Secondhand Smoke Exposure and Its Impact on Pediatric Lung Function, Aerobic Fitness, and Body Mass: Evidence from a Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.2.1. The Inclusion Criteria Included the Following
- Children between 10 and 14 years;
- Only children who were in good overall health, with no acute or chronic illnesses affecting respiratory or systemic health, were included;
- Children must have had no known respiratory infections for at least one month prior to testing to ensure accurate respiratory function measurements.
2.2.2. The Exclusion Criteria Included the Following
- Children diagnosed with chronic respiratory conditions or other chronic diseases;
- Children with any acute respiratory or systemic illness at the time of the study or within the one-month period prior to testing;
- Presence of systemic illnesses that could affect the respiratory system (e.g., cardiovascular diseases, neuromuscular disorders, immunodeficiency disorders);
- Children on medications that could affect respiratory function, such as bronchodilators or corticosteroids;
- Children whose parental smoking status could not be clearly determined through the questionnaire.
2.3. Data Collection
2.4. Anthropometric Measurements
2.5. Spirometry Measurements
2.6. Assessment of Motor Skills
2.7. Statistical Analyses
3. Results
3.1. Demographic Data
3.2. Association of SHS Exposure, Lung Function, and BMI
4. Discussion
Limitations of the Study and Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hancox, R.J.; Gray, A.R.; Poulton, R.; Sears, M.R. The Effect of Cigarette Smoking on Lung Function in Young Adults with Asthma. Am. J. Respir. Crit. Care Med. 2016, 194, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J.K.; Hernberg, S.; Lajunen, T.K.; Sripaijboonkij, P.; Malmberg, L.P.; Jaakkola, M.S. Smoking and lung function among adults with newly onset asthma. BMJ Open Respir. Res. 2019, 6, e000377. [Google Scholar] [CrossRef] [PubMed]
- Precioso, J.; Frias, S.; Silva, C.N.; Rocha, V.; Cunha-Machado, J.; Gonçalves, F.; Sousa, I. Prevalence of children exposed to secondhand smoke at home and in the car in Azores (Portugal). Pulmonology 2019, 25, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.B.U.; Rungta, N.M.N.; Shenoy, R.; Rao, A.; Shetty, M.K. Exposure to Second Hand Tobacco Smoke among 12 year old Adolescents in Mangalore, Karnataka—A Descriptive Study. Asian Pac. J. Cancer Prev. 2021, 22, 827–835. [Google Scholar] [CrossRef]
- Huang, L.; Cao, Y.; Zhang, Z. Status and correlates of children’s exposure to secondhand smoke at home: A survey in Chongqing, China. Tob. Induc. Dis. 2023, 21, 38. [Google Scholar] [CrossRef]
- Vanker, A.; Gie, R.P.; Zar, H.J. The association between environmental tobacco smoke exposure and childhood respiratory disease: A review. Expert. Rev. Respir. Med. 2017, 11, 661–673. [Google Scholar] [CrossRef]
