Review of Opsoclonus-Myoclonus Ataxia Syndrome in Pediatric Patients
Abstract
1. Introduction
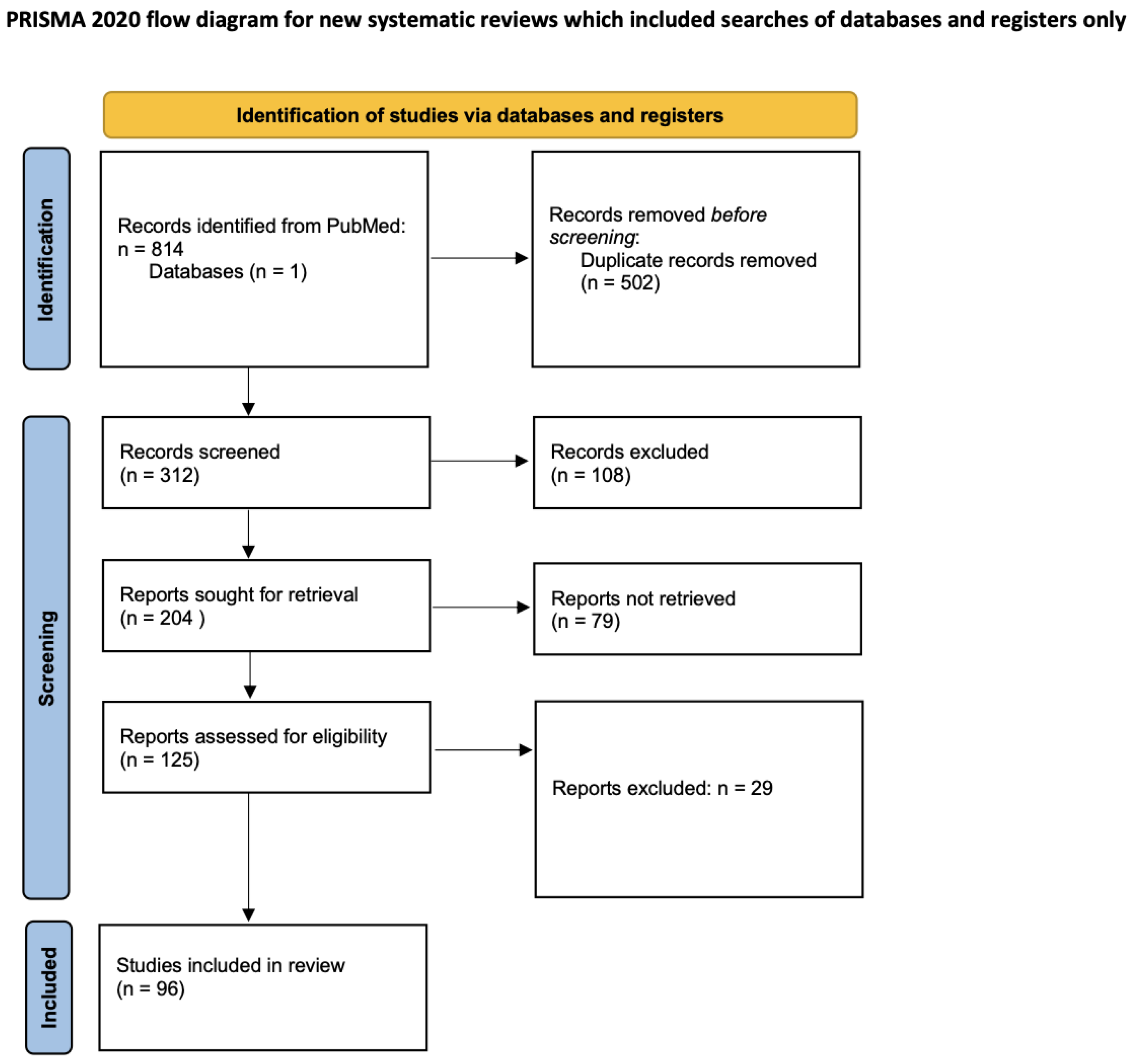
2. Materials and Methods
Case Report
3. Review
3.1. Overview and Etiologies
3.2. Diagnosis, Clinical Presentation, and Screening
3.3. Treatment
3.4. Prognosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pranzatelli, M.R.; Tate, E.D.; McGee, N.R. Demographic, Clinical, and Immunologic Features of 389 Children with Opsoclonus-Myoclonus Syndrome: A Cross-sectional Study. Front. Neurol. 2017, 8, 468. [Google Scholar] [CrossRef]
- Pang, K.K.; de Sousa, C.; Lang, B.; Pike, M.G. A prospective study of the presentation and management of dancing eye syndrome/opsoclonus-myoclonus syndrome in the United Kingdom. Eur. J. Paediatr. Neurol. 2010, 14, 156–161. [Google Scholar] [CrossRef]
- Hasegawa, S.; Matsushige, T.; Kajimoto, M.; Inoue, H.; Momonaka, H.; Oka, M.; Ohga, S.; Ichiyama, T. A nationwide survey of opsoclonus-myoclonus syndrome in Japanese children. Brain Dev. 2015, 37, 656–660. [Google Scholar] [CrossRef]
- Brunklaus, A.; Pohl, K.; Zuberi, S.M.; de Sousa, C. Outcome and prognostic features in opsoclonus-myoclonus syndrome from infancy to adult life. Pediatrics 2011, 128, e388–e394. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, A.B.; Berdon, W.E.; D’Angio, G.J.; Yamashiro, D.J.; Cowles, R.A. The association between neuroblastoma and opsoclonus-myoclonus syndrome: A historical review. Pediatr. Radiol. 2009, 39, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Mutch, L.S.; Johnston, D.L. Late presentation of opsoclonus-myoclonus-ataxia syndrome in a child with stage 4S neuroblastoma. J. Pediatr. Hematol. Oncol. 2005, 27, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Galgano, S.; Royal, S. Primary pancreatic neuroblastoma presenting with opsoclonus-myoclonus syndrome. Radiol. Case Rep. 2016, 11, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Rossor, T.; Yeh, E.A.; Khakoo, Y.; Angelini, P.; Hemingway, C.; Irani, S.R.; Schleiermacher, G.; Santosh, P.; Lotze, T.; Dale, R.C.; et al. Diagnosis and Management of Opsoclonus-Myoclonus-Ataxia Syndrome in Children: An International Perspective. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1153. [Google Scholar] [CrossRef]
- Sahu, J.K.; Prasad, K. The opsoclonus–myoclonus syndrome. Pract. Neurol. 2011, 11, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Blaes, F.; Fühlhuber, V.; Preissner, K.T. Identification of autoantigens in pediatric opsoclonus-myoclonus syndrome. Expert. Rev. Clin. Immunol. 2007, 3, 975–982. [Google Scholar] [CrossRef]
- Connolly, A.M.; Pestronk, A.; Mehta, S.; Pranzatelli, M.R., 3rd; Noetzel, M.J. Serum autoantibodies in childhood opsoclonus-myoclonus syndrome: An analysis of antigenic targets in neural tissues. J. Pediatr. 1997, 130, 878–884. [Google Scholar] [CrossRef]
- Cardesa-Salzmann, T.M.; Mora, J.; García Cazorla, M.A.; Cruz, O.; Muñoz, C.; Campistol, J. Epstein-Barr virus related opsoclonus-myoclonus-ataxia does not rule out the presence of occult neuroblastic tumors. Pediatr. Blood Cancer 2006, 47, 964–967. [Google Scholar] [CrossRef]
