Risk Factors for Urinary Tract Infections in Children with Hematuria in the Emergency Department
Abstract
:1. Introduction
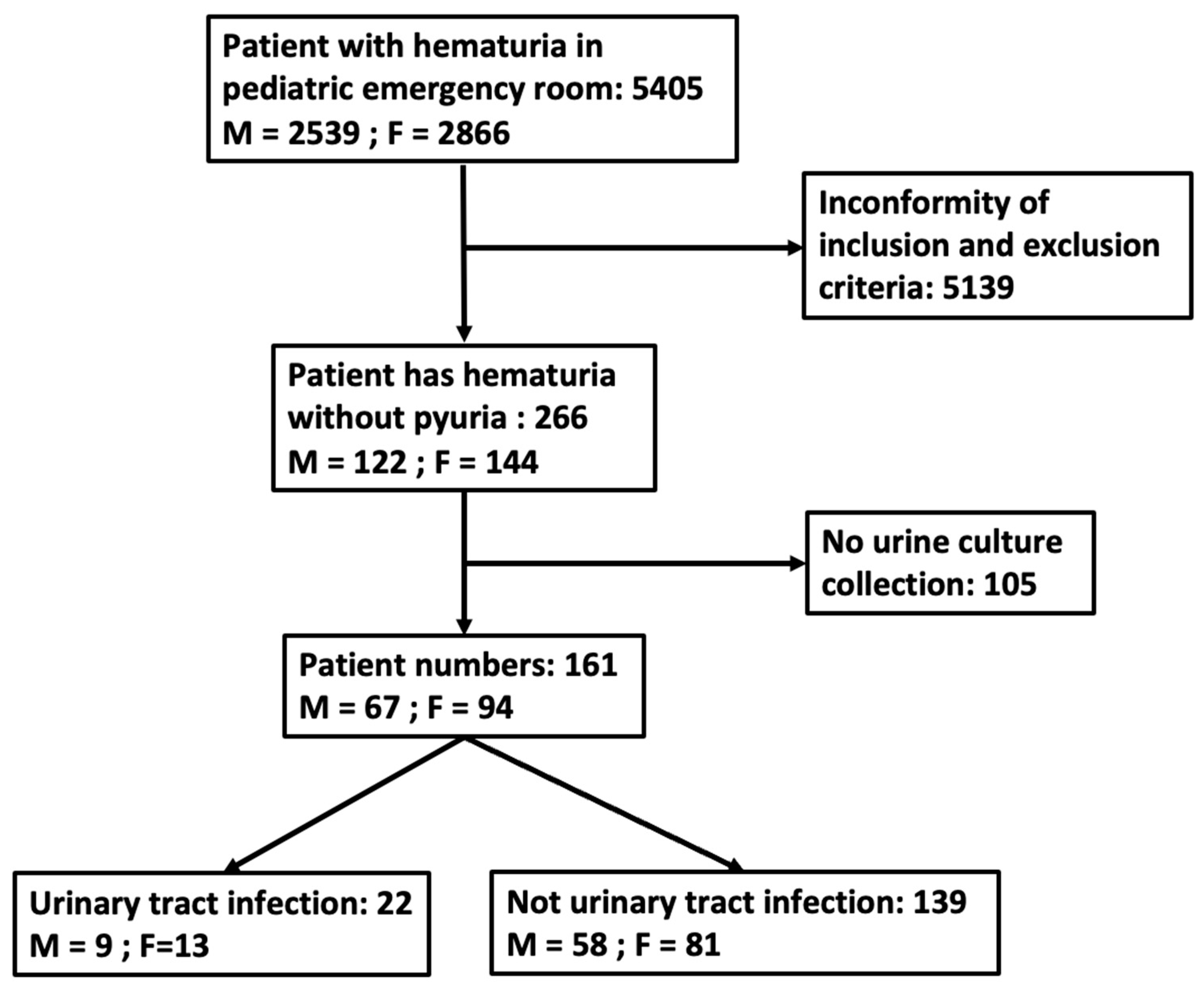
2. Materials and methods
2.1. Patient Population
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Demographics
3.2. Causes of Hematuria and UTI Pathogen
3.3. Presentation of Hematuria in Pediatric ED Patients without Pyuria
3.4. Prediction of UTIs in Pediatric Patients with Hematuria Based on the Two Age Groups
4. Discussion
4.1. Limitations
4.2. Future prospect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Leung, A.K.; Wong, A.H.; Leung, A.A.; Hon, K.L. Urinary tract infection in children. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 2–18. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, J.; Song, M.; Ma, L.; Pan, T.; Yang, Q.; Zhang, W. Clinicopathologic analysis of isolated hematuria in child/adolescent and adult patients. Pol. J. Pathol. 2015, 66, 353–360. [Google Scholar] [CrossRef]
- Ding, J.Y.; Ibañez, D.; Gladman, D.D.; Urowitz, M.B. Isolated hematuria and sterile pyuria may indicate systemic lupus erythematosus activity. J. Rheumatol. 2015, 42, 437–440. [Google Scholar] [CrossRef]
- Bignall, O.N.R., 2nd; Dixon, B.P. Management of Hematuria in Children. Curr. Treat. Options Pediatr. 2018, 4, 333–349. [Google Scholar] [CrossRef]
- Utsch, B.; Klaus, G. Urinalysis in children and adolescents. Dtsch. Ärztebl. Int. 2014, 111, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.-K.; Shenoy, M.A. Approach to the Child with Hematuria and/or Proteinuria. Pediatr. Nephrol. 2021, 235–252. [Google Scholar] [CrossRef]
- Viteri, B.; Reid-Adam, J. Hematuria and Proteinuria in Children. Pediatr. Rev. 2018, 39, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Balighian, E.; Burke, M. Urinary tract infections in children. Pediatr. Rev. 2018, 39, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Okarska-Napierala, M.; Wasilewska, A.; Kuchar, E. Urinary tract infection in children: Diagnosis, treatment, imaging—Comparison of current guidelines. J. Pediatr. Urol. 2017, 13, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Dogan, H.S.; Hoebeke, P.; Kocvara, R.; Nijman, R.J.; Radmayr, C.; Tekgul, S.; The European University Association; European Society for Paediatric Urology. Urinary tract infections in children: EAU/ESPU guidelines. Eur. Urol. 2015, 67, 546–558. [Google Scholar] [CrossRef] [PubMed]
