Furosemide and Ductus Arteriosus Closure in Very-Low-Birth-Weight Preterm Infants: A Comprehensive Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
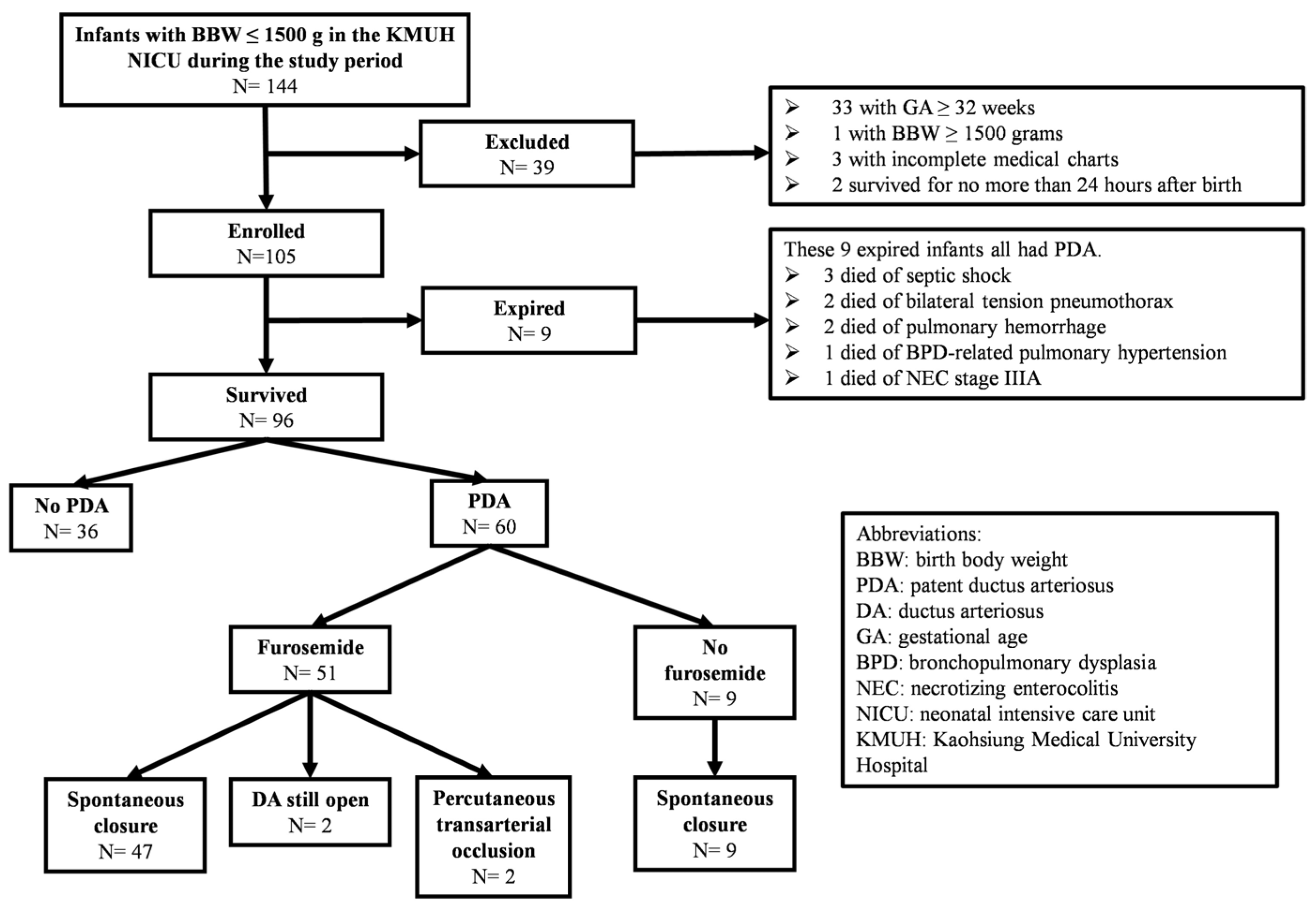
3.1. Mortality Rate of Enrolled Infants
3.2. Characteristics of VLBW Preterm Infants with/without PDA
3.3. Characteristics of VLBW Preterm Infants with PDA with/without Furosemide Exposure
3.4. Factors Associated with the Time Taken for Spontaneous Ductal Closure in VLBW Preterm Infants with Furosemide Exposure
3.5. Clinical Outcomes of VLBW Preterm Infants
3.6. Factors Associated with BPD and ROP in VLBW Preterm Infants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siew, M.L.; Kitchen, M.J.; Pas, A.B.T.; Harding, R.; Hooper, S.B. Chapter 13—Pulmonary Transition at Birth. In The Lung, 2nd ed.; Harding, R., Pinkerton, K.E., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 251–264. ISBN 9780127999418. [Google Scholar]
- Clyman, R.I.; Couto, J.; Murphy, G.M. Patent ductus arteriosus: Are current neonatal treatment options better or worse than no treatment at all? Semin. Perinatol. 2012, 36, 123–129. [Google Scholar] [CrossRef]
- Lemons, J.A.; Bauer, C.R.; Oh, W.; Korones, S.B.; Papile, L.A.; Stoll, B.J.; Verter, J.; Temprosa, M.; Wright, L.L.; Ehrenkranz, R.A.; et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001, 107, E1. [Google Scholar] [CrossRef] [PubMed]
- Kluckow, M.; Evans, N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J. Pediatr. 2000, 137, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.; Francescato, G.; Cappelleri, A.; Picciolli, I.; Mayer, A.; Mosca, F.; Fumagalli, M. Association between hemodynamically significant patent ductus arteriosus and bronchopulmonary dysplasia. J. Pediatr. 2015, 166, 1488–1492. [Google Scholar] [CrossRef]
- Gentle, S.J.; Travers, C.P.; Clark, M.; Carlo, W.A.; Ambalavanan, N. Patent Ductus Arteriosus and Development of Bronchopulmonary Dysplasia-associated Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2023, 207, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Cassady, G.; Crouse, D.T.; Kirklin, J.W.; Strange, M.J.; Joiner, C.H.; Godoy, G.; Odrezin, G.T.; Cutter, G.R.; Kirklin, J.K.; Pacifico, A.D.; et al. A randomized, controlled trial of very early prophylactic ligation of the ductus arteriosus in babies who weighed 1000 g or less at birth. N. Engl. J. Med. 1989, 320, 1511–1516. [Google Scholar] [CrossRef]
- Kluckow, M.; Evans, N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 82, F188–F194. [Google Scholar] [CrossRef] [PubMed]
- Majed, B.; Bateman, D.A.; Uy, N.; Lin, F. Patent ductus arteriosus is associated with acute kidney injury in the preterm infant. Pediatr. Nephrol. 2019, 34, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Runte, K.E.; Flyer, J.N.; Edwards, E.M.; Soll, R.F.; Horbar, J.D.; Yeager, S.B. Variation of Patent Ductus Arteriosus Treatment in Very Low Birth Weight Infants. Pediatrics 2021, 148, e2021052874. [Google Scholar] [CrossRef]