- Ertekin, V.; Tan, H. Opsoclonus-myoclonus syndrome attributable to hepatitis C infection. Pediatr. Neurol. 2010, 42, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Gurkas, E.; Gucuyener, K.; Yılmaz, U.; Havalı, C.; Demir, E. Opsoclonus-myoclonus syndrome following rotavirus gastroenteritis. Pediatr. Int. 2014, 56, e86–e87. [Google Scholar] [CrossRef] [PubMed]
- Syrbe, S.; Merkenschlager, A.; Bernhard, M.K.; Grosche, J.; Liebert, U.G.; Hirsch, W.; Härtig, W. Opsoclonus-myoclonus syndrome after adenovirus infection. Springerplus 2015, 4, 636. [Google Scholar] [CrossRef] [PubMed]
- Sahly, A.; Gauquelin, L.; Sébire, G. Rapid Resolution of Enterovirus 71-Associated Opsoclonus Myoclonus Syndrome on Intravenous Immunoglobulin. Child Neurol. Open 2017, 4, 2329048X17733215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, W.; Chen, L.; Hou, C.; Zeng, Y.; Tian, Y.; Shen, H.; Gao, Y.; Zhang, Y.; Peng, B.; et al. Clinical Analysis of Pediatric Opsoclonus-Myoclonus Syndrome in One of the National Children’s Medical Center in China. Front. Neurol. 2021, 12, 744041. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Cheuret, E.; Fiedler, L.; Mengelle, C.; Baudou, E.; Deiva, K. Acute transverse myelitis following an opsoclonus-myoclonus syndrome: An unusual presentation. Eur. J. Paediatr. Neurol. 2018, 22, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Bose, K.; Saha, S.; Islam, M.R.; Chakraborty, C.; Laskar, M. Opsoclonus myoclonus ataxia syndrome due to falciparum malaria in two Indian children. Indian J. Ophthalmol. 2016, 64, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S.E.; Mitchell, W.G.; Santoro, J.D. Immunotherapy responsive SARS-CoV-2 infection exacerbating opsoclonus myoclonus syndrome. Mult. Scler. Relat. Disord. 2021, 50, 102855. [Google Scholar] [CrossRef]
- van Toorn, R.; Rabie, H.; Warwick, J.M. Opsoclonus-myoclonus in an HIV-infected child on antiretroviral therapy--possible immune reconstitution inflammatory syndrome. Eur. J. Paediatr. Neurol. 2005, 9, 423–426. [Google Scholar] [CrossRef]
- Sharma, P.; Ganvir, S.P.; Vagha, K. Opsoclonus Myoclonus Syndrome in a Case of Severe Acute Malnutrition in Children: A Case Report. Cureus 2022, 14, e32578. [Google Scholar] [CrossRef]
- Lenti, C.; Bognetti, E.; Bonfanti, R.; Bonifacio, E.; Meschi, F. Myoclonic encephalopathy and diabetes mellitus in a boy. Dev. Med. Child Neurol. 1999, 41, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Ghia, T.; Kanhangad, M.; Alessandri, A.J.; Price, G.; Gera, P.; Nagarajan, L. Opsoclonus-Myoclonus Syndrome, Neuroblastoma, and Insulin-Dependent Diabetes Mellitus in a Child: A Unique Patient. Pediatr. Neurol. 2016, 55, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Alburaiky, S.; Dale, R.C.; Crow, Y.J.; Jones, H.F.; Wassmer, E.; Melki, I.; Boespflug-Tanguy, O.; Cao, J.D.; Gras, D.; Sharpe, C. Opsoclonus-myoclonus in Aicardi-Goutières syndrome. Dev. Med. Child Neurol. 2021, 63, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, H.; Shimizu, Y.; Saito, Y.; Sugai, K.; Inagaki, M.; Kaga, M.; Sasaki, M. Electrophysiological Evidence of Cerebral Dysfunction in Childhood Opsoclonus-Myoclonus Syndrome. Mov. Disord. 2010, 25, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Gallerini, S.; Marsili, L. Pediatric opsoclonus-myoclonus syndrome: The role of functional brain connectivity studies. Dev. Med. Child Neurol. 2016, 59, 14–15. [Google Scholar] [CrossRef]
