Timely Surgical Intervention Leads to Better Sustained Coverage after Reconstructive Hip Surgery in Patients with Cerebral Palsy
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical Data
3.3. Hip Development
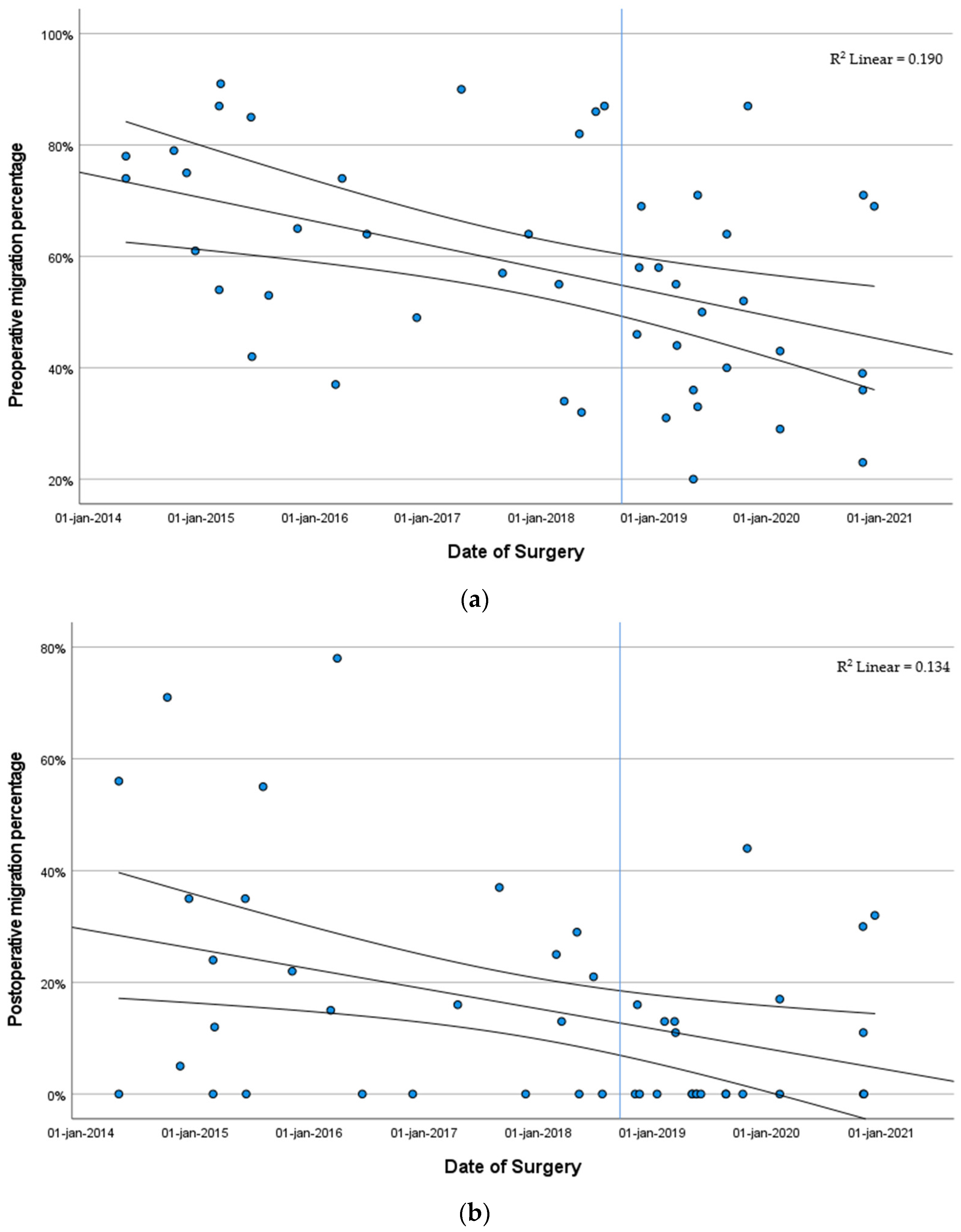
3.3.1. Head–Shaft Angle Stratified by Year of Surgery
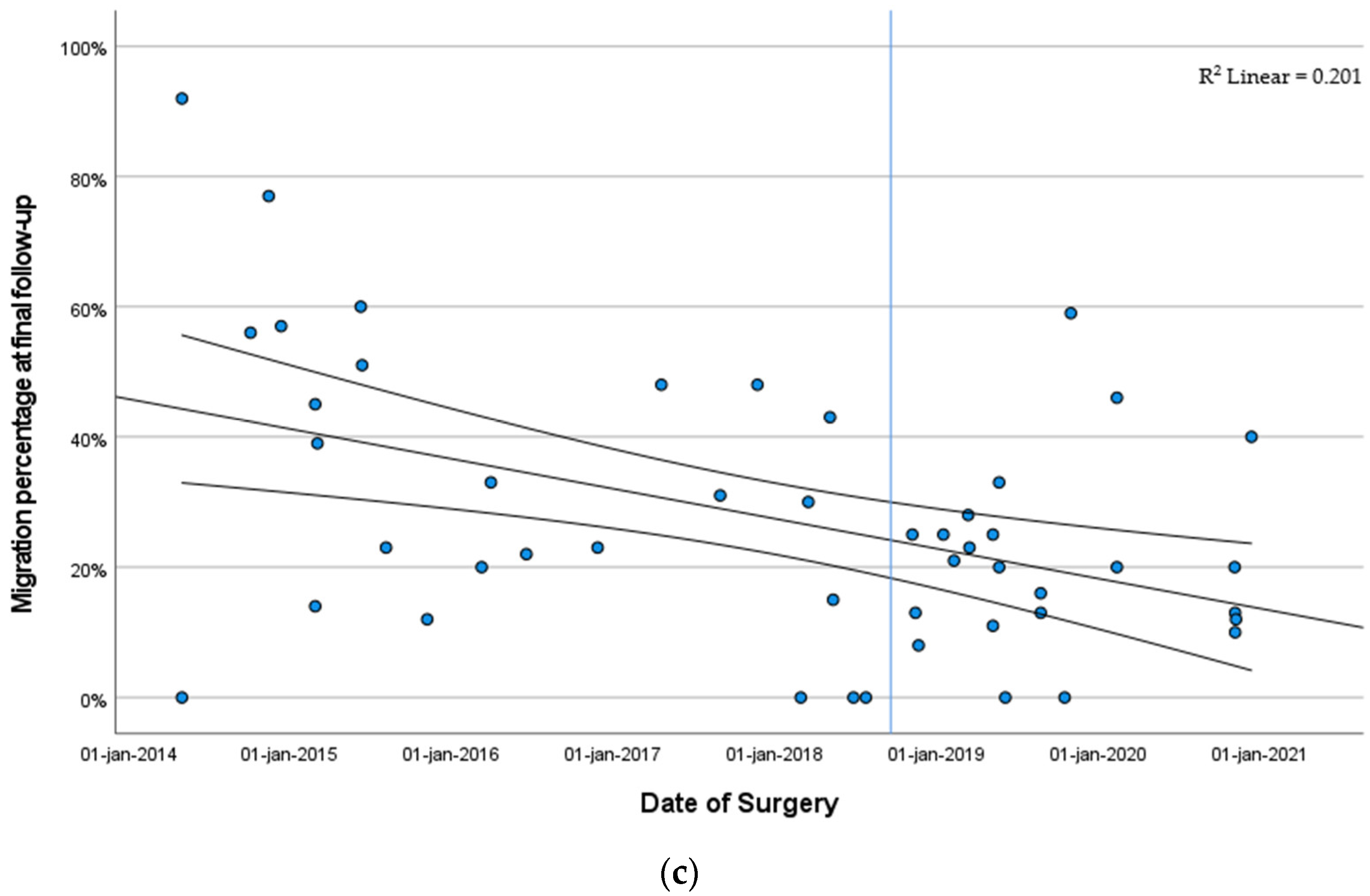
3.3.2. Migration Percentage Stratified by Year of Surgery
3.3.3. Risk Factors Impacting Hip Development
3.4. Melbourne Cerebral Palsy Hip Classification System
3.5. Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arneson, C.L.; Durkin, M.S.; Benedict, R.E.; Kirby, R.S.; Yeargin-Allsopp, M.; Van Naarden Braun, K.; Doernberg, N.S. Prevalence of cerebral palsy: Autism and Developmental Disabilities Monitoring Network, three sites, United States, 2004. Disabil. Health J. 2009, 2, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, T.K.; Brocksen, S.; Avchen, R.N.; Van Naarden Braun, K. Prevalence of four developmental disabilities among children aged 8 years—Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1996 and 2000. MMWR Surveill. Summ. 2006, 55, 1–9. [Google Scholar]
- Jonsson, U.; Eek, M.N.; Sunnerhagen, K.S.; Himmelmann, K. Cerebral palsy prevalence, subtypes, and associated impairments: A population-based comparison study of adults and children. Dev. Med. Child. Neurol. 2019, 61, 1162–1167. [Google Scholar] [CrossRef]
- Paneth, N.; Hong, T.; Korzeniewski, S. The descriptive epidemiology of cerebral palsy. Clin. Perinatol. 2006, 33, 251–267. [Google Scholar] [CrossRef]
- Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev. Med. Child. Neurol. 2000, 42, 816–824. [Google Scholar] [CrossRef]