- Flor, L.S.; Anderson, J.A.; Ahmad, N.; Aravkin, A.; Carr, S.; Dai, X.; Gil, G.F.; Hay, S.I.; Malloy, M.J.; McLaughlin, S.A.; et al. Health effects associated with exposure to secondhand smoke: A Burden of Proof study. Nat. Med. 2024, 30, 149–167. [Google Scholar] [CrossRef]
- Dai, S.; Chan, K.C. Associations of household environmental tobacco smoke exposure with respiratory symptoms and utilisation of medical services in healthy young children in Hong Kong. Tob. Induc. Dis. 2020, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Pavić, I.; Pavić, P.; Palčić, I.; Nenadić, N. Influence of passive smoking on functional abilities in children. Int. J. Environ. Health Res. 2012, 22, 355–361. [Google Scholar] [CrossRef]
- Pattenden, S.; Antova, T.; Neuberger, M.; Nikiforov, B.; De Sario, M.; Grize, L.; Heinrich, J.; Hruba, F.; Janssen, N.; Luttmann-Gibson, H.; et al. Parental smoking and children’s respiratory health: Independent effects of prenatal and postnatal exposure. Tob. Control. 2006, 15, 294–301. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, G.R.; Lang, J.J.; Tremblay, M.S.; Dale, M.; LeBlanc, A.G.; Belanger, K.; Ortega, F.B.; Léger, L. International normative 20 m shuttle run values from 1142026 children and youth representing 50 countries. Br. J. Sports Med. 2017, 51, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.C.V.; Pereira, G.V.A.; Silva, M.X.; Sbolli, K.; Ribeiro, E.R. Effects of passive smoking on the health of children and adolescents: A systematic review. Res. Soc. Dev. 2021, 10, e582101321275. [Google Scholar] [CrossRef]
- Eisner, M.D.; Forastiere, F. Passive smoking, lung function, and public health. Am. J. Respir. Crit. Care Med. 2006, 173, 1184–1185. [Google Scholar] [CrossRef]
- Li, Y.F.; Gilliland, F.D.; Berhane, K.; McConnell, R.; Gauderman, W.J.; Rappaport, E.B.; Peters, J.M. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Milanzi, E.B.; Koppelman, G.H.; Smit, H.A.; Wijga, A.H.; Vonk, J.M.; Brunekreef, B.; Gehring, U. Timing of secondhand smoke, pet, dampness or mould exposure and lung function in adolescence. Thorax 2020, 75, 153–163. [Google Scholar] [CrossRef]
- Strachan, D.P.; Cook, D.G. Health effects of passive smoking. 6. Parental smoking and childhood asthma: Longitudinal and case-control studies. Thorax 1998, 53, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Thacher, J.D.; Schultz, E.S.; Hallberg, J.; Hellberg, U.; Kull, I.; Thunqvist, P.; Pershagen, G.; Gustafsson, P.M.; Melén, E.; Bergström, A. Tobacco smoke exposure in early life and adolescence in relation to lung function. Eur. Respir. J. 2018, 51, 1702111. [Google Scholar] [CrossRef]
- Fernández-Plata, R.; Rojas-Martínez, R.; Martínez-Briseño, D.; García-Sancho, C.; Pérez-Padilla, R. Effect of Passive Smoking on the Growth of Pulmonary Function and Respiratory Symptoms in Schoolchildren. Rev. Investig. Clin. 2016, 68, 119–127. [Google Scholar]