- Hero, B.; Clement, N.; Øra, I.; Pierron, G.; Lapouble, E.; Theissen, J.; Pasqualini, C.; Valteau-Couanet, D.; Plantaz, D.; Michon, J.; et al. Genomic Profiles of Neuroblastoma Associated with Opsoclonus Myoclonus Syndrome. J. Pediatr. Hematol./Oncol. 2018, 40, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Turkel, S.B.; Brumm, V.L.; Mitchell, W.G.; Tavare, C.J. Mood and behavioral dysfunction with opsoclonus-myoclonus ataxia. J. Neuropsychiatry Clin. Neurosci. 2006, 18, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Heim, J.; Cornejo, P.; Kane, L.; Santiago, J.; Kruer, M.C. Opsoclonus-myoclonus-ataxia syndrome in children. J. Neurol. 2022, 269, 750–757. [Google Scholar] [CrossRef]
- Yıldırım, M.; Öncel, İ.; Bektaş, Ö.; Tanali, G.; Sahin, S.; Kutluk, T.; Teber, S.; Anlar, B. Clinical features and outcomes of opsoclonus myoclonus ataxia syndrome. Eur. J. Paediatr. Neurol. 2022, 41, 19–26. [Google Scholar] [CrossRef]
- Singhi, P.; Sahu, J.K.; Sarkar, J.; Bansal, D. Clinical profile and outcome of children with opsoclonus-myoclonus syndrome. J. Child Neurol. 2014, 29, 58–61. [Google Scholar] [CrossRef]
- Mizuno, T.; Kumada, S.; Naito, R. Sleep-Related Laryngeal Stridor in Opsoclonus Myoclonus Syndrome. Pediatr. Neurol. 2017, 77, 91. [Google Scholar] [CrossRef]
- Yonekawa, T.; Saito, Y.; Skuma, H.; Sugai, K.; Shimizu, Y.; Inagaki, M.; Sasaki, M. Augmented startle response in opsoclonus-myoclonus syndrome. Brain Dev. 2011, 33, 335–338. [Google Scholar] [CrossRef]
- Takenaka, J.; Hirata, K.; Watanabe, S.; Shiraishi, H.; Kudo, K. Neuroblastoma-related severe hypoperfusion in the cerebellum of an infant: A case of opsoclonus-myoclonus syndrome. Asia Ocean. J. Nucl. Med. Biol. 2023, 11, 93–96. [Google Scholar] [PubMed]
- Papero, P.H.; Pranzatelli, M.R.; Margolis, L.J.; Tate, E.; Wilson, L.A.; Glass, P. Neurobehavioral and psychosocial functioning of children with opsoclonus-myoclonus syndrome. Dev. Med. Child. Neurol. 1995, 37, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Badaki, O.B.; Schapiro, E.S. Dancing eyes, dancing feet: Opsoclonus-myoclonus in an 18-month-old child with neuroblastoma. Pediatr. Emerg. Care. 2007, 23, 885–888. [Google Scholar] [CrossRef]
- Huddar, A.; Bindu, P.S.; Nagappa, M.; Bharath, R.; Sinha, S.; Mathuranath, P.S.; Taly, A.B. Pediatric opsoclonus-myoclonus-ataxia syndrome: Experience from a tertiary care university hospital. Neurol. India 2018, 66, 1332–1337. [Google Scholar] [CrossRef]