- Van Naarden Braun, K.; Doernberg, N.; Schieve, L.; Christensen, D.; Goodman, A.; Yeargin-Allsopp, M. Birth Prevalence of Cerebral Palsy: A Population-Based Study. Pediatrics 2016, 137, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.; Autry, A.; Boyle, C.; Yeargin-Allsopp, M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics 2002, 110, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Connelly, A.; Flett, P.; Graham, H.K.; Oates, J. Hip surveillance in Tasmanian children with cerebral palsy. J. Paediatr. Child. Health 2009, 45, 437–443. [Google Scholar] [CrossRef]
- Hagglund, G.; Alriksson-Schmidt, A.; Lauge-Pedersen, H.; Rodby-Bousquet, E.; Wagner, P.; Westbom, L. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Jt. J. 2014, 96-B, 1546–1552. [Google Scholar] [CrossRef]
- Lonstein, J.E.; Beck, K. Hip dislocation and subluxation in cerebral palsy. J. Pediatr. Orthop. 1986, 6, 521–526. [Google Scholar] [CrossRef]
- Soo, B.; Howard, J.J.; Boyd, R.N.; Reid, S.M.; Lanigan, A.; Wolfe, R.; Reddihough, D.; Graham, H.K. Hip displacement in cerebral palsy. J. Bone Jt. Surg. Am. 2006, 88, 121–129. [Google Scholar] [CrossRef]
- Terjesen, T. The natural history of hip development in cerebral palsy. Dev. Med. Child. Neurol. 2012, 54, 951–957. [Google Scholar] [CrossRef]
- Wordie, S.J.; Bugler, K.E.; Bessell, P.R.; Robb, J.E.; Gaston, M.S. Hip displacement in children with cerebral palsy. Bone Jt. J. 2021, 103-B, 411–414. [Google Scholar] [CrossRef]
- Bagg, M.R.; Farber, J.; Miller, F. Long-term follow-up of hip subluxation in cerebral palsy patients. J. Pediatr. Orthop. 1993, 13, 32–36. [Google Scholar] [CrossRef]
