Scabies in Infants: Series of 51 Cases
Abstract
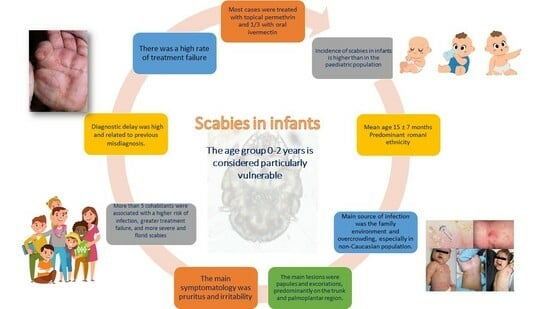
:1. Introduction
2. Materials and Methods
- Epidemiological variables: age, sex, number of household members, ethnicity, and source of contagion;
- Clinical variables: type of lesions, location of lesions, symptoms (e.g., pruritus, pain, and fever), and complications;
- Diagnosis and treatment variables: time to diagnosis, prescribed treatments, and time to healing.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, A.K.; Lam, J.M.; Leong, K.F. Scabies: A Neglected Global Disease. Curr. Pediatr. Rev. 2020, 16, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Wu, J.; Tizek, L.; Ziehfreund, S.; Zink, A. Prevalence of scabies worldwide—An updated systematic literature review in 2022. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Skayem, C.; Majda, A.; Gary, C.; Hemery, F.; Mahé, E.; Caux, F.; Dupin, N.; Senet, P.; Greder-Belan, A.; Hillion, B.; et al. Severe Scabies: A French Multi-centre Study Involving 95 Patients with Crusted and Profuse Disease and Review of the Literature. Acta Derm.-Venereol. 2023, 103, adv00878. [Google Scholar] [CrossRef] [PubMed]
- Trave, I.; Muracchioli, A.; Cozzani, E.; Parodi, A. Scabies revisited in the COVID-19 era. J. Eur. Acad. Dermatol. Venereol. 2022, 36, E760–E761. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, Ö.; Aktaş, H. The explosion in scabies cases during COVID-19 pandemic. Dermatol. Ther. 2020, 33, e13662. [Google Scholar] [CrossRef] [PubMed]
- Metin, N. Impact of Pandemic in the Frequency of Scabies: Possible Scabies Outbreak Scenario Aftermath COVID-19. Turk. J. Parasitol. 2021, 45, 190–194. [Google Scholar] [CrossRef]
- López-Sundh, A.E.; Gómez-Fernández, C.; Marlasca-SanMartín, P.; Pérez-González, D.; Reguero-DelCura, L.; Rubia-Fernández, L.; González-López, M.A. Neonatal scabies in times of confinement: An unexpected guest to be recognised. J. Paediatr. Child Health 2020, 57, 1505–1507. [Google Scholar] [CrossRef] [PubMed]
- Etiz, P.; Altunsu, A.T. The Scabies Epidemic during the COVID-19 Pandemic. Turk. J. Parasitol. 2023, 47, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Bravo, L.; Fernandez-Martinez, B.; Gómez-Barroso, D.; Gherasim, A.; García-Gómez, M.; Benito, A.; Herrador, Z. Scabies in Spain? A comprehensive epidemiological picture. PLoS ONE 2021, 16, e0258780. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Jradi, H.; AlBuraikan, D.A.; Almuqbil, B.I.; Albaijan, M.A.; Al-Shehri, A.M.; Al-Jahdali, H. Rate and factors for scabies recurrence in children in Saudi Arabia: A retrospective study. BMC Pediatr. 2019, 19, 187. [Google Scholar] [CrossRef]
- Bernigaud, C.; Fischer, K.; Chosidow, O. The Management of Scabies in the 21st Century: Past, Advances and Potentials. Acta Derm.-Venereol. 2020, 100, adv00112. [Google Scholar] [CrossRef]
- Hill, T.A.; Cohen, B. Scabies in babies. Pediatr. Dermatol. 2017, 34, 690–694. [Google Scholar] [CrossRef]
- Craig, N.B.; Burkhart, C.G.; Morrell, D.S. Infections, Infestations. In Dermatology, 4th ed.; Bolognia, J.L., Jorizzo, J.L., Schaffer, J.I., Torrelo, A., Eds.; Mosby-Elsevier: Amsterdam, The Netherlands, 2018; pp. 1291–1300. [Google Scholar]
- Riebenbauer, K.; Weber, P.B.; Haitel, A.; Walochnik, J.; Valencak, J.; Meyersburg, D.; Kinaciyan, T.; Handisurya, A. Comparison of Permethrin-Based Treatment Strategies against Scabies in Infants and Young Children. J. Pediatr. 2022, 245, 184–189. [Google Scholar] [CrossRef]
- Boralevi, F.; Diallo, A.; Miquel, J.; Guerin-Moreau, M.; Bessis, D.; Chiavérini, C.; Plantin, P.; Hubiche, T.; Maruani, A.; Lassalle, M.; et al. Clinical Phenotype of Scabies by Age. Pediatrics 2014, 133, e910–e916. [Google Scholar] [CrossRef]