- Tate, E.D.; Allison, T.J.; Pranzatelli, M.R.; Verhulst, S.J. Neuroepidemiologic trends in 105 US cases of pediatric opsoclonus-myoclonus syndrome. J. Pediatr. Oncol. Nurs. 2005, 22, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, K.; Thomas, M.; Yoganathan, S.; Sudhakar, S.V. Clinical Profile, Prognostic Indicators, and Therapeutic Outcomes of Pediatric Opsoclonus-Myoclonus-Ataxia Syndrome: A Single-Center Experience from South India. Ann. Indian Acad. Neurol. 2019, 22, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.E.; Ritchey, A.K.; Tersak, J.; Thakkar, K.; Lambore, S. Pediatrician’s Approach to Recognizing Neuroblastoma with Opsoclonus Myoclonus-Ataxia Syndrome. Clin. Pediatr. 2022, 62, 820–823. [Google Scholar] [CrossRef]
- Wilfong, A.A.; Parke, J.T.; McCrary, J.A. Opsoclonus-myoclonus with Beckwith-Wiedemann syndrome and hepatoblastoma. Pediatr. Neurol. 1992, 8, 77–79. [Google Scholar] [CrossRef]
- Haden, S.V.; McShane, M.A.; Holt, C.M. Opsoclonus myoclonus: A non-epileptic movement disorder that may present as status epilepticus. Arch. Dis. Child. 2009, 94, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Gerstle, K.; Siddiqui, A.; Schulte, J.J.; Cohn, S.L. Paraneoplastic opsoclonus myoclonus syndrome associated with inflammatory myofibroblastic tumor in a pediatric patient. Pediatr. Blood Cancer 2020, 67, e28218. [Google Scholar] [CrossRef]
- Player, B.; Harmelink, M.; Bordini, B.; Weisgerber, M.; Girolami, M.; Croix, M. Pediatric Opsoclonus-Myoclonus-Ataxia Syndrome Associated with Anti-N-methyl-D-aspartate Receptor Encephalitis. Pediatr. Neurol. 2015, 53, 456–458. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D.; Hoefgen, E.R.; Swan, J.A.; Colliver, J.A. Therapeutic down-regulation of central and peripheral B-cell-activating factor (BAFF) production in pediatric opsoclonus-myoclonus syndrome. Cytokine 2008, 44, 26–32. [Google Scholar] [CrossRef]
- Fühlhuber, V.; Bick, S.; Kirsten, A.; Hahn, A.; Gerriets, T.; Tschernatsch, M.; Kaps, M.; Preissner, K.; Blaes, F.; Altenkämper, S. Elevated B-cell activating factor, BAFF, but not APRIL, correlates with CSF cerebellar autoantibodies in pediatric opsoclonus-myoclonus syndrome. J. Neuroimmunol. 2009, 210, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pranzatelli, M.R.; Travelstead, A.L.; Tate, E.D.; Allison, T.J.; Verhulst, S.J. CSF B-cell expansion in opsoclonus-myoclonus syndrome: A biomarker of disease activity. Mov. Disord. 2004, 19, 770–777. [Google Scholar] [CrossRef]
- Storz, C.; Bares, R.; Ebinger, M.; Handgretinger, R.; Tsiflikas, I.; Schäfer, J.F. Diagnostic value of whole-body MRI in Opsoclonus-myoclonus syndrome: A clinical case series (3 case reports). BMC Med. Imaging 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Brunklaus, A.; Pohl, K.; Zuberi, S.M.; de Sousa, C. Investigating neuroblastoma in childhood opsoclonus-myoclonus syndrome. Arch. Dis. Child. 2012, 97, 461–463. [Google Scholar] [CrossRef]
- Swart, J.F.; de Kraker, J.; van der Lely, N. Metaiodobenzylguanidine total-body scintigraphy required for revealing occult neuroblastoma in opsoclonus-myoclonus syndrome. Eur. J. Pediatr. 2002, 161, 255–258. [Google Scholar] [CrossRef]