- Pritchett, J.W. The untreated unstable hip in severe cerebral palsy. Clin. Orthop. Relat. Res. 1983, 173, 169–172. [Google Scholar] [CrossRef]
- Ramstad, K.; Jahnsen, R.B.; Terjesen, T. Severe hip displacement reduces health-related quality of life in children with cerebral palsy. Acta Orthop. 2017, 88, 205–210. [Google Scholar] [CrossRef] [PubMed]
- DiFazio, R.; Vessey, J.A.; Miller, P.; Van Nostrand, K.; Snyder, B. Postoperative Complications After Hip Surgery in Patients With Cerebral Palsy: A Retrospective Matched Cohort Study. J. Pediatr. Orthop. 2016, 36, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Shrader, M.W.; Wimberly, L.; Thompson, R. Hip Surveillance in Children With Cerebral Palsy. J. Am. Acad. Orthop. Surg. 2019, 27, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Wynter, M.; Gibson, N.; Willoughby, K.L.; Love, S.; Kentish, M.; Thomason, P.; Graham, H.K.; National Hip Surveillance Working, G. Australian hip surveillance guidelines for children with cerebral palsy: 5-year review. Dev. Med. Child. Neurol. 2015, 57, 808–820. [Google Scholar] [CrossRef]
- Wynter, M.; Gibson, N.; Kentish, M.; Love, S.; Thomason, P.; Kerr Graham, H. The development of Australian Standards of Care for Hip Surveillance in Children with Cerebral Palsy: How did we reach consensus? J. Pediatr. Rehabil. Med. 2011, 4, 171–182. [Google Scholar] [CrossRef] [PubMed]
- British Columbia’s Consensus on Hip Surveillance for Children with Cerebral Palsy. Available online: https://www.childhealthbc.ca/sites/default/files/clinical_booket_hip_surveillance_march_2018.pdf (accessed on 1 June 2022).