- ‘t Hoen, L.A.; Bogaert, G.; Radmayr, C.; Dogan, H.S.; Nijman, R.J.M.; Quaedackers, J.; Rawashdeh, Y.F.; Silay, M.S.; Tekgul, S.; Bhatt, N.R.; et al. Update of the EAU/ESPU guidelines on urinary tract infections in children. J. Pediatr. Urol. 2021, 17, 200–207. [Google Scholar] [CrossRef]
- Hamilton, J.L.; John, S.P. Evaluation of fever in infants and young children. Am. Fam. Physician 2013, 87, 254–260. [Google Scholar]
- O’Brien, K.; Edwards, A.; Hood, K.; Butler, C.C. Prevalence of urinary tract infection in acutely unwell children in general practice: A prospective study with systematic urine sampling. Br. J. Gen. Pract. 2013, 63, e156–e164. [Google Scholar] [CrossRef]
- Robinson, J.L.; Finlay, J.C.; Lang, M.E.; Bortolussi, R.; Canadian Paediatric Society; Community Paediatrics Committee; Infectious Diseases and Immunization Committee. Urinary tract infection in infants and children: Diagnosis and management. Paediatr. Child Health 2014, 19, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Veauthier, B.; Miller, M.V. Urinary tract infections in young children and infants: Common questions and answers. Am. Fam. Physician 2020, 102, 278–285. [Google Scholar] [PubMed]
- White, B. Diagnosis and treatment of urinary tract infections in children. Am. Fam. Physician 2011, 83, 409–415. [Google Scholar] [PubMed]
- Marques, A.G.; Pasternak, J.; Damascena, M.D.S.; França, C.N.; Martino, M.D.V. Performance of the dipstick screening test as a predictor of negative urine culture. Einstein 2017, 15, 34–39. [Google Scholar] [CrossRef] [PubMed]
- St John, A.; Boyd, J.C.; Lowes, A.J.; Price, C.P. The use of urinary dipstick tests to exclude urinary tract infection: A systematic review of the literature. Am. J. Clin. Pathol. 2006, 126, 428–436. [Google Scholar] [CrossRef]
- Simati, B.; Kriegsman, B.; Safranek, S. Dipstick urinalysis for the diagnosis of acute UTI. Am. Fam. Physician 2013, 87. [Google Scholar]
- Bharara, T.; Sharma, A.; Gur, R.; Duggal, S.D.; Jena, P.P.; Kumar, A. Predictive Role of Proteinuria in Urinary Tract Infection. J. Clin. Diagn. Res. 2017, 11, DC01–DC03. [Google Scholar] [CrossRef]