- Kumar, R.; Vankadari, K.; Mittal, B.R.; Bansal, D.; Trehan, A.; Sahu, J.K.; Sankhyan, N. Diagnostic values of 68Ga-labelled DOTANOC PET/CT imaging in pediatric patients presenting with paraneoplastic opsoclonus myoclonus ataxia syndrome. Eur. Radiol. 2021, 31, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Cantarín-Extremera, V.; Jiménez-Legido, M.; Aguilera-Albesa, S.; Hedrera-Fernández, A.; Arrabal-Fernández, L.; Gorría-Redondo, N.; Martí-Carrera, I.; Yoldi-Pedtri, M.; Ilúrdoz, M.S.-D.; González-Gutiérrez-Solana, L. Opsoclonus-myoclonus syndrome: Clinical characteristics, therapeutic considerations, and prognostic factors in a Spanish paediatric cohort. Neurología (Engl. Ed.) 2023, 38, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Auconi, M.; Papetti, L.; Ruscitto, C.; Ferilli, M.A.N.; Ursitti, F.; Sforza, G.; Vigevano, F.; Valeriani, M. Opsoclonus-Myoclonus Syndrome in Children and Adolescents: A Therapeutic Challenge. Children 2021, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Mizia-Malarz, A.; Stolpa, W.; Sobol-Milejska, G. The Treatment of Opsoclonus-Myoclonus Syndrome Secondary to Neuroblastic Tumours-Single-Centre Experience and Literature Review. Medicina 2020, 56, 412. [Google Scholar] [CrossRef] [PubMed]
- Ertle, F.; Behnisch, W.; Al Mulla, N.A.; Bessisso, M.; Rating, D.; Mechtersheimer, G.; Hero, B.; Kulozik, A.E. Treatment of neuroblastoma-related opsoclonus-myoclonus-ataxia syndrome with high-dose dexamethasone pulses. Pediatr. Blood Cancer 2008, 50, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Oguma, M.; Morimoto, A.; Takada, A.; Kashii, Y.; Fukuda, T.; Mori, M.; Yamagata, T.; Sugie, H.; Momoi, M.Y. Another promising treatment option for neuroblastoma-associated opsoclonus-myoclonus syndrome by oral high-dose dexamethasone pulse: Lymphocyte markers as disease activity. Brain Dev. 2012, 34, 251–254. [Google Scholar] [CrossRef]
- de Alarcon, P.A.; Matthay, K.K.; London, W.B.; Naranjo, A.; Tenney, S.C.; Panzer, J.A.; Hogarty, M.D.; Park, J.R.; Maris, J.M.; Cohn, S.L. Intravenous immunoglobulin with prednisone and risk-adapted chemotherapy for children with opsoclonus myoclonus ataxia syndrome associated with neuroblastoma (ANBL00P3): A randomised, open-label, phase 3 trial. Lancet Child Adolesc. Health 2018, 2, 25–34. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D.; Travelstead, A.L.; Longee, D. Immunologic and clinical responses to rituximab in a child with opsoclonus-myoclonus syndrome. Pediatrics 2005, 115, e115–e119. [Google Scholar] [CrossRef]
- Bell, J.; Moran, C.; Blatt, J. Response to rituximab in a child with neuroblastoma and opsoclonus-myoclonus. Pediatr. Blood Cancer 2008, 50, 370–371. [Google Scholar] [CrossRef]
- Battaglia, T.; De Grandis, E.; Mirabelli-Badenier, M.; Boeri, L.; Morcaldi, G.; Barabino, P.; Intra, C.; Naselli, F.; Pistoia, V.; Veneselli, E.; et al. Response to rituximab in 3 children with opsoclonus-myoclonus syndrome resistant to conventional treatments. Eur. J. Paediatr. Neurol. 2012, 16, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Kord Valeshabad, A.; Moradveisi, B.; Aminasnafi, A.; Arzanian, M.T. Clinical responses to rituximab in a case of neuroblastoma with refractory opsoclonus myoclonus ataxia syndrome. Case Rep. Oncol. Med. 2012, 2012, 164082. [Google Scholar] [CrossRef][Green Version]
