The Thousand Faces of Invasive Group A Streptococcal Infections: Update on Epidemiology, Symptoms, and Therapy
Abstract
:1. Introduction
2. Epidemiology
3. Emm Types and Other Virulence Factors
4. Clinical Features
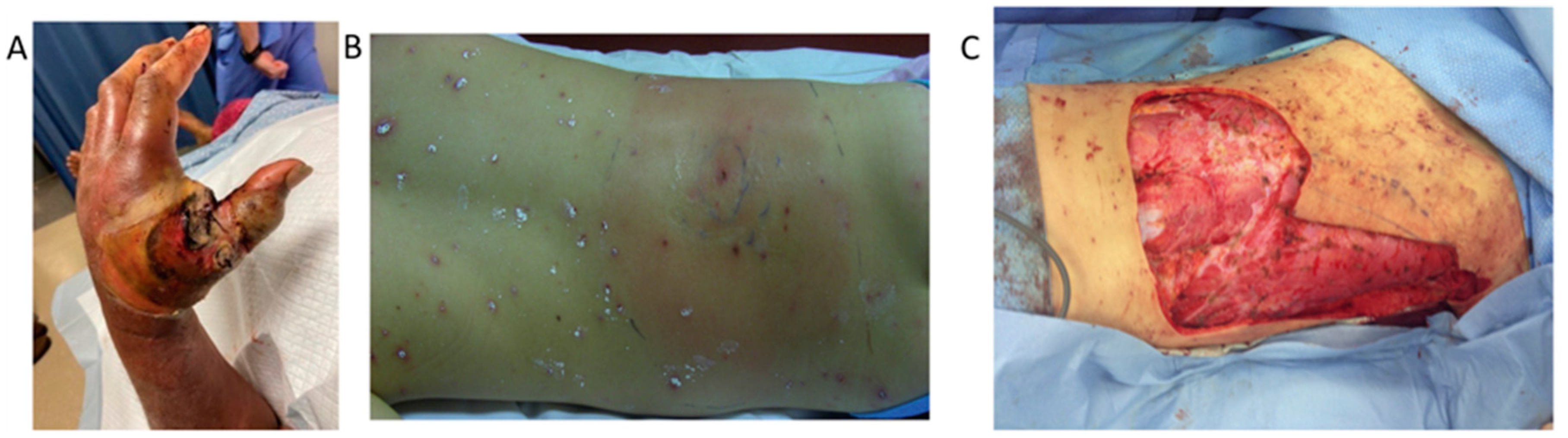
5. Treatment
6. Prevention
7. Contact Prophylaxis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steer, A.C.; Lamagni, T.; Curtis, N.; Carapetis, J.R. Invasive Group A Streptococcal Disease: Epidemiology, Pathogenesis and Management. Drugs 2012, 72, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Increased Incidence of Scarlet Fever and Invasive Group A Streptococcus Infection—Multi-Country. 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429 (accessed on 15 December 2022).
- GOV UK. Group A Streptococcal Infections: Report on Seasonal Activity in England, 2022 to 2023. 2023. Available online: https://www.gov.uk/government/publications/group-a-streptococcal-infections-activity-during-the-2022-to-2023-season/group-a-streptococcal-infections-report-on-seasonal-activity-in-england-2022-to-2023 (accessed on 29 June 2023).