- Engelman, D.; Yoshizumi, J.; Hay, R.; Osti, M.; Micali, G.; Norton, S.; Walton, S.; Boralevi, F.; Bernigaud, C.; Bowen, A.; et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br. J. Dermatol. 2020, 183, 808–820. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Keshri, A.; Sabaretnam, M. Selection of appropriate statistical methods for data analysis. Ann. Card. Anaesth. 2019, 22, 297–301. [Google Scholar] [CrossRef]
- Anderson, K.L.; Strowd, L.C. Epidemiology, Diagnosis, and Treatment of Scabies in a Dermatology Office. J. Am. Board Fam. Med. 2017, 30, 78–84. [Google Scholar] [CrossRef]
- Kaburi, B.B.; Ameme, D.K.; Adu-Asumah, G.; Dadzie, D.; Tender, E.K.; Addeh, S.V.; Aryee, T.; Addo-Lartey, A.; Sackey, S.O.; Wurapa, F.; et al. Outbreak of scabies among preschool children, Accra, Ghana, 2017. BMC Public Health 2019, 19, 746. [Google Scholar] [CrossRef]
- Chang, A.Y. On the importance of social determinants of health: A second look at scabies and failure to thrive in an immigrant female infant. Int. J. Women’s Dermatol. 2021, 7, 853–855. [Google Scholar] [CrossRef]
- Borràs-Batalla, M.; Macías-Aranda, F. Condiciones de Habitabilidad de la Población Gitana en España. Informe Final. Córdoba: KAMIRA, Federación de Asociaciones de Mujeres Gitanas, 2023. Available online: https://federacionkamira.com (accessed on 13 March 2024).
- Grodner, C.; Miquel, J.; Hadj-Rabia, S.; Mallet, S.; Boralevi, F.; Mazereeuw-Hautier, J.; Benzebouchi, N.; Dhers, M.; Goujon, E.; Bensaïd, P.; et al. Crusted scabies in children in France: A series of 20 cases. Eur. J. Pediatr. 2021, 181, 1167–1174. [Google Scholar] [CrossRef]
- Thompson, R.; Westbury, S.; Slape, D. Paediatrics: How to manage scabies. Drugs Context 2021, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salavastru, C.; Chosidow, O.; Boffa, M.; Janier, M.; Tiplica, G. European guideline for the management of scabies. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.C.; Mößner, R.; Schön, M.P.; Lippert, U. Topical scabies therapy with permethrin is effective and well tolerated in infants younger than two months. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Meyersburg, D.; Kaiser, A.; Bauer, J.W. Loss of efficacy of topical 5% permethrin for treating scabies: An Austrian single-center study. J. Dermatol. Treat. 2020, 33, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Pouessel, G.; Dumortier, J.; Lagrée, M.; Pierre, M.-H.; Ganga-Zandzou, P.-S.; Ythier, H.; Carpentier, O. La gale: Une infection fréquente en pédiatrie. Arch. Pediatr. 2012, 19, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Jittamala, P.; Monteiro, W.; Smit, M.R.; Pedrique, B.; Specht, S.; Chaccour, C.J.; Dard, C.; Del Giudice, P.; Khieu, V.; Maruani, A.; et al. Correction: A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? PLoS Neglected Trop. Dis. 2023, 17, e0011053. [Google Scholar] [CrossRef] [PubMed]
- Lobo, Y.; Wheller, L. A narrative review of the roles of topical permethrin and oral ivermectin in the management of infantile scabies. Australas. J. Dermatol. 2021, 62, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Lluch-Galcerá, J.; Carrascosa, J.; Boada, A. Epidemia de escabiosis: Los nuevos retos de una enfermedad ancestral. Actas Dermo-Sifiliogr. 2023, 114, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Martin, L.; Bursztejn, A.; Chiaverini, C.; Miquel, J.; Mahé, E.; Maruani, A.; Boralevi, F.; Groupe de Recherche de la Société Française de Dermatologie Pédiatrique. Ivermectin safety in infants and children under 15 kg treated for scabies: A multicentric observational study. Br. J. Dermatol. 2019, 182, 1003–1006. [Google Scholar] [CrossRef]
- Yürekli, A. Is there a really resistance to scabies treatment with permethrin? In vitro killing activity of permethrin on Sarcoptes scabiei from patients with resistant scabies. Dermatol. Ther. 2021, 35, e15260. [Google Scholar] [CrossRef]