- Wilbur, C.; Yea, C.; Licht, C.; Irwin, M.S.; Yeh, E.A. An upfront immunomodulatory therapy protocol for pediatric opsoclonus-myoclonus syndrome. Pediatr. Blood Cancer 2019, 66, e27776. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D.; Travelstead, A.L.; Barbosa, J.; Bergamini, R.A.; Civitello, L.; Franz, D.N.; Greffe, B.S.; Hanson, R.D.; Hurwitz, C.A.; et al. Rituximab (anti-CD20) adjunctive therapy for opsoclonus-myoclonus syndrome. J. Pediatr. Hematol. Oncol. 2006, 28, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Pranzatelli, M.R.; Tate, E.D.; Swan, J.A.; Travelstead, A.L.; Colliver, J.A.; Verhulst, S.J.; Carl, J.C.; William, D.G.; Suja, A.J.; Howard, M.K.; et al. B cell depletion therapy for new-onset opsoclonus-myoclonus. Mov. Disord. 2010, 25, 238–242. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D.; McGee, N.R.; MacArthur, C.A. Evaluation of Responsiveness to Reduced-Dose Rituximab in Corticotropin/Intravenous Immunoglobulin/Rituximab Combination Immunotherapy for Opsoclonus-Myoclonus Syndrome. Pediatr. Neurol. 2018, 85, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, D.; Morisada, N.; Takami, Y.; Kidokoro, H.; Nishiyama, M.; Nakagawa, T.; Ninchoji, T.; Nozu, K.; Takeshima, Y.; Takada, S.; et al. Rituximab treatment for relapsed opsoclonus-myoclonus syndrome. Brain Dev. 2016, 38, 346–349. [Google Scholar] [CrossRef]
- Wilken, B.; Baumann, M.; Bien, C.G.; Hero, B.; Rostasy, K.; Hanefeld, F. Chronic relapsing opsoclonus-myoclonus syndrome: Combination of cyclophosphamide and dexamethasone pulses. Eur. J. Paediatr. Neurol. 2008, 12, 51–55. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Allison, T.J.; Tate, E.D. Effect of low-dose cyclophosphamide, ACTH, and IVIG combination immunotherapy on neuroinflammation in pediatric-onset OMS: A retrospective pilot study. Eur. J. Paediatr. Neurol. 2018, 22, 586–594. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D.; Allison, T.J. 6-Mercaptopurine modifies cerebrospinal fluid T cell abnormalities in paediatric opsoclonus-myoclonus as steroid sparer. Clin. Exp. Immunol. 2017, 190, 217–225. [Google Scholar] [CrossRef]
- Yiu, V.W.; Kovithavongs, T.; McGonigle, L.F.; Ferreira, P. Plasmapheresis as an effective treatment for opsoclonus-myoclonus syndrome. Pediatr. Neurol. 2001, 24, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.B.; Robertson, P.L.; Castle, V.P. Delayed, recurrent opsoclonus-myoclonus syndrome responding to plasmapheresis. Pediatr. Neurol. 2005, 33, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Greensher, J.E.; Louie, J.; Fish, J.D. Therapeutic plasma exchange for a case of refractory opsoclonus myoclonus ataxia syndrome. Pediatr. Blood Cancer 2018, 65, e26819. [Google Scholar] [CrossRef]
- Johnston, D.L.; Murray, S.; Irwin, M.S.; Doyle, J.; Schechter, T. Autologous stem cell transplantation for refractory opsoclonus myoclonus ataxia syndrome. Pediatr. Blood Cancer 2018, 65, e27110. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, V.K.; Chandra, S.; Verma, R.; Kalita, J.; Misra, U.K. Clonazepam responsive opsoclonus myoclonus syndrome: Additional evidence in favour of fastigial nucleus disinhibition hypothesis? J. Neural Transm. 2010, 117, 613–615. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D.; Shenoy, S.; Travelstead, A.L. Ofatumumab for a rituximab-allergic child with chronic-relapsing paraneoplastic opsoclonus-myoclonus. Pediatr. Blood Cancer 2012, 58, 988–991. [Google Scholar] [CrossRef]