- De Gier, B.; Marchal, N.; De Beer-Schuurman, I.; Te Wierik, M.; Hooiveld, M.; ISIS-AR Study Group; GAS Study Group; De Melker, H.E.; Van Sorge, N.M. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Eurosurveillance 2023, 28, 2200941. [Google Scholar] [CrossRef] [PubMed]
- Holdstock, V.; Twynam-Perkins, J.; Bradnock, T.; Dickson, E.M.; Harvey-Wood, K.; Kalima, P.; King, J.; Olver, W.J.; Osman, M.; Sabharwal, A.; et al. National case series of group A streptococcus pleural empyema in children: Clinical and microbiological features. Lancet Infect. Dis. 2023, 23, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.I.; Shewmaker, P.L.; Facklam, R.R.; Farley, M.M.; Beneden, C.; Beall, B. Identification of superantigen genes speM, ssa, and smeZ in invasive strains of beta-hemolytic group C and G streptococci recovered from humans. FEMS Microbiol. Lett. 2003, 229, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lintges, M.; Van Der Linden, M.; Hilgers, R.; Arlt, S.; Al-Lahham, A.; Reinert, R.R.; Plücken, S.; Rink, L. Superantigen Genes Are More Important than the emm Type for the Invasiveness of Group A Streptococcus Infection. J. Infect. Dis. 2010, 202, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, E.; Vergnano, S.; Kakuchi, I.; Bruce, M.G.; Chaurasia, S.; David, S.; Dramowski, A.; Georges, S.; Guy, R.; Lamagni, T.; et al. Invasive group A streptococcal disease in pregnant women and young children: A systematic review and meta-analysis. Lancet Infect. Dis. 2022, 22, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Meehan, M.; Murchan, S.; Gavin, P.J.; Drew, R.J.; Cunney, R. Epidemiology of an upsurge of invasive group A streptococcal infections in Ireland, 2012–2015. J. Infect. 2018, 77, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zangarini, L.; Martiny, D.; Miendje Deyi, V.Y.; Hites, M.; Maillart, E.; Hainaut, M.; Delforge, M.; Botteaux, A.; Matheeussen, V.; Goossens, H.; et al. Incidence and clinical and microbiological features of invasive and probable invasive streptococcal group A infections in children and adults in the Brussels-Capital Region, 2005–2020. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Tapiainen, T.; Launonen, S.; Renko, M.; Saxen, H.; Salo, E.; Korppi, M.; Kainulainen, L.; Heiskanen-Kosma, T.; Lindholm, L.; Vuopio, J.; et al. Invasive Group A Streptococcal Infections in Children: A Nationwide Survey in Finland. Pediatr. Infect. Dis. J. 2016, 35, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, L.M.; Ingels, H.; Schønheyder, H.C.; Hoffmann, S. Nationwide laboratory-based surveillance of invasive beta-haemolytic streptococci in Denmark from 2005 to 2011. Clin. Microbiol. Infect. 2014, 20, O216–O223. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Vázquez, E.; Aguilera-Alonso, D.; Carbayo, T.; Figueroa-Ospina, L.M.; Sanz-Santaeufemia, F.; Baquero-Artigao, F.; Vázquez-Ordoñez, C.; Carrasco-Colom, J.; Blázquez-Gamero, D.; Jiménez-Montero, B.; et al. Epidemiology and clinical features of Streptococcus pyogenes bloodstream infections in children in Madrid, Spain. Eur. J. Pediatr. 2023, 182, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.M.; Hutton, S.; Peterson, E.; Blackstock, A.J.; Hahn, C.G.; Turner, K.; Carter, K.K. Increasing Incidence of Invasive Group A Streptococcus Disease, Idaho, USA, 2008–2019. Emerg. Infect. Dis. 2022, 28, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.M.; Langworthy, K.; Manning, L. The Australian burden of invasive group A streptococcal disease: A narrative review. Intern. Med. J. 2021, 51, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, A.B.; Jansen Van Rensburg, M.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.J.; Van Der Linden, M.P.G.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [CrossRef] [PubMed]
- CDC Gov. Active Bacterial Core Surveillance (ABCs), Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/abcs/reports-findings/surv-reports.html (accessed on 19 May 2020).
- European Centre for Disease Prevention and Control. Increase in Invasive Group A Streptococcal Infections among Children in Europe, Including Fatalities. 2022. Available online: https://www.ecdc.europa.eu/en/news-events/increase-invasive-group-streptococcal-infections-among-children-europe-including (accessed on 12 December 2022).