- Balestri, R.; Magnano, M.; Infusino, S.D.; Rizzoli, L.; Girardelli, C.R.; Rech, G. Use of oral ivermectin in permethrin-resistant scabies: A pilot study. Dermatol. Ther. 2022, 35, e15495. [Google Scholar] [CrossRef] [PubMed]
- Trave, I.; Cozzani, E.; Parodi, A. Topical treatment failure in scabies: A survey in resistant patients. Ital. J. Dermatol. Venereol. 2023, 158, 163–164. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | n (%) * |
|---|---|
| Female sex | 32 (63%) |
| Mean age (months) ± SD | 15 ± 7 |
| Age ≥ 12 months | 26 (51%) |
| Mean number of household members | 6 ± 1 |
| Risk factors for scabies | |
| ≥5 household members | 26 (51%) |
| Atopic dermatitis | 14 (27%) |
| Prematurity | 4 (8%) |
| Previous corticosteroid therapy | 4 (8%) |
| Ethnicity | |
| European | 18 (35.3%) |
| Romani | 22 (43.2%) |
| Latin American | 7 (13.7%) |
| Middle Eastern/North African | 4 (7.8%) |
| Source of contagion | |
| Family | 48 (94%) |
| Daycare | 3 (6%) |
| Characteristics | n (%) |
|---|---|
| Type of lesions | |
| Papules | 47 (92%) |
| Pustules | 29 (57%) |
| Burrows | 28 (55%) |
| Vesicles | 24 (47%) |
| Scaly areas | 36 (71%) |
| Excoriation | 38 (75%) |
| Nodules | 18 (35%) |
| Location | |
| Head | 20 (39%) |
| Trunk | 42 (82%) |
| Arms | 29 (57%) |
| Folds | 23 (45%) |
| Palms/soles | 32 (63%) |
| Symptoms | |
| Pruritus | 48 (94%) |
| Irritability | 35 (69%) |
| Respiratory symptoms | 8 (16%) |
| Fever | 8 (16%) |
| A. Confirmed scabies At least one of: A1: Mites, eggs, or faces on light microscopy of skin samples A2: Mites, eggs, or faces visualized on an individual using a high-powered imaging device A3: Mite visualized on an individual using dermoscopy B. Clinical scabies At least one of: B1: Scabies burrows B2: Typical lesions affecting male genitalia B3: Typical lesions in a typical distribution and two history features C. Suspected scabies One of: C1: Typical lesions in a typical distribution and one history feature C2: Atypical lesions or atypical distribution and two history features History features H1: Itch H2: Positive contact history |
| Characteristics | n (%) * |
|---|---|
| Confirmed diagnosis | 23 (45%) |
| Clinical diagnosis | 24 (47%) |
| Suspected diagnosis | 4 (8%) |
| Mean time to diagnosis (days) ± SD | 27 ± 9 |
| Diagnosis ≤ 2 weeks | 28 (55%) |
| Diagnosis > 2 weeks | 23 (45%) |
| Evaluation by dermatologist | 38 (75%) |
| Treatment | n (%) |
|---|---|
| Scabicides | |
| Permethrin | 50 (98%) |
| Permethrin (>2 cycles) | 1 (2%) |
| Ivermectin | 18 (35%) |
| Ivermectin (>1 cycle) | 5 (10%) |
| Benzyl benzoate | 8 (16%) |
| Sulphur | 8 (16%) |
| Other treatments | |
| Oral antihistamines | 38 (75%) |
| Topical corticosteroids | 29 (57%) |
| Oral corticosteroids | 10 (20%) |
| Topical antibiotics | 23 (45%) |
| Oral antibiotics | 12 (24%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betlloch-Mas, I.; Boluda-Verdú, E.; Jara-Rico, N.; Sánchez-García, V.; Berbegal-De Gracia, L.; Chiner-Vives, E. Scabies in Infants: Series of 51 Cases. Children 2024, 11, 443. https://doi.org/10.3390/children11040443
Betlloch-Mas I, Boluda-Verdú E, Jara-Rico N, Sánchez-García V, Berbegal-De Gracia L, Chiner-Vives E. Scabies in Infants: Series of 51 Cases. Children. 2024; 11(4):443. https://doi.org/10.3390/children11040443
Chicago/Turabian StyleBetlloch-Mas, Isabel, Elena Boluda-Verdú, Noelia Jara-Rico, Verónica Sánchez-García, Laura Berbegal-De Gracia, and Eusebi Chiner-Vives. 2024. "Scabies in Infants: Series of 51 Cases" Children 11, no. 4: 443. https://doi.org/10.3390/children11040443
APA StyleBetlloch-Mas, I., Boluda-Verdú, E., Jara-Rico, N., Sánchez-García, V., Berbegal-De Gracia, L., & Chiner-Vives, E. (2024). Scabies in Infants: Series of 51 Cases. Children, 11(4), 443. https://doi.org/10.3390/children11040443