- Patel, H.P.; Bissler, J.J. Hematuria in children. Pediatr. Clin. 2001, 48, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Mambatta, A.K.; Jayarajan, J.; Rashme, V.L.; Harini, S.; Menon, S.; Kuppusamy, J. Reliability of dipstick assay in predicting urinary tract infection. J. Fam. Med. Prim. Care 2015, 4, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Magrini, L.; Gagliano, G.; Travaglino, F.; Vetrone, F.; Marino, R.; Cardelli, P.; Salerno, G.; Di Somma, S. Comparison between white blood cell count, procalcitonin and C reactive protein as diagnostic and prognostic biomarkers of infection or sepsis in patients presenting to emergency department. Clin. Chem. Lab. Med. 2014, 52, 1465–1472. [Google Scholar] [CrossRef]
- Bergstein, J.; Leiser, J.; Andreoli, S. The clinical significance of asymptomatic gross and microscopic hematuria in children. Arch. Pediatr. Adolesc. Med. 2005, 159, 353–355. [Google Scholar] [CrossRef]
- Greenfield, S.P.; Williot, P.; Kaplan, D. Gross hematuria in children: A ten-year review. Urology 2007, 69, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Parray, N.A.; Malla, R.A.; Rasool, S.; Ahmed, K. Hematuria in children. Int. J. Clin. Pediatr. 2013, 2, 51–60. [Google Scholar] [CrossRef]
- Ingelfinger, J.R.; Davis, A.E.; Grupe, W.E. Frequency and etiology of gross hematuria in a general pediatric setting. Pediatrics 1977, 59, 557–561. [Google Scholar] [CrossRef]
- Chung, H.-M.; Liao, Y.-M.; Tsai, Y.-C.; Liu, M.-C. Microscopic hematuria in children. Urol. Sci. 2011, 22, 93–96. [Google Scholar] [CrossRef]
- Sepahi, M.A.; Sepahi, M.H.A. Etiology of Hematuria in Children: A Review Article. J. Pediatr. Nephrol. 2022, 10, 149. [Google Scholar] [CrossRef]
- Kallash, M.; Rheault, M.N. Approach to persistent microscopic hematuria in children. Kidney360 2020, 1, 1014. [Google Scholar] [CrossRef]
- Santangelo, L.; Netti, G.S.; Giordano, P.; Carbone, V.; Martino, M.; Torres, D.D.; Rossini, M.; Di Palma, A.M.; Gesualdo, L.; Giordano, M. Indications and results of renal biopsy in children: A 36-year experience. World J. Pediatr. 2018, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Timeline–COVID19; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Binagwaho, A.; Senga, J. Children and adolescent mental health in a time of COVID-19: A forgotten priority. Ann. Glob. Health 2021, 87, 57. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M. Multisystem inflammatory syndrome in children (MIS-C). Curr. Allergy Asthma Rep. 2022, 22, 53–60. [Google Scholar] [CrossRef]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar]