- Guy, R.; Henderson, K.L.; Coelho, J.; Hughes, H.; Mason, E.L.; Gerver, S.M.; Demirjian, A.; Watson, C.; Sharp, A.; Brown, C.S.; et al. Increase in invasive group A streptococcal infection notifications, England, 2022. Eurosurveillance 2023, 28, 2200942. [Google Scholar] [CrossRef] [PubMed]
- Van Der Putten, B.C.L.; Bril-Keijzers, W.C.M.; Rumke, L.W.; Vestjens, S.M.T.; Koster, L.A.M.; Willemsen, M.; Van Houten, M.A.; Rots, N.Y.; Vlaminckx, B.J.M.; De Gier, B.; et al. Novel emm4 lineage associated with an upsurge in invasive group A streptococcal disease in The Netherlands, 2022. Microb. Genom. 2023, 9, 001026. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, T.B.; Munkstrup, C.; Edslev, S.M.; Baig, S.; Nielsen, S.; Funk, T.; Kristensen, D.K.; Jacobsen, L.H.; Ravn, S.F.; Bindslev, N.; et al. Increase in invasive group A streptococcal infections and emergence of novel, rapidly expanding sub-lineage of the virulent Streptococcus pyogenes M1 clone, Denmark, 2023. Eurosurveillance 2023, 28, 2300291. [Google Scholar] [CrossRef] [PubMed]
- Peltola, V.T.; Murti, K.G.; McCullers, J.A. Influenza Virus Neuraminidase Contributes to Secondary Bacterial Pneumonia. J. Infect. Dis. 2005, 192, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wissinger, E.; Goulding, J.; Hussell, T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin. Immunol. 2009, 21, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Sandrini, S.; Datta, S.; Freestone, P.; Shafeeq, S.; Radhakrishnan, P.; Williams, G.; Glenn, S.M.; Kuipers, O.P.; Hirst, R.A.; et al. Respiratory Syncytial Virus Increases the Virulence of Streptococcus pneumoniae by Binding to Penicillin Binding Protein 1a. A New Paradigm in Respiratory Infection. Am. J. Respir. Crit. Care Med. 2014, 190, 196–207. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.J.; Drèze, P.-A.; Vu, T.; Bessen, D.E.; Guglielmini, J.; Steer, A.C.; Carapetis, J.R.; Van Melderen, L.; Sriprakash, K.S.; Smeesters, P.R. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin. Microbiol. Infect. 2013, 19, E222–E229. [Google Scholar] [CrossRef] [PubMed]
- Steer, A.C.; Law, I.; Matatolu, L.; Beall, B.W.; Carapetis, J.R. Global emm type distribution of group A streptococci: Systematic review and implications for vaccine development. Lancet Infect. Dis. 2009, 9, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Smeesters, P.R.; McMillan, D.J.; Sriprakash, K.S. The streptococcal M protein: A highly versatile molecule. Trends Microbiol. 2010, 18, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Sagar, V.; Toor, D.; Lyngdoh, V.; Nongrum, G.; Kapoor, M.; Chakraborti, A. Group A Streptococcus Infections: Their Mechanisms, Epidemiology, and Current Scope of Vaccines. Cureus 2022, 14, e33146. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Wotton, J.T.; Shet, A.; Kaplan, E.L. A Comparison of Group A Streptococci from Invasive and Uncomplicated Infections: Are Virulent Clones Responsible for Serious Streptococcal Infections? J. Infect. Dis. 2002, 185, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.J.; Musser, J.M. Molecular Pathogenesis of Necrotizing Fasciitis. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Luca-Harari, B.; Darenberg, J.; Neal, S.; Siljander, T.; Strakova, L.; Tanna, A.; Creti, R.; Ekelund, K.; Koliou, M.; Tassios, P.T.; et al. Clinical and Microbiological Characteristics of Severe Streptococcus pyogenes Disease in Europe. J. Clin. Microbiol. 2009, 47, 1155–1165. [Google Scholar] [CrossRef]
- Mistou, M.Y.; Dramsi, S.; Brega, S.; Poyart, C.; Trieu-Cuot, P. Centre National de Référence des Streptococques-Rapport d’Activité 2009. 2009. Available online: https://cnr-strep.fr/images/CNR-STREP/rapport/Rapport_CNR-Strep_2007-2010.pdf (accessed on 15 February 2024).