- The American Academy of Cerebral Palsy and Developmental Medicine: Hip Surveillance Care Pathways. Available online: https://www.aacpdm.org/publications/care-pathways/hip-surveillance-in-cerebral-palsy (accessed on 1 June 2022).
- Spastische Cerebrale Parese BIJ Kinderen: Screening ter Preventie van Heupluxatie. Available online: https://richtlijnendatabase.nl/richtlijn/spastische_cerebrale_parese_bij_kinderen/behandeling_gericht_op_verbetering_van_mobiliteit/effect_van_orthopedisch_chirurgie_op_mobiliteit/screening_ter_preventie_van_heupluxatie.html (accessed on 1 June 2022).
- Rutz, E.; Vavken, P.; Camathias, C.; Haase, C.; Junemann, S.; Brunner, R. Long-term results and outcome predictors in one-stage hip reconstruction in children with cerebral palsy. J. Bone Jt. Surg. Am. 2015, 97, 500–506. [Google Scholar] [CrossRef]
- Minaie, A.; Gordon, J.E.; Schoenecker, P.; Hosseinzadeh, P. Failure of Hip Reconstruction in Children With Cerebral Palsy: What Are the Risk Factors? J. Pediatr. Orthop. 2022, 42, e78–e82. [Google Scholar] [CrossRef]
- Bayusentono, S.; Choi, Y.; Chung, C.Y.; Kwon, S.S.; Lee, K.M.; Park, M.S. Recurrence of hip instability after reconstructive surgery in patients with cerebral palsy. J. Bone Jt. Surg. Am. 2014, 96, 1527–1534. [Google Scholar] [CrossRef]
- Chang, F.M.; May, A.; Faulk, L.W.; Flynn, K.; Miller, N.H.; Rhodes, J.T.; Zhaoxing, P.; Novais, E.N. Outcomes of Isolated Varus Derotational Osteotomy in Children With Cerebral Palsy Hip Dysplasia and Predictors of Resubluxation. J. Pediatr. Orthop. 2018, 38, 274–278. [Google Scholar] [CrossRef]
- Terjesen, T. To what extent can soft-tissue releases improve hip displacement in cerebral palsy? Acta Orthop. 2017, 88, 695–700. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child. Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Reimers, J. The stability of the hip in children. A radiological study of the results of muscle surgery in cerebral palsy. Acta Orthop. Scand. Suppl. 1980, 184, 1–100. [Google Scholar] [CrossRef]
- Pons, C.; Remy-Neris, O.; Medee, B.; Brochard, S. Validity and reliability of radiological methods to assess proximal hip geometry in children with cerebral palsy: A systematic review. Dev. Med. Child. Neurol. 2013, 55, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Southwick, W.O. Osteotomy through the lesser trochanter for slipped capital femoral epiphysis. J. Bone Jt. Surg. Am. 1967, 49, 807–835. [Google Scholar] [CrossRef]