- Generalić, A.; Davidović, M.; Kos, I.; Vrljičak, K.; Lamot, L. Hematuria as an early sign of multisystem inflammatory syndrome in children: A case report of a boy with multiple comorbidities and review of literature. Front. Pediatr. 2021, 9, 760070. [Google Scholar] [CrossRef] [PubMed]
- Massengill, S.F. Hematuria. Pediatr. Rev. 2008, 29, 342. [Google Scholar] [CrossRef]
- Wise, G.J.; Schlegel, P.N. Sterile pyuria. N. Engl. J. Med. 2015, 372, 1048–1054. [Google Scholar] [CrossRef]
- Saleem, M.O.; Hamawy, K. Hematuria. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]

| Items | N (%/Mean ± SD) |
|---|---|
| Numbers | 161 |
| Mean age (years) | 6.0 ± 5.5 |
| Gender (M/F) | 67: 94 |
| Clinical presentation | |
| Fever (%) | 83 (51.6) |
| Fever (highest temperature at PER, °C) | 39.3 ± 0.8 |
| Gross hematuria | 54 (33.5) |
| Cough | 26 (16.1) |
| Rhinorrhea | 26 (16.1) |
| Nausea or vomiting | 17 (10.6) |
| Diarrhea | 3 (1.9) |
| Upper or periumbilical pain | 5 (3.1) |
| Lower abdominal pain | 10 (6.2) |
| Flank pain | 17 (10.6) |
| Dysuria | 40 (24.8) |
| Urine frequency | 10 (6.2) |
| Urine discharge | 2 (1.2) |
| Laboratory data examinations | |
| WBC (/mm3) | 89 (55.2/10,991 ± 5364) |
| Neutrophil (%) | 89 (55.2/59.7 ± 16.4) |
| CRP (mg/dl) | 85 (52.8/3.46 ± 5.69) |
| Urine RBC (/µL) | 161 (100/271 ± 410) |
| Urine WBC (/µL) | 161 (100/10 ± 8) |
| Hospitalization (days) | 44 (27.3/4.8 ± 3.7) |
| Antibiotics treatment | 90(55.9) |
| Items | N (%/mean ± SD) | p-Value | |||
|---|---|---|---|---|---|
| Ages (Years) | Age < 2 | 2 ≤ Age < 7 | 7 ≤ Age < 13 | 13 ≤ Age < 18 | |
| Mean age (years) a | 0.7 ± 0.5 | 3.5 ± 1.2 | 8.5 ± 1.4 | 15.6 ± 1.3 | <0.001 |
| Numbers | 38 | 67 | 24 | 32 | |
| Gender (M/F) | 16/22 | 27/40 | 9/15 | 15/17 | 0.908 |
| Clinical presentation | |||||
| Fever a | 36 (94.7) | 35 (52.2) | 7 (29.1) | 5 (15.6) | <0.001 |
| Fever (highest temperature at PER, °C) | 39.3 ± 0.9 | 39.4 ± 0.7 | 39.3 ± 0.6 | 38.6 ± 0.5 | 0.068 |
| Gross hematuria a | 3 (7.9) | 22 (32.8) | 10 (41.7) | 19 (59.4) | <0.001 |
| Cough | 6 (15.8) | 12 (18.0) | 6 (25.0) | 2 (6.3) | 0.255 |
| Rhinorrhea | 6 (15.8) | 11 (16.4) | 7 (29.2) | 2 (6.3) | 0.156 |
| Nausea or vomiting | 4 (10.5) | 8 (12.0) | 2 (8.3) | 3 (9.4) | 1.000 |
| Diarrhea | 2 (5.2) | 1 (1.5) | 0 (0) | 0 (0) | 0.341 |
| Upper or periumbilical pain | 0 (0) | 3 (4.5) | 1 (4.2) | 1 (3.1) | 0.651 |
| Lower abdominal pain a | 0 (0) | 0 (0) | 1 (4.2) | 9 (28.1) | <0.001 |