- Goić-Barišić, I.; Bradarić, A.; Erceg, M.; Barišić, I.; Foretić, N.; Pavlov, N. Influence of Passive Smoking on Basic Anthropometric Characteristics and Respiratory Function in Young Athletes. Coll. Antropol. 2006, 30, 615–619. Available online: https://hrcak.srce.hr/27574 (accessed on 5 September 2024).
- Li, Y.; Hecht, S.S. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, Y.; Qian, H.; Zheng, X.; Huang, C.; Zhang, Y.; Li, B.; Zhao, Z.; Deng, Q.; Yang, X.; Sun, Y.; et al. Effects of parental smoking and indoor tobacco smoke exposure on respiratory outcomes in children. Sci. Rep. 2020, 10, 4311. [Google Scholar] [CrossRef] [PubMed]
- Topalušić, I.; Stipić Marković, A.; Artuković, M.; Dodig, S.; Bucić, L.; Lugović Mihić, L. Divergent Trends in the Prevalence of Children’s Asthma, Rhinitis and Atopic Dermatitis and Environmental Influences in the Urban Setting of Zagreb, Croatia. Children 2022, 9, 1788. [Google Scholar] [CrossRef]
- Moshammer, H.; Hoek, G.; Luttmann-Gibson, H.; Neuberger, M.A.; Antova, T.; Gehring, U.; Hruba, F.; Pattenden, S.; Rudnai, P.; Slachtova, H.; et al. Parental smoking and lung function in children: An international study. Am. J. Respir. Crit. Care Med. 2006, 173, 1255–1263. [Google Scholar] [CrossRef]
- López-Gil, J.F.; Del Pozo-Cruz, J.; Del Pozo Cruz, B.; Tárraga-López, P.J.; García-Hermoso, A. Environmental tobacco smoke exposure and 24-h movement guidelines in Spanish young people. Transl. Pediatr. 2023, 12, 1327–1335. [Google Scholar] [CrossRef]
- Parnell, M.; Gee, I.; Foweather, L.; Whyte, G.; Knowles, Z.; Dickinson, J. The Impact of Environmental Tobacco Smoke Exposure on Cardiorespiratory Fitness in Children: A Pilot Study. Int. J. Environ. Impacts Manag. Mitig. Recover. 2019, 2, 240–248. [Google Scholar] [CrossRef]
- Pavić, I.; Jurica, S.A.; Pavić, P.; Bogović, J.C.; Krmek, M.; Dodig, S. The Effects of Parental Smoking on Anthropometric Parameters, Peak Expiratory Flow Rate and Physical Condition in School Children. Coll. Antropol. 2014, 38, 189–194. [Google Scholar]
- Erkelenz, N.; Schreiber, A.; Kobel, S. Relationship of parental health-related behaviours and physical fitness in girls and boys. J. Public. Health 2014, 22, 407–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaakkola, J.M.; Rovio, S.P.; Pahkala, K.; Viikari, J.; Rönnemaa, T.; Jula, A.; Niinikoski, H.; Mykkänen, J.; Juonala, M.; Hutri-Kähönen, N.; et al. Childhood exposure to parental smoking and life-course overweight and central obesity. Ann. Med. 2021, 53, 208–216. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Juonala, M.; Ronnemaa, T. Cohort profile: The cardiovascular risk in young Finns study. Int. J. Epidemiol. 2008, 37, 1220–1226. [Google Scholar] [CrossRef]