- Dice, J.E.; Bhatia, J. Patent ductus arteriosus: An overview. J. Pediatr. Pharmacol. Ther. 2007, 12, 138–146. [Google Scholar] [CrossRef]
- Porstmann, W.; Wierny, L.; Warnke, H. Closure of persistent ductus arteriosus without thoracotomy. Ger. Med. Mon. 1967, 12, 259–261. [Google Scholar] [PubMed]
- Clyman, R.I. Ibuprofen and patent ductus arteriosus. N. Engl. J. Med. 2000, 343, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Dudley, S.; Sen, S.; Hanson, A.; El Khuffash, A.; Levy, P.T. The role of furosemide and fluid management for a hemodynamically significant patent ductus arteriosus in premature infants. J. Perinatol. 2022, 42, 1703–1707. [Google Scholar] [CrossRef]
- Gillam-Krakauer, M.; Hagadorn, J.I.; Reese, J. Pharmacological closure of the patent ductus arteriosus: When treatment still makes sense. J. Perinatol. 2019, 39, 1439–1441. [Google Scholar] [CrossRef]
- Semberova, J.; Sirc, J.; Miletin, J.; Kucera, J.; Berka, I.; Sebkova, S.; O’Sullivan, S.; Franklin, O.; Stranak, Z. Spontaneous Closure of Patent Ductus Arteriosus in Infants ≤1500 g. Pediatrics 2017, 140, e20164258. [Google Scholar] [CrossRef]
- Chung, H.W.; Yang, S.T.; Liang, F.W.; Chen, H.L. Taiwan Premature Infant Follow-up Network. Clinical outcomes of different patent ductus arteriosus treatment in preterm infants born between 28 and 32 weeks in Taiwan. Pediatr. Neonatol. 2023, 64, 411–419. [Google Scholar] [CrossRef]
- Hsieh, E.M.; Hornik, C.P.; Clark, R.H.; Laughon, M.M.; Benjamin, D.K.; Smith, P. Medication use in the neonatal intensive care unit. Am. J. Perinatol. 2014, 31, 811–822. [Google Scholar] [CrossRef]
- Iacobelli, S.; Lorrain, S.; Gouyon, B.; Gambacorta, S.; Laforgia, N.; Gouyon, J.B.; Bonsante, F. Drug exposure for PDA closure in France: A prospective, cohort-based, analysis. Eur. J. Clin. Pharm. 2020, 76, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Miyanoshita, A.; Terada, M.; Endou, H. Furosemide directly stimulates prostaglandin E2 production in the thick ascending limb of Henle’s loop. J. Pharmacol. Exp. Ther. 1989, 251, 1155–1159. [Google Scholar]
- Clyman, R.I.; Mauray, F.; Roman, C.; Rudolph, A.M.; Heymann, M.A. Circulating prostaglandin E2 concentrations and patent ductus arteriosus in fetal and neonatal lambs. J. Pediatr. 1980, 97, 455–461. [Google Scholar] [CrossRef]
- Toyoshima, K.; Momma, K.; Nakanishi, T. In vivo dilatation of the ductus arteriosus induced by furosemide in the rat. Pediatr. Res. 2010, 67, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Lasix (Furosemide) Tablets Label—Accessdata.fda.gov. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/016273s061lbl.pdf (accessed on 16 May 2024).