| Flank pain a | 0 (0) | 2 (3.0) | 2 (8.3) | 13 (31.3) | <0.001 |
| Dysuria a | 1 (2.6) | 21 (31.3) | 9 (37.5) | 9 (28.1) | <0.001 |
| Urine frequency | 0 (0) | 4 (6.0) | 1 (4.2) | 5 (15.6) | 0.056 |
| Urine discharge | 1 (2.6) | 1 (1.5) | 0 (0) | 0 (0) | 1.000 |
| Laboratory data examinations | |||||
| WBC (/mm3) | 11,445 ± 7670 | 11,762 ± 5259 | 9503 ± 2510 | 10,337 ± 3376 | 0.300 |
| Neutrophil (%) a | 49.4 ± 18.7 | 60.6 ± 14.2 | 65.3 ± 14.3 | 66.2 ± 12.2 | 0.007 |
| CRP (mg/dL) a | 4.44 ± 8.34 | 4.26 ± 4.92 | 2.13 ± 2.70 | 1.39 ± 2.25 | 0.018 |
| Urine RBC (/µL) a | 44 ± 160 | 225 ± 372 | 300 ± 395 | 642 ± 460 | <0.001 |
| Urine WBC (/µL) | 12 ± 8 | 10 ± 7 | 8 ± 7 | 10 ± 8 | 0.158 |
| Hospitalization (numbers) a | 18 | 20 | 3 | 3 | 0.001 |
| Hospitalization (days) a | 4.4 ± 1.9 | 5.2 ± 5.1 | 6.3 ± 3.2 | 2.7 ± 0.6 | 0.048 |
| Antibiotics treatment a | 14 (36.8) | 40 (59.7) | 17 (70.8) | 19 (59.4) | 0.040 |
| Variables | UTI (n = 22) | Other Causes, non-UTI (n = 139) | p-Value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age | ||||||
| 0–2 | 5 | 22.7 | 33 | 27.4 | 1.000 | |
| 2–7 | 7 | 31.8 | 60 | 43.2 | 0.316 | |
| 7–13 | 1 | 4.6 | 23 | 16.6 | 0.252 | |
| 13–18 a | 9 | 40.9 | 23 | 16.6 | 0.008 | |
| Male sex | 9 | 40.9 | 58 | 41.7 | 0.942 | |
| Clinical symptoms | ||||||
| Fever | 9 | 40.9 | 74 | 53.2 | 0.282 | |
| Gross hematuria | 8 | 36.4 | 46 | 33.1 | 0.746 | |
| Cough | 1 | 4.6 | 25 | 18.0 | 0.201 | |
| Rhinorrhea | 1 | 4.6 | 25 | 18.0 | 0.201 | |
| Nausea or vomiting | 2 | 9.1 | 15 | 10.8 | 1.000 | |
| Diarrhea | 0 | 0 | 3 | 2.2 | 1.000 | |
| Urine discharge | 0 | 0 | 2 | 1.4 | 1.000 | |
| Urine frequency | 3 | 13.6 | 7 | 5.0 | 0.281 | |
| Dysuria a | 11 | 50 | 29 | 20.9 | 0.003 | |
| Upper or periumbilical pain | 0 | 0 | 5 | 3.6 | 0.808 | |
| Lower abdominal pain | 3 | 13.6 | 7 | 5.0 | 0.281 | |
| Flank pain | 4 | 18.2 | 13 | 9.4 | 0.380 | |
| Hospitalization | 7 | 31.8 | 37 | 26.6 | 0.611 | |
| Urine examination | ||||||
| Urine OB | + | 17 | 77.3 | 79 | 56.2 | 0.114 |
| Urine protein | + | 13 | 59.1 | 73 | 52.5 | 0.566 |
| Urine nitrite | + | 2 | 9.1 | 2 | 1.4 | 0.160 |
| Urine esterase a | + | 5 | 22.7 | 5 | 3.6 | 0.003 |
| Urine bacteria | + | 1 | 4.6 | 4 | 2.9 | 1.000 |
| Cutoff values | ||||||
| Age > 8 years a | 10 | 45.5 | 32 | 23.0 | 0.026 | |
| Urine protein > 30 mg/dL a | 7 | 31.8 | 13 | 9.4 | 0.003 | |
