. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, R.M. Ureaplasma species: Role in diseases of prematurity. Clin. Perinatol. 2010, 37, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Van Mechelen, K.; van Westering-Kroon, E.; Hütten, M.; Mahieu, L.; Villamor, E. Placing Ureaplasma within the Context of Bronchopulmonary Dysplasia Endotypes and Phenotypes. Children 2023, 10, 256. [Google Scholar] [CrossRef]
- Sun, T.; Fu, J. Analysis of the Clinical Features of Intrauterine Ureaplasma urealyticum Infection in Preterm Infants: A Case-Control Study. Front. Pediatr. 2021, 9, 774150. [Google Scholar] [CrossRef]
- Viscardi, R.M.; Magder, L.S.; Terrin, M.L.; Davis, N.L. Predicting the likelihood of lower respiratory tract Ureaplasma infection in preterms. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 250–255. [Google Scholar] [CrossRef]
- Chen, X.; Huang, X.; Lin, Y.; Lin, B.; Yang, C.; Huang, Z.; Yang, C. Association of Ureaplasma infection pattern and azithromycin treatment effect with bronchopulmonary dysplasia in Ureaplasma positive infants: A cohort study. BMC Pulm. Med. 2023, 23, 229. [Google Scholar] [CrossRef]
- Cultrera, R.; Seraceni, S.; Germani, R.; Contini, C. Molecular evidence of Ureaplasma urealyticum and Ureaplasma parvum colonization in preterm infants during respiratory distress syndrome. BMC Infect. Dis. 2006, 6, 166. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Luo, C.; Xi, J.; Wang, X.; Hu, Y.; Zhu, C.; Jin, Z. Epidemiological and Clinical Characteristics of Neonatal Ureaplasma urealyticum Infection. Infect. Drug Resist. 2024, 17, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Cavoretto, P.I.; Barros, F.C.; Romero, R.; Papageorghiou, A.T.; Kennedy, S.H. Etiologically Based functional Taxonomy of the Preterm Birth Syndrome. Clin. Perinatol. 2024, 51, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Aughey, H.; Jardine, J.; Knight, H.; Gurol-Urganci, I.; Walker, K.; Harris, T.; van der Meulen, J.; Hawdon, J.; Pasupathy, D. Iatrogenic and spontaneous preterm birth in England: A population-based cohort study. BJOG 2023, 130, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, J.Y.; Yun, B.; Lee, B.; Choi, C.W.; Kim, B.I. Interstitial pneumonia pattern on day 7 chest radiograph predicts bronchopulmonary dysplasia in preterm infants. BMC Pediatr. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, C.; Zhang, L.; Mikhael, M. Newer bronchopulmonary dysplasia definitions and prediction of health economics impacts in very preterm infants. Pediatr. Pulmonol. 2021, 56, 409–417. [Google Scholar] [CrossRef]
- Sobouti, B.; Fallah, S.; Mobayen, M.; Noorbakhsh, S.; Ghavami, Y. Colonization of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women and their transmission to offspring. Iran. J. Microbiol. 2014, 6, 219–224. [Google Scholar]
- Gracie, S.; Pennell, C.; Ekman-Ordeberg, G.; Lye, S.; McManaman, J.; Williams, S.; Palmer, L.; Kelley, M.; Menon, R.; Gravett, M. An integrated systems biology approach to the study of preterm birth using “-omic” technology—A guideline for research. BMC Pregnancy Childbirth 2011, 11, 71. [Google Scholar] [CrossRef]
- Nuss, E.E.; Spiegelman, J.; Turitz, A.L.; Gyamfi-Bannerman, C. Childhood neurodevelopment after spontaneous versus indicated preterm birth. Am. J. Obstet. Gynecol. MFM 2020, 2, 100082. [Google Scholar] [CrossRef]
- Daskalakis, G.; Psarris, A.; Koutras, A.; Fasoulakis, Z.; Prokopakis, I.; Varthaliti, A.; Karasmani, C.; Ntounis, T.; Domali, E.; Theodora, M.; et al. Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications. Children 2023, 10, 907. [Google Scholar] [CrossRef]
- Bae, J.; Kim, S.; Hwang, I.; Park, J. Comparison between Cervical Ureaplasma spp. Colonization and the Intensity of Inflammatory Mediators in the Amniotic Fluid Retrieved during Cesarean Delivery in Preterm Birth. Int. J. Environ. Res. Public Health 2021, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.L.; Kallapur, S.G.; Meawad, S.; Gisslen, T.; Stephenson, S.A.; Jobe, A.H.; Knox, C.L. Ureaplasma Species Multiple Banded Antigen (MBA) Variation Is Associated with the Severity of Inflammation In vivo and In vitro in Human Placentae. Front. Cell Infect. Microbiol. 2017, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.L.; Dando, S.J.; Kallapur, S.G.; Knox, C.L. The Human Ureaplasma Species as Causative Agents of Chorioamnionitis. Clin. Microbiol. Rev. 2017, 30, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.; Vial, Y.; Hohlfeld, P.; Witkin, S.S. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J. Infect. Dis. 2003, 187, 518–521. [Google Scholar] [CrossRef]
- Kataoka, S.; Yamada, T.; Chou, K.; Nishida, R.; Morikawa, M.; Minami, M.; Yamada, H.; Sakuragi, N.; Minakami, H. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J. Clin. Microbiol. 2006, 44, 51–55. [Google Scholar] [CrossRef]