- Chougule, S.; Dabis, J.; Petrie, A.; Daly, K.; Gelfer, Y. Is head-shaft angle a valuable continuous risk factor for hip migration in cerebral palsy? J. Child. Orthop. 2016, 10, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, M.; Hagglund, G.; Riad, J.; Wagner, P. Head-shaft angle is a risk factor for hip displacement in children with cerebral palsy. Acta Orthop. 2015, 86, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Tonnis, D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clin. Orthop. Relat. Res. 1976, 119, 39–47. [Google Scholar]
- Robin, J.; Graham, H.K.; Baker, R.; Selber, P.; Simpson, P.; Symons, S.; Thomason, P. A classification system for hip disease in cerebral palsy. Dev. Med. Child. Neurol. 2009, 51, 183–192. [Google Scholar] [CrossRef]
- Herndon, W.A.; Bolano, L.; Sullivan, J.A. Hip stabilization in severely involved cerebral palsy patients. J. Pediatr. Orthop. 1992, 12, 68–73. [Google Scholar] [CrossRef]
- Zhang, S.; Wilson, N.C.; Mackey, A.H.; Stott, N.S. Radiological outcome of reconstructive hip surgery in children with gross motor function classification system IV and V cerebral palsy. J. Pediatr. Orthop. B 2014, 23, 430–434. [Google Scholar] [CrossRef]
- DiFazio, R.; Shore, B.; Vessey, J.A.; Miller, P.E.; Snyder, B.D. Effect of Hip Reconstructive Surgery on Health-Related Quality of Life of Non-Ambulatory Children with Cerebral Palsy. J. Bone Jt. Surg. Am. 2016, 98, 1190–1198. [Google Scholar] [CrossRef] [PubMed]

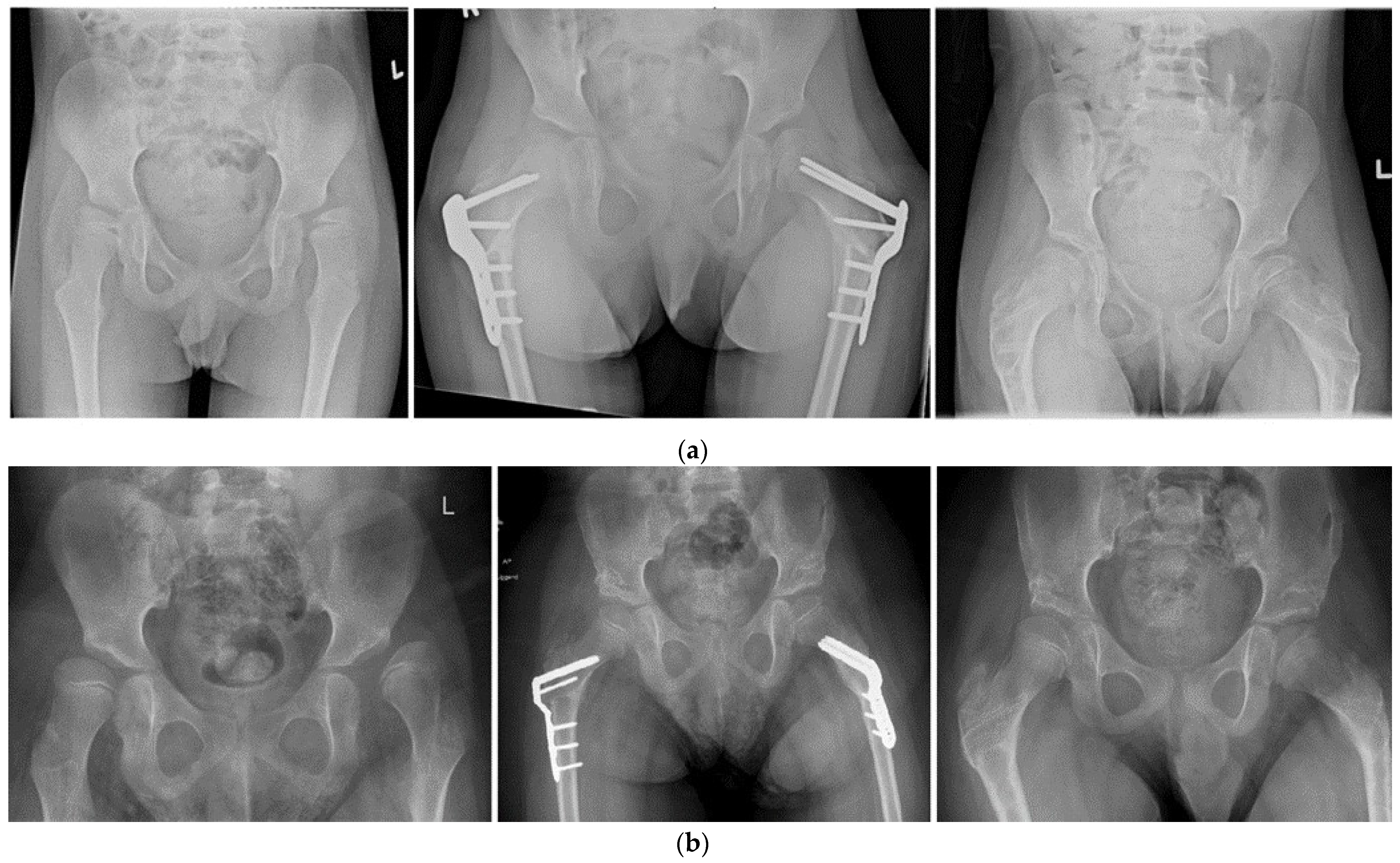
| Median Age (IQR *) at Time of Surgery, Years | 8.0 (IQR 7.0; 9.6) |
|---|---|
| Male: Female | 29: 19 |
| GMFCS ‡ level, n (%) | |
| I | 0 |
| II | 1 (2.1%) |
| III | 2 (4.2%) |
| IV | 26 (54.2%) |
| V | 19 (39.6) |
| CP type, n (%) | |
| Spastic | 45 (93.8%) |
| Dystonic | 3 (6.3%) |
| CP extent, n (%) | |
| Hemiplegia | 1 (2.1%) |
| Diplegia | 14 (29.2%) |
| Quadriplegia | 33 (68.8%) |
| Tone management, n (%) | |
| Missing/unknown | 1 (2.1%) |
| None | 32 (66.7%) |
| Oral Baclofen | 14 (29.2%) |
| Baclofen pump | 1 (2.1%) |
| Median follow-up of all primary reconstructive hip surgeries, years (IQR) | 4.4 (3.6; 6.1) |