- Lynskey, N.N.; Jauneikaite, E.; Li, H.K.; Zhi, X.; Turner, C.E.; Mosavie, M.; Pearson, M.; Asai, M.; Lobkowicz, L.; Chow, J.Y.; et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: A population-based molecular epidemiological study. Lancet Infect. Dis. 2019, 19, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, J.P.; Lin, Q.; Lammens, C.; Smeesters, P.R.; Van Kleef-van Koeveringe, S.; Matheeussen, V.; Malhotra-Kumar, S. Increase in bloodstream infections caused by emm1 group A Streptococcus correlates with emergence of toxigenic M1UK, Belgium, May 2022 to August 2023. Eurosurveillance 2023, 28, 2300422. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, C.; Bajanca-Lavado, M.P.; Mamede, R.; Carvalho, A.A.; Rodrigues, F.; Melo-Cristino, J.; Ramirez, M.; Friães, A.; Portuguese Group for the Study of Streptococcal Disease. Sustained increase of paediatric invasive Streptococcus pyogenes infections dominated by M1UK and diverse emm12 isolates, Portugal, September 2022 to May 2023. Eurosurveillance 2023, 28, 2300427. [Google Scholar] [CrossRef] [PubMed]
- Steer, A.C.; Jenney, A.J.W.; Oppedisano, F.; Batzloff, M.R.; Hartas, J.; Passmore, J.; Russell, F.M.; Kado, J.H.H.; Carapetis, J.R. High burden of invasive β-haemolytic streptococcal infections in Fiji. Epidemiol. Infect. 2008, 136, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, A.; Duke, T.; Steer, A.; Smeesters, P.R. Severe group A streptococcal infections in a paediatric intensive care unit. J. Paediatr. Child Health 2014, 50, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Davies, H.D.; Low, D.E.; Schwartz, B.; Green, K.; the Ontario Group A Streptococcal Study Group; McGeer, A. Invasive Group A Streptococcal Disease in Children and Association With Varicella-Zoster Virus Infection. Pediatrics 2000, 105, e60. [Google Scholar] [CrossRef] [PubMed]
- CDC. Streptococcal Toxic Shock Syndrome. In STSS Clinical Case Definition; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- Johansson, L.; Thulin, P.; Low, D.E.; Norrby-Teglund, A. Getting under the Skin: The Immunopathogenesis of Streptococcus pyogenes Deep Tissue Infections. Clin. Infect. Dis. 2010, 51, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Bingöl-Koloğlu, M.; Yıldız, R.V.; Alper, B.; Yağmurlu, A.; Çiftçi, E.; Gökçora, İ.H.; İnce, E.; Emiroğlu, M.; Dindar, H. Necrotizing fasciitis in children: Diagnostic and therapeutic aspects. J. Pediatr. Surg. 2007, 42, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Lamagni, T.L.; Darenberg, J.; Luca-Harari, B.; Siljander, T.; Efstratiou, A.; Henriques-Normark, B.; Vuopio-Varkila, J.; Bouvet, A.; Creti, R.; Ekelund, K.; et al. Epidemiology of Severe Streptococcus pyogenes Disease in Europe. J. Clin. Microbiol. 2008, 46, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Lamagni, T.L.; Neal, S.; Keshishian, C.; Alhaddad, N.; George, R.; Duckworth, G.; Vuopio-Varkila, J.; Efstratiou, A. Severe Streptococcus pyogenes Infections, United Kingdom, 2003–2004. Emerg. Infect. Dis. 2008, 14, 202–209. [Google Scholar] [CrossRef]
- Jamal, N.; Teach, S.J. Necrotizing Fasciitis. Pediatr. Emerg. Care 2011, 27, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Zundel, S.; Lemaréchal, A.; Kaiser, P.; Szavay, P. Diagnosis and Treatment of Pediatric Necrotizing Fasciitis: A Systematic Review of the Literature. Eur. J. Pediatr. Surg. 2016, 27, 127–137. [Google Scholar] [CrossRef]
- Eneli, I.; Davies, H.D. Epidemiology and Outcome of Necrotizing Fasciitis in Children: An Active Surveillance Study of the Canadian Paediatric Surveillance Program. J. Pediatr. 2007, 151, 79–84.e1. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Kapoor, R.; Yadav, P.S.; Saxena, S.; Agarwal, K.; Solanki, R.S.; Gupta, A.; Choudhury, S.R.; Chadha, R. Morbidity and Mortality of Necrotizing Fasciitis and Their Prognostic Factors in Children. J. Indian Assoc. Pediatr. Surg. 2022, 27, 577–584. [Google Scholar]