- Ketterl, T.G.; Messinger, Y.H.; Niess, D.R.; Gilles, E.; Engel, W.K.; Perkins, J.L. Ofatumumab for refractory opsoclonus-myoclonus syndrome following treatment of neuroblastoma. Pediatr. Blood Cancer 2013, 60, E163–E165. [Google Scholar] [CrossRef]
- Ki Pang, K.; Lynch, B.J.; Osborne, J.P.; Pike, M.G. Dancing Eye Syndrome associated with spontaneous recovery and normal neurodevelopment. Eur. J. Paediatr. Neurol. 2010, 14, 178–181. [Google Scholar] [CrossRef]
- Galstyan, A.; Wilbur, C.; Selby, K.; Hukin, J. Opsoclonus-Myoclonus Syndrome: A New Era of Improved Prognosis? Pediatr. Neurol. 2017, 72, 65–69. [Google Scholar] [CrossRef]
- Tate, E.D.; Pranzatelli, M.R.; Verhulst, S.J.; Markwell, S.J.; Franz, D.N.; Graf, W.D.; Joseph, S.A.; Khakoo, Y.N.; Lo, W.D.; Mitchell, W.G.; et al. Active comparator-controlled, rater-blinded study of corticotropin-based immunotherapies for opsoclonus-myoclonus syndrome. J. Child Neurol. 2012, 27, 875–884, Erratum in J. Child Neurol. 2012, 27, 1364. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D. Dexamethasone, Intravenous Immunoglobulin, and Rituximab Combination Immunotherapy for Pediatric Opsoclonus-Myoclonus Syndrome. Pediatr. Neurol. 2017, 73, 48–56, Erratum in Pediatr Neurol. 2017, 73, 48–56. https://doi.org/10.1016/j.pediatrneurol.2017.04.027 . Correction in Pediatr. Neurol. 2018, 78, 84. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Nakamura, R.; Yamaga, A.; Haraki, T.; Yasuda, T.; Hamada, H.; Kawamoto, M. Transient symptomatic worsening by atropine in opsoclonus-myoclonus syndrome. Pediatr. Int. 2017, 59, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.P.; Seth, R.; Chakrabarty, B.; Gulati, S.; Agrawala, S.; Naranje, P. Neuroblastoma presenting as opsoclonus-myoclonus: A series of six cases and review of literature. J. Pediatr. Neurosci. 2016, 11, 373–377. [Google Scholar] [CrossRef]
- Anand, S.M.; Agarwala, S.M.; Jain, V.M.; Bakhshi, S.D.; Dhua, A.M.; Gulati, S.; Seth, R.; Srinivas, M.M.; Jana, M.; Kandasamy, D.; et al. Neuroblastoma with Opsoclonus-Myoclonus-Ataxia Syndrome: Role of Chemotherapy in the Management: Experience from a Tertiary Care Center in a Resource-limited Setting. J. Pediatr. Hematol. Oncol. 2021, 43, e924–e929. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Bai, C.; Li, K.; Dong, K.; Yao, W. Comparison of mediastinal and non-mediastinal neuroblastoma and ganglioneuroblastoma associated with opsoclonus-myoclonus syndrome; a systematic review and meta analysis. Transl. Cancer Res. 2022, 11, 3741–3753. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.; Jeremy, R.J.; Jenkins, S.; Barkovich, A.J.; Gultekin, S.; Kramer, J.; Crittenden, M.; Matthay, K.K. Long-term neurobehavioral outcomes in children with neuroblastoma and opsoclonus-myoclonus-ataxia syndrome: Relationship to MRI findings and anti-neuronal antibodies. J. Pediatr. 