- Simell, O.; Niinikoski, H.; Rönnemaa, T. Cohort profile: The STRIP study (special Turku coronary risk factor intervention project), an infancy-onset dietary and life-style intervention trial. Int. J. Epidemiol. 2009, 38, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Trinh, T.A.; Hallam, K.T. The links between parental smoking and childhood obesity: Data of the longitudinal study of Australian children. BMC Public. Health 2024, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Nadhiroh, S.R.; Djokosujono, K.; Utari, D.M. The association between secondhand smoke exposure and growth outcomes of children: A systematic literature review. Tob. Induc. Dis. 2020, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, A.; Smith, L.; Oh, H.; Yang, L.; Jackson, S.E.; Haro, J.M.; Shin, J.I.I.; Carvalho, A.F.; Jacob, L. Secondhand Smoking and Obesity Among Nonsmoking Adolescents Aged 12–15 Years From 38 Low- and Middle-Income Countries. Nicotine Tob. Res. 2020, 22, 2014–2021. [Google Scholar] [CrossRef]
- Lisboa, P.C.; de Oliveira, E.; de Moura, E.G. Obesity and endocrine dysfunction programmed by maternal smoking in pregnancy and lactation. Front. Physiol. 2012, 3, 437. [Google Scholar] [CrossRef]
- Alkerwi, A.; Baydarlioglu, B.; Sauvageot, N.; Stranges, S.; Lemmens, P.; Shivappa, N.; Hébert, J.R. Smoking status is inversely associated with overall diet quality: Findings from the ORISCAV-LUX study. Clin. Nutr. 2017, 36, 1275–1282. [Google Scholar] [CrossRef]
- Hancox, R.J.; Rasmussen, F. Does physical fitness enhance lung function in children and young adults? Eur. Respir. J. 2018, 51, 1701374. [Google Scholar] [CrossRef]
- Becher, H.; Zatonski, W.; Jöckel, K.H. Passive smoking in Germany and Poland: Comparison of exposure levels, sources of exposure, validity, and perception. Epidemiology 1992, 3, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E.; Preston-Martin, S.; Sarracci, R.; Haley, N.J.; Trichopoulos, D.; Becher, H.; Burch, J.D.; Fontham, E.T.; Gao, Y.T.; Jindal, S.K.; et al. Exposure to nonsmoking women to environmental tobacco smoke: A 10-country collaborative study. Cancer Causes Control 1990, 1, 243–252. [Google Scholar] [CrossRef]
- Jurado, D.; Muñoz, C.; Luna, J.D.D.; Fernández-Crehuet, M. Environmental tobacco smoke exposure in children: Parental perception of smokiness at home and other factors associated with urinary cotinine in preschool children. J. Expo. Sci. Environ. Epidemiol. 2004, 14, 330–336. [Google Scholar] [CrossRef]
| Mean | Standard Deviation (SD) | ||
|---|---|---|---|
| Age (years) | 12.55 | 1.05 | |
| Median | Minimum | Maximum | |
| BMI (z-score) | 0.17 | −2.71 | 2.33 |
| FVC (z-score) | −0.05 | −2.64 | 1.92 |
| FEV1 (z-score) | −0.40 | −2.69 | 2.13 |
| FEV1/FVC (Tiffeneau) z-score | −0.20 | −3.34 | 2.28 |
| PEF (z-score) | −0.72 | −3.64 | 2.96 |