| Blood WBC amount > 9300/mm3 a | 13 | 86.7 | 40 | 53.3 | 0.042 | |
| Variables’ population means | Mean ± SD (Number) | Mean ± SD (Number) | ||||
| Age (years) a | 8.1 ± 6.4 (22) | 5.6 ± 5.3 (139) | 0.133 | |||
| Fever | ||||||
| Days | 2.2 ± 1.6 (9) | 2.1 ± 1.7 (74) | 0.905 | |||
| Highest temperature, °C | 39.0 ± 0.8 (9) | 39.4 ± 0.8 (74) | 0.236 | |||
| Urine examinations | ||||||
| Gravity | 1.018 ± 0.009 (22) | 1.020 ± 0.009 (139) | 0.297 | |||
| PH | 6.2 ± 0.5 (22) | 6.3 ± 0.6 (139) | 0.567 | |||
| Protein a | 65.0 ± 106.6 (22) | 19.8 ± 45.2 (139) | 0.256 | |||
| RBC (/µL) | 403 ± 473 (22) | 250 ± 367 (139) | 0.176 | |||
| WBC (/µL) | 12 ± 7 (22) | 10 ± 8 (139) | 0.123 | |||
| Blood | ||||||
| WBC (/mm3) | 12,579 ± 5216 (15) | 10,669 ± 5372 (74) | 0.108 | |||
| Hb (g/dL) | 12.3 ± 1.7 (15) | 12.6 ± 1.5 (73) | 0.859 | |||
| PLT (×103/mm3) | 264.533 ± 89.443 (15) | 272.192 ± 82.128 (73) | 0.811 | |||
| Neutrophil (%) | 63.4 ± 17.7 (15) | 58.9 ± 16.1 (74) | 0.278 | |||
| BUN (mg/dL) | 12.1 ± 3.6 (9) | 12.4 ± 3.5 (23) | 0.849 | |||
| Creatinine (mg/dL) | 0.59 ± 0.18 (12) | 0.51 ± 0.25 (47) | 0.134 | |||
| CRP (mg/dL) | 3.96 ± 9.91 (14) | 2.73 ± 4.03 (71) | 0.247 | |||
| PT(s) | 11.0 ± 0.6 (4) | 11.2 ± 0.6 (11) | 0.947 | |||
| APTT(s) | 31.3 ± 1.0 (4) | 28.9 ± 2.9 (11) | 0.226 | |||
| Days of hospitalization | 4.6 ± 2.3 (7) | 4.8 ± 3.9 (38) | 0.830 | |||
| Variables | UTI (N = 17) | Other Causes, non-UTI (N = 106) | p-Value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age | ||||||
| 2–7 | 7 | 41.2 | 60 | 56.6 | 0.236 | |
| 7–13 | 1 | 5.9 | 23 | 21.7 | 0.231 | |
| 13–18 a | 9 | 52.9 | 23 | 21.7 | 0.006 | |
| Male sex | 5 | 29.4 | 46 | 43.4 | 0.411 | |
| Clinical symptoms | ||||||
| Fever | 4 | 23.5 | 43 | 40.6 | 0.283 | |
| Gross hematuria | 7 | 41.2 | 44 | 41.5 | 0.979 | |
| Cough | 1 | 5.9 | 19 | 17.9 | 0.371 | |
| Rhinorrhea | 1 | 5.9 | 19 | 17.9 | 0.371 | |
| Nausea or vomiting | 1 | 5.9 | 12 | 11.3 | 0.801 | |
| Diarrhea | 0 | 0 | 1 | 0.94 | 1.000 | |
| Urine discharge | 0 | 0 | 1 | 100 | 0.293 | |
| Urine frequency | 3 | 17.6 | 7 | 6.6 | 0.285 | |
| Dysuria a | 11 | 64.7 | 28 | 26.4 | 0.002 | |
| Upper or periumbilical pain | 0 | 0 | 5 | 4.7 | 0.800 | |
| Lower abdominal pain | 3 | 17.6 | 7 | 6.6 | 0.285 | |
| Flank pain | 4 | 23.5 | 13 | 9.4 | 0.384 | |
| Hospitalization | 4 | 23.5 | 22 | 20.8 | 1.000 | |
| Urine examinations | ||||||