- Wang, J.-M.; Lim, H.-K. Necrotizing fasciitis: Eight-year experience and literature review. Braz. J. Infect. Dis. 2014, 18, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Liese, J.G.; Schoen, C.; Van Der Linden, M.; Lehmann, L.; Goettler, D.; Keller, S.; Maier, A.; Segerer, F.; Rose, M.A.; Streng, A. Changes in the incidence and bacterial aetiology of paediatric parapneumonic pleural effusions/empyema in Germany, 2010–2017: A nationwide surveillance study. Clin. Microbiol. Infect. 2019, 25, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Megged, O. Characteristics of Streptococcus pyogenes Versus Streptococcus pneumoniae Pleural Empyema and Pneumonia With Pleural Effusion in Children. Pediatr. Infect. Dis. J. 2020, 39, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Hutton, D.; Kameda-Smith, M.; Afshari, F.T.; Elawadly, A.; Hogg, F.; Mehta, S.; Samarasekara, J.; Aquilina, K.; Jeelani, N.U.O.; Tahir, M.Z.; et al. Intracranial invasive group A streptococcus: A neurosurgical emergency in children. J. Neurosurg. Pediatr. 2023, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, E.; Woytanowski, J.; Sibliss, K.; Sheffer, I. Streptococcus pyogenes and invasive central nervous system infection. SAGE Open Med. Case Rep. 2018, 6, 2050313X1877558. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, R.E.; Roberson, A.; Cieslak, P.R.; Lynfield, R.; Gershman, K.; Craig, A.; Albanese, B.A.; Farley, M.M.; Barrett, N.L.; Spina, N.L.; et al. The Epidemiology of Invasive Group A Streptococcal Infection and Potential Vaccine Implications: United States, 2000–2004. Clin. Infect. Dis. 2007, 45, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Van De Beek, D.; De Gans, J.; Spanjaard, L.; Sela, S.; Vermeulen, M.; Dankert, J. Group A Streptococcal Meningitis in Adults: Report of 41 Cases and a Review of the Literature. Clin. Infect. Dis. 2002, 34, e32–e36. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Torres, R.S.L.; Fedalto, L.E.; De Almeida Torres, R.F.; Steer, A.C.; Smeesters, P.R. Group A Streptococcus Meningitis in Children. Pediatr. Infect. Dis. J. 2013, 32, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Baraldés, M.A.; Domingo, P.; Mauri, A.; Monmany, J.; Castellanos, M.; Pericas, R.; Vázquez, G. Group A Streptococcal Meningitis in the Antibiotic Era. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.R.; Bradley, J.S.; Chatterjee, A.; Copley, L.A.; Robinson, J.; Kronman, M.P.; Arrieta, A.; Fowler, S.L.; Harrison, C.; Carrillo-Marquez, M.A.; et al. Clinical Practice Guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on Diagnosis and Management of Acute Hematogenous Osteomyelitis in Pediatrics. J. Pediatr. Infect. Dis. Soc. 2021, 10, 801–844. [Google Scholar] [CrossRef] [PubMed]
- Gornitzky, A.L.; Kim, A.E.; O’Donnell, J.M.; Swarup, I. Diagnosis and Management of Osteomyelitis in Children: A Critical Analysis Review. JBJS Rev. 2020, 8, e19.00202. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Wood, J.B.; Creech, C.B.; Williams, D.; Jimenez-Truque, N.; Yildirim, I.; Sederdahl, B.; Daugherty, M.; Hussaini, L.; Munye, M.; et al. Clinical Epidemiology and Outcomes of Pediatric Musculoskeletal Infections. J. Pediatr. 2021, 234, 236–244.e2. [Google Scholar] [CrossRef] [PubMed]