- Guideline for the Management of Patent Ductus Arteriosus (PDA). North West Neonatal Operational Delivery Network. Available online: https://www.neonatalnetwork.co.uk/nwnodn/wp-content/uploads/2020/10/GL-ODN-09-NW-Guideline-for-the-Management-of-PDA.pdf (accessed on 31 July 2023).
- Bestic, M.L.; Reed, M.D. Common Diuretics Used in the Preterm and Term Infant: What’s Changed? Neoreviews 2012, 13, e410–e419. [Google Scholar] [CrossRef]
- Green, T.P.; Thompson, T.R.; Johnson, D.E.; Lock, J.E. Furosemide promotes patent ductus arteriosus in premature infants with the respiratory-distress syndrome. N. Engl. J. Med. 1983, 308, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Green, T.P.; Thompson, T.R.; Johnson, D.; Lock, J.E. Furosemide use in premature infants and appearance of patent ductus arteriosus. Am. J. Dis. Child. 1981, 135, 239–243. [Google Scholar] [CrossRef]
- Green, T.P.; Johnson, D.E.; Bass, J.L.; Landrum, B.G.; Ferrara, T.B.; Thompson, T.R. Prophylactic furosemide in severe respiratory distress syndrome: Blinded prospective study. J. Pediatr. 1988, 112, 605–612. [Google Scholar] [CrossRef]
- Belik, J.; Spitzer, A.R.; Clark, B.J.; Gewitz, M.H.; Fox, W.W. Effect of early furosemide administration in neonates with respiratory distress syndrome. Pediatr. Pulmonol. 1987, 3, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Neu, J. Necrotizing enterocolitis: The search for a unifying pathogenic theory leading to prevention. Pediatr. Clin. N. Am. 1996, 43, 409–432. [Google Scholar] [CrossRef]
- Sodini, D.; Baragatti, B.; Barogi, S.; Laubach, V.E.; Coceani, F. Indomethacin promotes nitric oxide function in the ductus arteriosus in the mouse. Br. J. Pharmacol. 2008, 153, 1631–1640. [Google Scholar] [CrossRef]
- Smith, G.C.; McGrath, J.C. Prostaglandin E2 and fetal oxygen tension synergistically inhibit response of isolated fetal rabbit ductus arteriosus to norepinephrine. J. Cardiovasc. Pharmacol. 1991, 17, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Gu, H.; Hagiwara, N.; Momma, K. Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ. Res. 1993, 72, 1218–1228. [Google Scholar] [CrossRef]
- Thébaud, B.; Michelakis, E.D.; Wu, X.C.; Moudgil, R.; Kuzyk, M.; Dyck, J.R.; Harry, G.; Hashimoto, K.; Haromy, A.; Rebeyka, I.; et al. Oxygen-sensitive Kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: Implications for infants with patent ductus arteriosus. Circulation 2004, 110, 1372–1379. [Google Scholar] [CrossRef]
- Ovalı, F. Molecular and Mechanical Mechanisms Regulating Ductus Arteriosus Closure in Preterm Infants. Front. Pediatr. 2020, 8, 516. [Google Scholar] [CrossRef]