| Urine OB | + | 14 | 82.3 | 66 | 62.3 | 0.181 |
| Urine protein | + | 12 | 70.6 | 56 | 52.8 | 0.172 |
| Urine nitrite | + | 0 | 0 | 2 | 1.9 | 1.000 |
| Urine esterase | + | 3 | 17.6 | 4 | 3.8 | 0.084 |
| Urine bacteria | + | 0 | 0 | 3 | 2.8 | 1.000 |
| Cutoff values | ||||||
| Age> 8 years a | 10 | 58.8 | 32 | 30.2 | 0.021 | |
| Urine protein > 30mg/dL a | 7 | 41.2 | 11 | 10.4 | 0.001 | |
| Urine RBC > 373/µL a | 9 | 52.9 | 30 | 28.3 | 0.043 | |
| Variables’ population means | Mean ± SD (Number) | Mean ± SD (Number) | ||||
| Age (years) a | 10.4 ± 5.6 (17) | 7.2 ± 5.1 (106) | 0.030 | |||
| Fever | ||||||
| Days | 3.0± 1.8 (4) | 2.2 ± 1.9 (43) | 0.236 | |||
| Highest temperature, °C | 39.0 ± 0.8 (4) | 39.4 ± 0.7 (43) | 0.552 | |||
| Urine examinations | ||||||
| Gravity | 1.022 ± 0.007 (17) | 1.018 ± 0.009 (106) | 0.112 | |||
| PH | 6.3 ± 0.4 (17) | 6.4 ± 0.6 (106) | 0.484 | |||
| Protein a | 83.5 ± 115.3 (17) | 22.4 ± 50.9 (106) | 0.045 | |||
| RBC (/µL) | 516 ± 483 (17) | 314 ± 425 (106) | 0.059 | |||
| WBC (/µL) | 11 ± 6 (17) | 9 ± 7 (106) | 0.138 | |||
| Blood | ||||||
| WBC (/mm3) | 12,255 ± 3950 (11) | 10,533 ± 4321 (54) | 0.149 | |||
| Hb (g/dL) | 12.7 ± 1.6 (11) | 12.9 ± 1.4(54) | 0.739 | |||
| PLT (×103/mm3) | 269.000 ± 47.722 (11) | 275.463 ± 77.124 (54) | 1.000 | |||
| Neutrophil (%) | 67.3 ± 13.0 (11) | 62.7 ± 13.9 (54) | 0.416 | |||
| BUN (mg/dL) | 12.7 ± 3.5 (7) | 12.3 ± 3.5 (23) | 0.941 | |||
| Creatinine (mg/dL) | 0.64 ± 0.16 (10) | 0.57 ± 0.24 (37) | 0.198 | |||
| CRP (mg/dL) | 1.49 ± 2.25 (10) | 2.63 ± 4.06 (52) | 0.237 | |||
| PT (s) | 11.0 ± 0.7 (3) | 11.2 ± 0.6 (11) | 0.874 | |||
| APTT (s) | 31.5 ± 1.1 (3) | 28.9 ± 2.9 (11) | 0.170 | |||
| Days of hospitalization | 3.8 ± 1.5 (4) | 5.3 ± 5.0 (22) | 0.741 | |||
| Variables | UTI (N = 5) | Other Causes, non-UTI (N = 33) | p-Value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Male sex | 4 | 80 | 12 | 36.4 | 0.175 | |
| Clinical symptoms | ||||||
| Fever | 5 | 100 | 31 | 93.4 | 1.000 | |
| Cough | 0 | 0 | 6 | 18.2 | 0.703 | |
| Rhinorrhea | 0 | 0 | 6 | 18.2 | 0.703 | |
| Nausea or vomiting | 1 | 20 | 3 | 9.1 | 1.000 | |
| Diarrhea | 0 | 0 | 2 | 6.1 | 0.583 | |
| Gross hematuria | 1 | 20 | 2 | 6.1 | 0.851 | |
| Hospitalization | 3 | 60 | 15 | 45.5 | 0.899 | |
| Urine examinations | ||||||
| Urine OB | + | 3 | 60 | 13 | 39.4 | 0.701 |
| Urine protein | + | 1 | 20 | 17 | 51.5 | 0.404 |