- Bréhin, C.; Claudet, I.; Dubois, D.; Sales De Gauzy, J.; Vial, J.; Chaix, Y.; Grouteau, E. Assessing the management of pediatric bone and joint infections according to French guidelines. Méd. Mal. Infect. 2020, 50, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Kuong, E.E.; To, M.; Yuen, M.H.; Choi, A.K.Y.; Fong, C.M.; Chow, W. Pitfalls in diagnosing septic arthritis in Hong Kong children: Ten years’ experience. Hong Kong Med. J. 2012, 18, 482–487. [Google Scholar] [PubMed]
- Dartnell, J.; Ramachandran, M.; Katchburian, M. Haematogenous acute and subacute paediatric osteomyelitis: A systematic review of the literature. J. Bone Jt. Surg. Br. Vol. 2012, 94-B, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Volzke, J.; Schultz, D.; Kordt, M.; Müller, M.; Bergmann, W.; Methling, K.; Kreikemeyer, B.; Müller-Hilke, B.; KoInfekt Study Group. Inflammatory Joint Disease Is a Risk Factor for Streptococcal Sepsis and Septic Arthritis in Mice. Front. Immunol. 2020, 11, 579475. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-L.; Wang, S.-M.; Yang, Y.-J.; Tsai, C.-H.; Liu, C.-C. Septic arthritis in children: Relationship of causative pathogens, complications, and outcome. J. Microbiol. Immunol. Infect. 2003, 36, 41–46. [Google Scholar] [PubMed]
- Yu, D.; Gao, W.; Guo, D.; Lu, Q.; Chen, Y.; Zheng, Y.; Wang, W.; Yang, Y. Case Report: Septic arthritis in children caused by Streptococcus pyogenes—Rational use of antibiotics. Front. Cell. Infect. Microbiol. 2023, 12, 1117217. [Google Scholar] [CrossRef] [PubMed]
- Krzysztofiak, A.; Roversi, M.; Musolino, A.; Cirillo, M.; Toniolo, R.M.; Mazza, O.; Gargiullo, L.; Lancella, L.; Rossi, P.; Villani, A.; et al. Clinical report and predictors of sequelae of 319 cases of pediatric bacterial osteomyelitis. Sci. Rep. 2022, 12, 14846. [Google Scholar] [CrossRef] [PubMed]
- Von Heideken, J.; Bennet, R.; Eriksson, M.; Hertting, O. A 10-year retrospective survey of acute childhood osteomyelitis in Stockholm, Sweden. J. Paediatr. Child Health 2020, 56, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, J.; Kuester, V.; Reznicek, J. Pediatric Necrotizing Fasciitis. J. Pediatr. Orthop. Soc. N. Am. 2023, 5, 728. [Google Scholar] [CrossRef]
- Pfeifle, V.A.; Gros, S.J.; Holland-Cunz, S.; Kämpfen, A. Necrotizing fasciitis in children due to minor lesions. J. Pediatr. Surg. Case Rep. 2017, 25, 52–55. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Executive Summary: Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Gibbons, A.E.; Bergstrom, R.; Winn, V. The Eagle Effect Revisited: Efficacy of Clindamycin, Erythromycin, and Penicillin in the Treatment of Streptococcal Myositis. J. Infect. Dis. 1988, 158, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lepoutre, A.; Doloy, A.; Bidet, P.; Leblond, A.; Perrocheau, A.; Bingen, E.; Trieu-Cuot, P.; Bouvet, A.; Poyart, C.; Lévy-Bruhl, D.; et al. Epidemiology of Invasive Streptococcus pyogenes Infections in France in 2007. J. Clin. Microbiol. 2011, 49, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Lapthorne, S.; McWade, R.; Scanlon, N.; Ní Bhaoill, S.; Page, A.; O’Donnell, C.; Dornikova, G.; Hannan, M.; Lynch, B.; Lynch, M.; et al. Rising Clindamycin Resistance in Group A Streptococcus in an Irish Healthcare Institution. Access Microbiol. 2024. [Google Scholar] [CrossRef]
- Seppälä, H.; Skurnik, M.; Soini, H.; Roberts, M.C.; Huovinen, P. A Novel Erythromycin Resistance Methylase Gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 1998, 42, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.; Petitpas, J.; Dib-Hajj, F.; Yuan, W.; Cronan, M.; Kamath, A.V.; Bergeron, J.; Retsema, J.A. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol. Microbiol. 1996, 22, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Platform, JMI Labs SENTRY Microbiology Visualization 2022. Available online: https://sentry-mvp.jmilabs.com/ (accessed on 1 January 2022).