- Rittenschober-Boehm, J.; Fuiko, R.; Farr, A.; Willinger, B.; Berger, A.; Goeral, K. Intrauterine Detection of Ureaplasma Species after Vaginal Colonization in Pregnancy and Neonatal Outcome. Neonatology 2024, 121, 187–194. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Kukla, R.; Bolehovska, R.; Bostik, P.; Matulova, J.; Mls, J.; Stranik, J.; Jacobsson, B.; Musilova, I. Prevalence and Load of Cervical Ureaplasma Species with Respect to Intra-amniotic Complications in Women with Preterm Prelabor Rupture of Membranes Before 34 weeks. Front. Pharmacol. 2022, 13, 860498. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, S.D.; Jang, I.H.; Uh, Y.; Lee, A. The prevalence of vaginal microorganisms in pregnant women with preterm labor and preterm birth. Ann. Lab. Med. 2012, 32, 194–200. [Google Scholar] [CrossRef]
- Sánchez, P.J.; Regan, J.A. Vertical transmission of Ureaplasma urealyticum from mothers to preterm infants. Pediatr. Infect. Dis. J. 1990, 9, 398–401. [Google Scholar] [CrossRef]
- Ozdemır, R.; Sarı, F.N.; Tunay, Z.O.; Erdeve, O.; Canpolat, F.E.; Oguz, S.S.; Uras, N.; Dılmen, U. The association between respiratory tract Ureaplasma urealyticum colonization and severe retinopathy of prematurity in preterm infants ≤ 1250 g. Eye 2012, 26, 992–996. [Google Scholar] [CrossRef]
- Panero, A.; Pacifico, L.; Rossi, N.; Roggini, M.; Chiesa, C. Ureaplasma urealyticum as a cause of pneumonia in preterm infants: Analysis of the white cell response. Arch. Dis. Child. Fetal Neonatal Ed. 1995, 73, F37–F40. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Savasan, Z.A.; Chaiworapongsa, T.; Berry, S.M.; Kusanovic, J.P.; Hassan, S.S.; Yoon, B.H.; Edwin, S.; Mazor, M. Hematologic profile of the fetus with systemic inflammatory response syndrome. J. Perinat. Med. 2011, 40, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Groneck, P.; Goetze-Speer, B.; Speer, C.P. Inflammatory bronchopulmonary response of preterm infants with microbial colonisation of the airways at birth. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 74, F51–F55. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, C.; Aydemir, H.; Kokturk, F.; Kulah, C.; Mungan, A.G. The cut-off levels of procalcitonin and C-reactive protein and the kinetics of mean platelet volume in preterm neonates with sepsis. BMC Pediatr. 2018, 18, 253. [Google Scholar] [CrossRef] [PubMed]
- Meadows, J.T.; Kopp, B.T., Jr.; Shook, L.A.; Ballard, H.O.; Hayes, D., Jr. Elevated high-sensitivity C-reactive protein in preterm infants with pulmonary colonization with Ureaplasma. J. Thorac. Dis. 2013, 5, 223–227. [Google Scholar]
- Mukhopadhyay, S.; Puopolo, K.M. Risk assessment in neonatal early onset sepsis. Semin. Perinatol. 2012, 36, 408–415. [Google Scholar] [CrossRef]
- Viscardi, R.M.; Hashmi, N.; Gross, G.W.; Sun, C.C.; Rodriguez, A.; Fairchild, K.D. Incidence of invasive ureaplasma in VLBW infants: Relationship to severe intraventricular hemorrhage. J. Perinatol. 2008, 28, 759–765. [Google Scholar] [CrossRef]
- Theilen, U.; Lyon, A.J.; Fitzgerald, T.; Hendry, G.M.; Keeling, J.W. Infection with Ureaplasma urealyticum: Is there a specific clinical and radiological course in the preterm infant? Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F163–F167. [Google Scholar] [CrossRef]
- Kakoo Brioso, E.; Moscoso, J.; Malveiro, D.; Aguiar, M.; Tuna, M. Bronchopulmonary Dysplasia: A Five-Year Retrospective Cohort Study on Differences in Clinical Characteristics and Morbidities According to Severity Grading. Cureus 2023, 15, e42720. [Google Scholar] [CrossRef]
- Baier, R.J.; Loggins, J.; Kruger, T.E. Failure of erythromycin to eliminate airway colonization with ureaplasma urealyticum in very low birth weight infants. BMC Pediatr. 2003, 3, 10. [Google Scholar] [CrossRef]
- Lyon, A.J.; McColm, J.; Middlemist, L.; Fergusson, S.; McIntosh, N.; Ross, P.W. Randomised trial of erythromycin on the development of chronic lung disease in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 78, F10–F14. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, R.M.; Terrin, M.L.; Magder, L.S.; Davis, N.L.; Dulkerian, S.J.; Waites, K.B.; Ambalavanan, N.; Kaufman, D.A.; Donohue, P.; Tuttle, D.J.; et al. Randomised trial of azithromycin to eradicate Ureaplasma in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Ballard, H.O.; Shook, L.A.; Bernard, P.; Anstead, M.I.; Kuhn, R.; Whitehead, V.; Grider, D.; Crawford, T.N.; Hayes, D., Jr. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: A randomized, double-blind, placebo controlled trial. Pediatr. Pulmonol. 2011, 46, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Gillespie, D.; Aboklaish, A.; Lau, T.M.M.; Consoli, C.; Babu, M.; Goddard, M.; Hood, K.; Klein, N.; Thomas-Jones, E.; et al. Azithromycin therapy for prevention of chronic lung disease of prematurity (AZTEC): A multicentre, double-blind, randomised, placebo-controlled trial. Lancet Respir. Med. 2024, 12, 608–618. [Google Scholar] [CrossRef]
- Motomura, K.; Romero, R.; Xu, Y.; Theis, K.R.; Galaz, J.; Winters, A.D.; Slutsky, R.; Garcia-Flores, V.; Zou, C.; Levenson, D.; et al. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio 2020, 11, e00797-20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).