- Chen, H.L.; Yang, R.C.; Lee, W.T.; Lee, P.L.; Hsu, J.H.; Wu, J.R.; Dai, Z.K. Lung function in very preterm infants with patent ductus arteriosus under conservative management: An observational study. BMC Pediatr. 2015, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.R.; Aldenryd, A.E.; Hagstrøm, S.; Pedersen, L.M.; Brix, N. The chance of spontaneous patent ductus arteriosus closure in preterm infants born before 32 weeks of gestation is high and continues to increase until 5 years of follow-up. Acta Paediatr. 2022, 111, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.L.; Cua, C.L.; Notestine, J.L.; Rivera, B.K.; Marzec, L.; Hade, E.M.; Maitre, N.L.; Klebanoff, M.A.; Ilgenfritz, M.; Le, V.T.; et al. Early prediction of spontaneous Patent Ductus Arteriosus (PDA) closure and PDA-associated outcomes: A prospective cohort investigation. BMC Pediatr. 2019, 19, 333. [Google Scholar] [CrossRef]
- Herrman, K.; Bose, C.; Lewis, K.; Laughon, M. Spontaneous closure of the patent ductus arteriosus in very low birth weight infants following discharge from the neonatal unit. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F48–F50. [Google Scholar] [CrossRef]
- Weber, S.C.; Weiss, K.; Bührer, C.; Hansmann, G.; Koehne, P.; Sallmon, H. Natural History of Patent Ductus Arteriosus in Very Low Birth Weight Infants after Discharge. J. Pediatr. 2015, 167, 1149–1151. [Google Scholar] [CrossRef]
- Laughon, M.M.; Chantala, K.; Aliaga, S.; Herring, A.H.; Hornik, C.P.; Hughes, R.; Clark, R.H.; Smith, P.B. Diuretic exposure in premature infants from 1997 to 2011. Am. J. Perinatol. 2015, 32, 49–56. [Google Scholar] [CrossRef]
- Lee, B.S.; Byun, S.Y.; Chung, M.L.; Chang, J.Y.; Kim, H.Y.; Kim, E.A.; Kim, K.S.; Pi, S.Y. Effect of furosemide on ductal closure and renal function in indomethacin-treated preterm infants during the early neonatal period. Neonatology 2010, 98, 191–199. [Google Scholar] [CrossRef]
- Thompson, E.J.; Greenberg, R.G.; Kumar, K.; Laughon, M.; Smith, P.B.; Clark, R.H.; Crowell, A.; Shaw, L.; Harrison, L.; Scales, G.; et al. Association between Furosemide Exposure and Patent Ductus Arteriosus in Hospitalized Infants of Very Low Birth Weight. J. Pediatr. 2018, 199, 231–236. [Google Scholar] [CrossRef]
- Jensen, E.A.; Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Liebowitz, M.; Clyman, R.I. Prophylactic Indomethacin Compared with Delayed Conservative Management of the Patent Ductus Arteriosus in Extremely Preterm Infants: Effects on Neonatal Outcomes. J. Pediatr. 2017, 187, 119–126.e1. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.C.; Gantz, M.G.; Carper, B.; Higgins, R.D.; Keszler, M.; Laughon, M.M.; Poindexter, B.B.; Stoll, B.J.; Walsh, M.C.; et al. Association between Use of Prophylactic Indomethacin and the Risk for Bronchopulmonary Dysplasia in Extremely Preterm Infants. J. Pediatr. 2017, 186, 34–40.e2. [Google Scholar] [CrossRef]
- Seiberth, V.; Linderkamp, O. Risk factors in retinopathy of prematurity—A multivariate statistical analysis. Ophthalmologica 2000, 214, 131–135. [Google Scholar] [CrossRef]
| Characteristics | PDA (N = 60) | No PDA (N = 36) | p Value |
|---|---|---|---|
| Birth body weight (gm) Mean (SD) | 992.4 (267.3) | 1174.5 (207.3) | 0.001 |