- Coyle, E.A.; Cha, R.; Rybak, M.J. Influences of Linezolid, Penicillin, and Clindamycin, Alone and in Combination, on Streptococcal Pyrogenic Exotoxin A Release. Antimicrob. Agents Chemother. 2003, 47, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, C.G. Virulence factor expression by Gram-positive cocci exposed to subinhibitory concentrations of linezolid. J. Antimicrob. Chemother. 2002, 50, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Rac, H.; Bojikian, K.D.; Lucar, J.; Barber, K.E. Successful Treatment of Necrotizing Fasciitis and Streptococcal Toxic Shock Syndrome with the Addition of Linezolid. Case Rep. Infect. Dis. 2017, 2017, 5720708. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yanagihara, K.; Kaneko, Y.; Ohno, H.; Higashiyama, Y.; Miyazaki, Y.; Hirakata, Y.; Tomono, K.; Tashiro, T.; Kohno, S. A Case of Invasive Group A Streptococcus Infection Which was Successfully Treated with Linezolid. kansenshogakuzasshi 2004, 78, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Vannice, K.S.; Ricaldi, J.; Nanduri, S.; Fang, F.C.; Lynch, J.B.; Bryson-Cahn, C.; Wright, T.; Duchin, J.; Kay, M.; Chochua, S.; et al. Streptococcus pyogenes pbp2x Mutation Confers Reduced Susceptibility to β-Lactam Antibiotics. Clin. Infect. Dis. 2020, 71, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Chochua, S.; Metcalf, B.; Li, Z.; Mathis, S.; Tran, T.; Rivers, J.; Fleming-Dutra, K.E.; Li, Y.; McGee, L.; Beall, B. Invasive Group A Streptococcal Penicillin Binding Protein 2× Variants Associated with Reduced Susceptibility to β-Lactam Antibiotics in the United States, 2015–2021. Antimicrob. Agents Chemother. 2022, 66, e00802-22. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr. Crit. Care Med. 2020, 21, e52–e106. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; McGeer, A.; Norrby-Teglund, A.; Kotb, M.; Schwartz, B.; O’Rourke, K.; Talbot, J.; Low, D.E.; The Canadian Streptococcal Study Group. Intravenous Immunoglobulin Therapy for Streptococcal Toxic Shock Syndrome—A Comparative Observational Study. Clin. Infect. Dis. 1999, 28, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Adalat, S.; Dawson, T.; Hackett, S.J.; Clark, J.E.; In Association with the British Paediatric Surveillance Unit. Toxic shock syndrome surveillance in UK children. Arch. Dis. Child. 2014, 99, 1078–1082. [Google Scholar] [CrossRef]
- Linner, A.; Darenberg, J.; Sjolin, J.; Henriques-Normark, B.; Norrby-Teglund, A. Clinical Efficacy of Polyspecific Intravenous Immunoglobulin Therapy in Patients With Streptococcal Toxic Shock Syndrome: A Comparative Observational Study. Clin. Infect. Dis. 2014, 59, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Darenberg, J.; Ihendyane, N.; Sjolin, J.; Aufwerber, E.; Haidl, S.; Follin, P.; Andersson, J.; Norrby-Teglund, A.; The Streptlg Study Group. Intravenous Immunoglobulin G Therapy in Streptococcal Toxic Shock Syndrome: A European Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2003, 37, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lesko, S.M.; O’Brien, K.L.; Schwartz, B.; Vezina, R.; Mitchell, A.A. Invasive Group A Streptococcal Infection and Nonsteroidal Antiinflammatory Drug Use Among Children With Primary Varicella. Pediatrics 2001, 107, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E.; Hackett, S.P.; Chang, A.; Peer, G.; Kosanke, S.; Emerson, T.; Hinshaw, L. Group A streptococcal bacteremia: The role of tumor necrosis factor in shock and organ failure. J. Infect. Dis. 1996, 173, 619–626. [Google Scholar] [CrossRef] [PubMed]
- LaRock, D.L.; Russell, R.; Johnson, A.F.; Wilde, S.; LaRock, C.N. Group A Streptococcus Infection of the Nasopharynx Requires Proinflammatory Signaling through the Interleukin-1 Receptor. Infect. Immun. 2020, 88, e00356-20. [Google Scholar] [CrossRef] [PubMed]
- Vallette, J.D.; Goldberg, R.N.; Suguihara, C.; Moral, T.D.; Martinez, O.; Lin, J.; Thompso, R.C.; Bancalari, E. Effect of an Interleukin-1 Receptor Antagonist on the Hemodynamic Manifestations of Group B Streptococcal Sepsis. Pediatr. Res. 1995, 38, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Walker, M.J. Update on group A streptococcal vaccine development. Curr. Opin. Infect. Dis. 2020, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Giersing, B.K.; Vekemans, J.; Nava, S.; Kaslow, D.C.; Moorthy, V. Report from the World Health Organization’s third Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 8–10th June 2016. Vaccine 2019, 37, 7315–7327. [Google Scholar] [CrossRef] [PubMed]