2001, 139, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Schmitt, B.; Boltshauser, E. Long-term outcome of ten children with opsoclonus-myoclonus syndrome. Eur. J. Pediatr. 2007, 166, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Catsman-Berrevoets, C.E.; Aarsen, F.K.; van Hemsbergen, M.L.; van Noesel, M.M.; Hakvoort-Cammel, F.G.; van den Heuvel-Eibrink, M.M. Improvement of neurological status and quality of life in children with opsoclonus myoclonus syndrome at long-term follow-up. Pediatr. Blood Cancer 2009, 53, 1048–1053. [Google Scholar] [CrossRef]
- Elzomor, H.; El Menawi, S.; Elwady, H.; Elkinaai, N.; Elshafie, M.; Refaat, A.; Ghareeb, H.; Fawzy, M. Neuroblastoma-associated Opsoclonus Myoclonus Ataxia Syndrome: Profile and Outcome Report on 15 Egyptian Patients. J. Pediatr. Hematol./Oncol. 2023, 45, e194–e199. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.; Xie, Y.; Wu, P.; Li, S.; Zhao, W. Long-term neurological outcomes of children with neuroblastoma with opsoclonus-myoclonus syndrome. Transl. Pediatr. 2022, 11, 368–374. [Google Scholar] [CrossRef]
- Goh, E.L.; Scarff, K.; Satariano, S.; Lim, M.; Anand, G. Evolving Cognitive Dysfunction in Children with Neurologically Stable Opsoclonus-Myoclonus Syndrome. Children 2020, 7, 103. [Google Scholar] [CrossRef]
- Green, D.; Lim, M.; Turk, J. Sensory Processing Difficulties in Opsoclonus-Myoclonus Syndrome: A Pilot Project of Presentation and Possible Prevalence. J. Child. Neurol. 2016, 31, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.; Schleiermacher, G.; Michon, J.; Valteau-Couanet, D.; Brisse, H.; Peuchmaur, M.; Sarnacki, S.; Martelli, H.; Desguerre, I.; Tardieu, M. Opsoclonus-myoclonus in children associated or not with neuroblastoma. Eur. J. Paediatr. Neurol. 2010, 14, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Morales La Madrid, A.; Rubin, C.M.; Kohrman, M.; Pytel, P.; Cohn, S.L. Opsoclonus-myoclonus and anti-Hu positive limbic encephalitis in a patient with neuroblastoma. Pediatr. Blood Cancer 2012, 58, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Pranzatelli, M.R.; Tate, E.D. Trends and tenets in relapsing and progressive opsoclonus-myoclonus syndrome. Brain Dev. 2016, 38, 439–449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.; Tejani, I.; Shah, N.; Olaosebikan, R.; Kumar, A.; Naik, S. Review of Opsoclonus-Myoclonus Ataxia Syndrome in Pediatric Patients. Children 2024, 11, 367. https://doi.org/10.3390/children11030367
Hsu M, Tejani I, Shah N, Olaosebikan R, Kumar A, Naik S. Review of Opsoclonus-Myoclonus Ataxia Syndrome in Pediatric Patients. Children. 2024; 11(3):367. https://doi.org/10.3390/children11030367
Chicago/Turabian StyleHsu, Mandy, Isbaah Tejani, Nidhi Shah, Rasaq Olaosebikan, Ashutosh Kumar, and Sunil Naik. 2024. "Review of Opsoclonus-Myoclonus Ataxia Syndrome in Pediatric Patients" Children 11, no. 3: 367. https://doi.org/10.3390/children11030367
APA StyleHsu, M., Tejani, I., Shah, N., Olaosebikan, R., Kumar, A., & Naik, S. (2024). Review of Opsoclonus-Myoclonus Ataxia Syndrome in Pediatric Patients. Children, 11(3), 367. https://doi.org/10.3390/children11030367