| Count (N) | Percentage % | ||
| Sex | Boys | 89 | 56.7% |
| Girls | 68 | 43.3% | |
| Active sport | No | 80 | 51.0% |
| Yes | 77 | 49.0% | |
| Every day secondhand smoke exposure in a household | No | 59 | 37.6% |
| Yes | 98 | 62.4% | |
| Father–smoking status | No | 82 | 52.2% |
| Yes | 75 | 47.8% | |
| Mother–smoking status | No | 78 | 49.7% |
| Yes | 79 | 50.3% | |
| Parents–smoking status | No | 59 | 37.6% |
| Only one parent | 42 | 26.7% | |
| Both mother and father | 56 | 35.7% | |
| Mother–smoking status during pregnancy | No | 97 | 61.8% |
| Yes | 60 | 38.2% | |
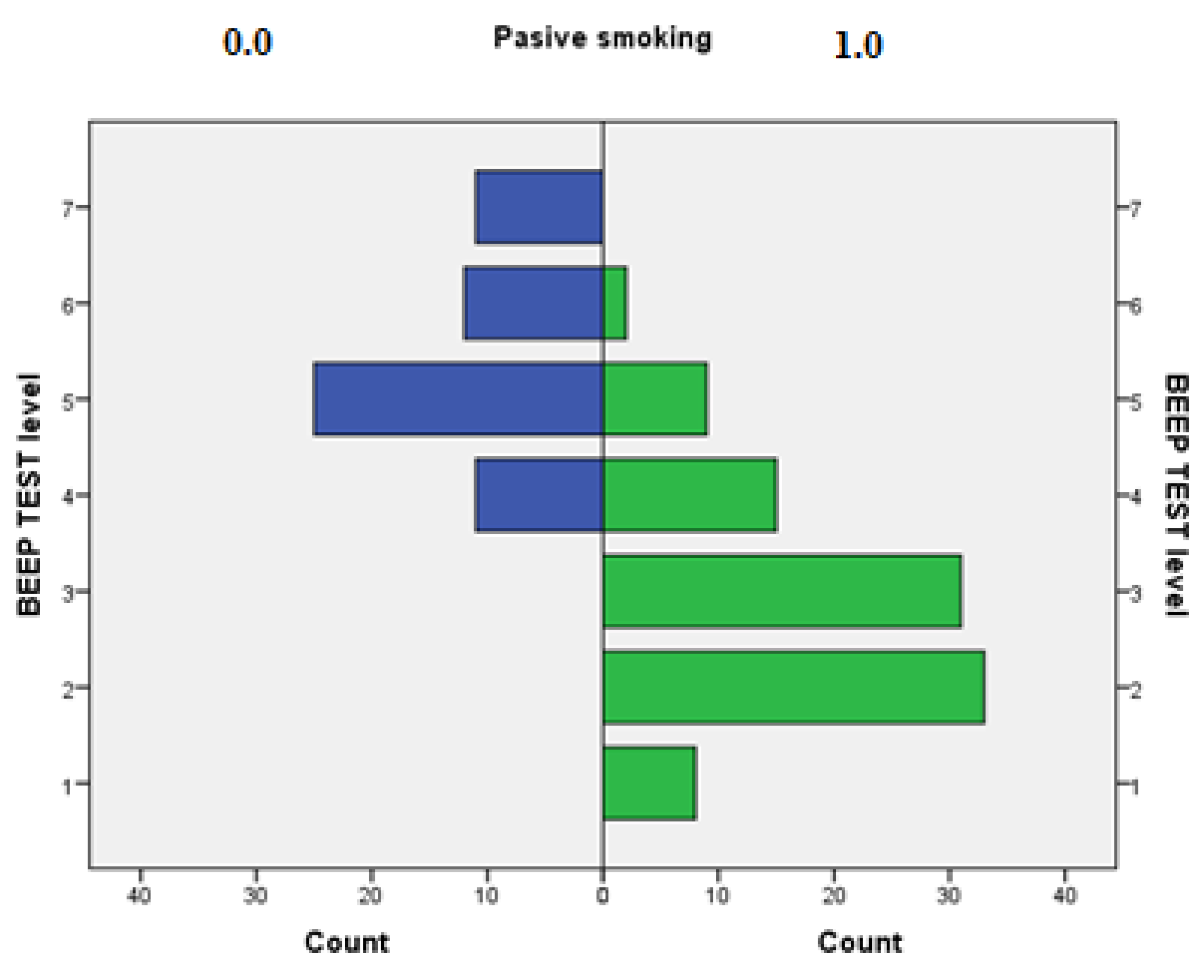
| BEEP TEST level | <P5 = very poor (level 1) | 8 | 5.1% |
| P5–P20 = poor (level 2) | 33 | 21.0% | |
| P20–P40 = fair (level 3) | 31 | 19.7% | |
| P40–P60 = average (level 4) | 26 | 16.6% | |
| P60–P80 = good (level 5) | 34 | 21.7% | |
| P80–P95 = very good (level 6) | 14 | 8.9% | |
| >P95 = excellent (level 7) | 11 | 7.0% | |
| FVC (z-Score) | FEV1 (z-Score) | FEV1/FVC (Tiffeneau) z-Score | PEF (z-Score) | BMI (z-Score) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (Minimum, Maximum) | p-Value | Median (Minimum, Maximum) | p-Value | Median (Minimum, Maximum) | p-Value | Median (Minimum, Maximum) | p-Value | Median (Minimum, Maximum) | p-Value | ||
| Sex | male | −0.01 (−2.39. 1.32) | p = 0.694 | −0.42 (−2.69. 1.71) | p = 0.347 | −0.34 (−2.84. 2.28) | p = 0.572 | −0.87 (−3.64. 2.53) | p = 0.134 | 0.17 (−2.71. 2.33) | p = 0.441 |
| female | −0.15 (−2.64. 1.92) | −0.19 (−2.54. 2.13) | 0.005 (−3.34. 2.25) | −0.53 (−2.54. 2.96) | 0.105 (−2.12. 1.96) | ||||||
| Active in sport | No | −0.115 (−2.64. 1.92) | p = 0.178 | −0.4 (−2.69. 2.13) | p = 0.112 | −0.145 (−3.34. 2.28) | p = 0.368 | −0.675 (−3.31. 2.04) | p= 0.384 | 0.12 (−2.71. 2.33) | p = 0.601 |
| Yes | 0.06 (−1.94. 1.32) | −0.35 (−1.99. 1.85) | −0.2 (−2.25. 2.25) | −0.74 (−3.64. 2.96) | 0.17 (−2.08. 1.93) | ||||||
| Passive smoking exposure | No | 0.47 (−0.65. 1.92) | p < 0.001 | 0.57 (−0.4. 2.13) | p < 0.001 | 0.51 (−0.91. 2.28) | p < 0.001 | 0.83 (−0.43. 2.96) | p < 0.001 | −0.03 (−2.38. 1.92) | p = 0.018 |
| Yes | −0.395 (−2.64. 1.2) | −0.88 (−2.69. 1.22) | −0.73 (−3.34. 1.65) | −1.19 (−3.64. 0.53) | 0.59 (−2.71. 2.33) | ||||||
| Father–smoking status | No | 0.265 (−2.64. 1.92) | p < 0.001 | 0.325 (−1.71. 2.13) | p < 0.001 | 0.34 (−2.84. 2.28) | p < 0.001 | 0.485 (−2.54. 2.96) | p < 0.001 | 0 (−2.71. 2.08) | p = 0.046 |
| Yes | −0.39 (−2.39. 1.2) | −0.89 (−2.69. 1.22) | −0.7 (−3.34. 1.65) | −1.17 (−3.64. 0.53) | 0.57 (−2.08. 2.33) | ||||||
| Mother–smoking status | No | 0.32 (−2.11. 1.92) | p < 0.001 | 0.375 (−1.61. 2.13) | p < 0.001 | 0.395 (−2.25. 2.28) | p < 0.001 | 0.515 (−3.64. 2.96) | p < 0.001 | −0.02 (−2.38. 1.92) | p = 0.011 |
| Yes | −0.55 (−2.64. 1.2) | −0.93 (−2.69. 0.37) | −0.75 (−3.34. 1.07) | −1.18 (−3.31. 0.04) | 0.64 (−2.71. 2.33) | ||||||