- Pastural, É.; McNeil, S.A.; MacKinnon-Cameron, D.; Ye, L.; Langley, J.M.; Stewart, R.; Martin, L.H.; Hurley, G.J.; Salehi, S.; Penfound, T.A.; et al. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: A randomized, controlled phase I study. Vaccine 2020, 38, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Chiang, E.Y.; Lederer, J.W. Recombinant tetravalent group A streptococcal M protein vaccine. J. Immunol. 1993, 151, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Simmons, M.; Chiang, E.C.; Chiang, E.Y. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 1996, 14, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 1999, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Walls, M.A.; Stroop, S.D.; Reddish, M.A.; Beall, B.; Dale, J.B. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 2002, 70, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Sekuloski, S.; Batzloff, M.R.; Griffin, P.; Parsonage, W.; Elliott, S.; Hartas, J.; O’Rourke, P.; Marquart, L.; Pandey, M.; Rubin, F.A.; et al. Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS ONE 2018, 13, e0198658. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Hsueh, P.-R.; Lin, T.-Y.; Yan, D.-C.; Hsia, S.-H. A Family Cluster of Streptococcal Toxic Shock Syndrome in Children: Clinical Implication and Epidemiological Investigation. Pediatrics 2001, 107, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, A.; Romanus, V.; Burman, L.G. Outbreak of Group A Streptococcal Bacteremia in Sweden: An Epidemiologic and Clinical Study. J. Infect. Dis. 1991, 164, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.B. Outbreak of Invasive Group A Streptococcal Infections in a Nursing Home: Lessons on Prevention and Control. Arch. Intern. Med. 1992, 152, 1017. [Google Scholar] [CrossRef] [PubMed]
- UK Guidelines for the Management of Contacts of Invasive Group A Streptococcus (iGAS) Infection in Community Settings. March 2023. Available online: https://assets.publishing.service.gov.uk/media/64071ec5d3bf7f25fa417a91/Management-of-contacts-of-invasive-group-a-streptococcus.pdf (accessed on 7 March 2023).
- Moore, D.L.; Allen, U.D.; Mailman, T. Invasive group A streptococcal disease: Management and chemoprophylaxis. Paediatr. Child Health 2019, 24, 128. [Google Scholar] [CrossRef] [PubMed]
- The Prevention of Invasive Group A Streptococcal Infections Workshop Participants Prevention of Invasive Group A Streptococcal Disease among Household Contacts of Case Patients and among Postpartum and Postsurgical Patients: Recommendations from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2002, 35, 950–959. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercadante, S.; Ficari, A.; Romani, L.; De Luca, M.; Tripiciano, C.; Chiurchiù, S.; Calo Carducci, F.I.; Cursi, L.; Di Giuseppe, M.; Krzysztofiak, A.; et al. The Thousand Faces of Invasive Group A Streptococcal Infections: Update on Epidemiology, Symptoms, and Therapy. Children 2024, 11, 383. https://doi.org/10.3390/children11040383
Mercadante S, Ficari A, Romani L, De Luca M, Tripiciano C, Chiurchiù S, Calo Carducci FI, Cursi L, Di Giuseppe M, Krzysztofiak A, et al. The Thousand Faces of Invasive Group A Streptococcal Infections: Update on Epidemiology, Symptoms, and Therapy. Children. 2024; 11(4):383. https://doi.org/10.3390/children11040383
Chicago/Turabian StyleMercadante, Stefania, Andrea Ficari, Lorenza Romani, Maia De Luca, Costanza Tripiciano, Sara Chiurchiù, Francesca Ippolita Calo Carducci, Laura Cursi, Martina Di Giuseppe, Andrzej Krzysztofiak, and et al. 2024. "The Thousand Faces of Invasive Group A Streptococcal Infections: Update on Epidemiology, Symptoms, and Therapy" Children 11, no. 4: 383. https://doi.org/10.3390/children11040383
APA StyleMercadante, S., Ficari, A., Romani, L., De Luca, M., Tripiciano, C., Chiurchiù, S., Calo Carducci, F. I., Cursi, L., Di Giuseppe, M., Krzysztofiak, A., Bernardi, S., & Lancella, L. (2024). The Thousand Faces of Invasive Group A Streptococcal Infections: Update on Epidemiology, Symptoms, and Therapy. Children, 11(4), 383. https://doi.org/10.3390/children11040383