| Urine nitrite a | + | 2 | 40 | 0 | 0 | 0.008 |
| Urine esterase a | + | 2 | 40 | 1 | 3.0 | 0.049 |
| Urine bacteria | + | 1 | 20 | 1 | 3.0 | 0.611 |
| Cutoff values | ||||||
| Urine RBC > 8/uL a | 2 | 40 | 30 | 90.9 | 0.024 | |
| Variables’ population means | Mean ± SD (Number) | Mean ± SD (Number) | ||||
| Age (years) | 0.6 ± 0.5 (5) | 0.7 ± 0.5 (33) | 0.793 | |||
| Fever | ||||||
| Days | 1.6 ± 1.3 (5) | 1.9 ± 1.2 (31) | 0.342 | |||
| Highest temperature, °C | 39.0 ± 0.8 (5) | 39.4 ± 0.9 (31) | 0.311 | |||
| Urine examinations | ||||||
| Gravity | 1.014 ± 0.012 (5) | 1.018 ± 0.008 (33) | 0.385 | |||
| PH | 5.8 ± 0.7 (5) | 5.9± 0.5 (33) | 0.491 | |||
| Protein | 2.0 ± 4.5 (5) | 11.8 ± 14.9 (33) | 0.145 | |||
| RBC (/µL) | 20 ± 25 (5) | 47 ± 171 (33) | 0.123 | |||
| WBC (/µL) | 15 ± 9 (5) | 12 ± 8 (33) | 0.375 | |||
| Blood | ||||||
| WBC (/mm3) | 13,472.5 ± 8574.4 (4) | 11,039 ± 7653 (20) | 0.575 | |||
| Hb (g/dL) | 10.9 ± 1.6 (4) | 11.8 ± 1.3 (19) | 0.239 | |||
| PLT (×103/mm3) | 252.250 ± 171.661 (4) | 262.895 ± 96.657 (19) | 0.903 | |||
| Creatinine (mg/dL) | 52.4 ± 26.1 (4) | 48.8 ± 17.7 (20) | 0.615 | |||
| CRP (mg/dL) | 10.13 ± 18.41 (4) | 3.01 ± 4.04 (19) | 0.715 | |||
| Days of hospitalization | 5.7 ± 3.1 (3) | 4.1 ± 1.6 (15) | 0.427 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.-C.; Chen, C.-Y.; Huang, W.-Y.; Lin, W.-Y.; Chen, Y.-J.; Lee, T.-A.; Lin, M.-J.; Wu, H.-P. Risk Factors for Urinary Tract Infections in Children with Hematuria in the Emergency Department. Children 2024, 11, 248. https://doi.org/10.3390/children11020248
Guo B-C, Chen C-Y, Huang W-Y, Lin W-Y, Chen Y-J, Lee T-A, Lin M-J, Wu H-P. Risk Factors for Urinary Tract Infections in Children with Hematuria in the Emergency Department. Children. 2024; 11(2):248. https://doi.org/10.3390/children11020248
Chicago/Turabian StyleGuo, Bei-Cyuan, Chun-Yu Chen, Wun-Yan Huang, Wen-Ya Lin, Ying-Ju Chen, Tai-An Lee, Mao-Jen Lin, and Han-Ping Wu. 2024. "Risk Factors for Urinary Tract Infections in Children with Hematuria in the Emergency Department" Children 11, no. 2: 248. https://doi.org/10.3390/children11020248
APA StyleGuo, B.-C., Chen, C.-Y., Huang, W.-Y., Lin, W.-Y., Chen, Y.-J., Lee, T.-A., Lin, M.-J., & Wu, H.-P. (2024). Risk Factors for Urinary Tract Infections in Children with Hematuria in the Emergency Department. Children, 11(2), 248. https://doi.org/10.3390/children11020248