| Smoking exposure during pregnancy | No | 0.17 (−2.11. 1.92) | p < 0.018 | 0.19 (−1.88. 2.13) | p < 0.046 | 0.1 (−2.25. 2.28) | p < 0.001 | 0.26 (−3.64. 2.96) | p < 0.016 | 0 (−2.71. 2.12) | p = 0.009 |
| Yes | −0.505 (−2.64. 1.2) | −0.885 (−2.69. −0.03) | −0.76 (−3.34. 1.07) | −1.21 (−3.31. −0.03) | 0.76 (−2.12. 2.33) | ||||||
| FVC (z-Score) | FEV1 (z-Score) | FEV1/FVC (Tiffeneau) z-Score | PEF (z-Score) | BMI (z-Score) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (Minimum, Maximum) | p | Median (Minimum, Maximum | p | Median (Minimum, Maximum | p | Median (Minimum, Maximum | p | Median (Minimum, Maximum | ||
| BEEP TEST level | 1 | −0.83 (−1.69. −0.24) | p < 0.001 | −1.065 (−1.5. −0.08) | p < 0.001 | −0.62 (−0.94. 1.02) | p < 0.001 | −1.44 (−2.4. −0.21) | p < 0.001 | 1.655 (−0.29. 2.33) |
| 2 | −0.32 (−2.64. 1.11) | −0.79 (−2.69. 1.22) | −0.68 (−2.84. 1.65) | −1.03 (−3.64. 0.53) | 1.01 (−1.1. 2.08) | |||||
| 3 | −0.48 (−1.8. 1.2) | −0.93 (−2.54. −0.12) | −0.98 (−3.34. 0.99) | −1.24 (−3.31. −0.45) | 0.16 (−2.71. 1.61) | |||||
| 4 | 0.005 (−1.42. 1.32) | −0.3 (−1.81. 1.71) | −0.28 (−2.72. 1.57) | −0.3 (−2.53. 1.59) | −0.02 (−2.12. 1.92) | |||||
| 5 | 0.25 (−1.85. 1.92) | 0.4 (−1.99. 2.13) | 0.12 (−1.2. 2.28) | 0.825 (−2.9. 2.96) | 0.135 (−2.08. 1.77) | |||||
| 6 | 0.45 (−0.31. 1.32) | 0.45 (−0.2. 1.25) | 0.42 (−0.2. 1.7) | 0.595 (−1.08. 2.04) | −0.375 (−2.38. 0.97) | |||||
| 7 | 0.51 (−0.3. 1.3) | 0.57 (−0.13. 1.56) | 0.43 (−0.4. 1.29) | 0.7 (−0.43. 2.53) | −0.61 (−1.37. 0.39) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavić, I.; Topalušić, I.; Poljičanin, T.; Hofmann Jaeger, O.; Žaja, S.; Stipić Marković, A. Secondhand Smoke Exposure and Its Impact on Pediatric Lung Function, Aerobic Fitness, and Body Mass: Evidence from a Cross-Sectional Study. Children 2024, 11, 1250. https://doi.org/10.3390/children11101250
Pavić I, Topalušić I, Poljičanin T, Hofmann Jaeger O, Žaja S, Stipić Marković A. Secondhand Smoke Exposure and Its Impact on Pediatric Lung Function, Aerobic Fitness, and Body Mass: Evidence from a Cross-Sectional Study. Children. 2024; 11(10):1250. https://doi.org/10.3390/children11101250
Chicago/Turabian StylePavić, Ivan, Iva Topalušić, Tamara Poljičanin, Ozana Hofmann Jaeger, Sara Žaja, and Asja Stipić Marković. 2024. "Secondhand Smoke Exposure and Its Impact on Pediatric Lung Function, Aerobic Fitness, and Body Mass: Evidence from a Cross-Sectional Study" Children 11, no. 10: 1250. https://doi.org/10.3390/children11101250
APA StylePavić, I., Topalušić, I., Poljičanin, T., Hofmann Jaeger, O., Žaja, S., & Stipić Marković, A. (2024). Secondhand Smoke Exposure and Its Impact on Pediatric Lung Function, Aerobic Fitness, and Body Mass: Evidence from a Cross-Sectional Study. Children, 11(10), 1250. https://doi.org/10.3390/children11101250