| Median follow-up of all primary hip surgeries, including salvage procedures, years (IQR) | 4.2 (2.8; 5.3) |
| Surgical Data | n (%) |
|---|---|
| Varus derotation osteotomy | 48 hips (100%) |
| + adductor tenotomies | 36 hips (75%) |
| + pelvic osteotomy | 30 hips (62.5%) |
| Dega pelvic osteotomy | 28 hips (58.3%) |
| Pemberton type osteotomy | 1 hip (2.1%) |
| Salter type osteotomy | 1 hip (2.1%) |
| Median blood loss, ml (IQR °) | 190 mL (IQR 100; 250) |
| Median surgical time, minutes (IQR) | 178 min (IQR 161; 222) |
| Preoperative | Postoperative | Final Follow-Up | |
|---|---|---|---|
| MP, % | 57.5 | 11.0 | 23.0 |
| (40.5; 74.0) | (0.0; 24.8) | (13.0; 42.3) | |
| HSA, degrees | 165.0 | 121.5 | 140.1 |
| (157.3; 169.0) | (114.0; 135.3) | (114.0; 153.6) | |
| AI, degrees | 28.5 | 21.0 | * |
| (22.0; 32.0) | (15.5; 27.5) | ||
| MCPHCS, grades | IV | III | |
| (IV; IV) | (III; IV) |
| Beta | 95% Confidence Interval | p Value | |
|---|---|---|---|
| Age at time of surgery | 2.13 | −0.87 to 5.12 | p = 0.157 |
| GMFCS ‡ II-III vs. IV-V | −2.03 | −25.10 to 20.91 | p = 0.855 |
| Single vs. Double surgeon | −8.58 | −20.28 to 3.11 | p = 0.144 |
| Tone management | −14.10 | −26.90 to −1.30 | p = 0.032 |
| Postoperative MP | 0.46 | 0.17 to 0.75 | p = 0.002 |
| Total available follow-up | 1.43 | −1.46 to 4.32 | p = 0.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Stralen, R.A.; Kempink, D.R.J.; Titulaer, A.F.; Eygendaal, D.; Reijman, M.; Tolk, J.J. Timely Surgical Intervention Leads to Better Sustained Coverage after Reconstructive Hip Surgery in Patients with Cerebral Palsy. Children 2024, 11, 272. https://doi.org/10.3390/children11030272
van Stralen RA, Kempink DRJ, Titulaer AF, Eygendaal D, Reijman M, Tolk JJ. Timely Surgical Intervention Leads to Better Sustained Coverage after Reconstructive Hip Surgery in Patients with Cerebral Palsy. Children. 2024; 11(3):272. https://doi.org/10.3390/children11030272
Chicago/Turabian Stylevan Stralen, Renée Anne, Dagmar Raymond Jacques Kempink, Alexandra Frederika Titulaer, Denise Eygendaal, Max Reijman, and Jaap Johannes Tolk. 2024. "Timely Surgical Intervention Leads to Better Sustained Coverage after Reconstructive Hip Surgery in Patients with Cerebral Palsy" Children 11, no. 3: 272. https://doi.org/10.3390/children11030272