| Gestational age (weeks) Mean (SD) | 27.1 (2.2) | 28.4 (2.0) | 0.003 |
| 1st min Apgar score Mean (SD) | 4.0 (1.9) | 4.8 (1.8) | 0.025 |
| 5th min Apgar score Mean (SD) | 5.9 (2.2) | 6.7 (2.0) | 0.098 |
| Male, n (%) | 32 (53.3) | 20 (55.6) | 0.832 |
| SGA, n (%) | 2 (3.3) | 0 (0.0) | 0.268 |
| LGA, n (%) | 2 (3.3) | 2 (5.6) | 0.598 |
| Antenatal steroid exposure, n (%) | 49 (81.7) | 32 (88.9) | 0.345 |
| Intubation at birth, n (%) | 34 (56.7) | 10 (27.8) | 0.006 |
| Characteristics | Furosemide (N = 51) | No Furosemide (N = 9) | p Value |
|---|---|---|---|
| Birth body weight (gm) Mean (SD) | 931.7 (238.8) | 1336.4 (117.5) | <0.001 |
| Gestational age (weeks) Mean (SD) | 26.6 (2.0) | 29.6 (1.2) | <0.001 |
| 1st min Apgar score Mean (SD) | 3.8 (1.9) | 5.0 (1.5) | 0.069 |
| 5th min Apgar score Mean (SD) | 5.7 (2.3) | 7.2 (1.0) | 0.002 |
| Male, n (%) | 27 (52.9) | 5 (55.6) | 0.885 |
| SGA, n (%) | 2 (3.9) | 0 (0.0) | 0.546 |
| LGA, n (%) | 2 (3.9) | 0 (0.0) | 0.546 |
| Antenatal steroid exposure, n (%) | 42 (82.4) | 7 (77.8) | 0.744 |
| Intubation at birth, n (%) | 31 (60.8) | 3 (33.3) | 0.125 |
| PDA spontaneous closure, n (%) | 47 (92.2) | 9 (100.0) | 0.384 |
| Time taken for PDA spontaneous closure * (days) Mean (SD) | 55.1 (33.3) | 28.0 (18.4) | 0.022 |
| PMA at PDA spontaneous closure * (weeks) Mean (SD) | 35.0 (4.1) | 34.1 (3.5) | 0.558 |
| Factors | Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RC | Beta Value | R, R2 | 95% CI | p Value | RC | Beta Value | R, R2 | 95% CI | p Value | |||
| Lower | Upper | Lower | Upper | |||||||||
| Furosemide cumulative dose | 0.458 | 0.199 | 0.458, 0.209 | 0.083 | 0.315 | 0.001 | −0.090 | −0.039 | 0.648, 0.420 | −0.222 | 0.144 | 0.668 |
| Duration of furosemide treatment | 0.603 | 0.741 | 0.603, 0.364 | 0.447 | 1.035 | <0.001 | 0.547 | 0.672 | 0.083 | 1.261 | 0.026 | |
| Birth body weight | −0.365 | −0.050 | 0.365, 0.133 | −0.089 | −0.012 | 0.012 | 0.193 | 0.027 | −0.026 | 0.079 | 0.317 | |
| Gestational age | −0.525 | −8.725 | 0.525, 0.276 | −12.968 | −4.483 | <0.001 | −0.384 | −6.370 | −13.066 | 0.326 | 0.062 | |
| 1st min Apgar score | −0.167 | −2.902 | 0.167, 0.028 | −8.044 | 2.240 | 0.262 | ||||||
| 5th min Apgar score | −0.125 | −1.796 | 0.125, 0.016 | −6.075 | 2.482 | 0.402 | ||||||
| Male | 0.105 | 6.937 | 0.105, 0.011 | −12.734 | 26.607 | 0.481 | ||||||
| Antenatal steroid exposure | 0.116 | 10.170 | 0.116, 0.013 | −15.963 | 36.302 | 0.437 | ||||||
| Intubation at birth | 0.152 | 10.278 | 0.152, 0.023 | −9.828 | 30.383 | 0.309 | ||||||
| Outcomes | PDA | No PDA (N = 36) | p Value # | |||
|---|---|---|---|---|---|---|
| Furosemide (N = 51) | No Furosemide (N = 9) | p Value * | Total (N = 60) | |||
| IVH, n (%) | 31 (60.8) | 5 (55.6) | 0.768 | 36 (60.0) | 24 (66.7) | 0.514 |
| BPD, n (%) | 40 (78.4) | 4 (44.4) | 0.034 | 44 (73.3) | 21 (58.3) | 0.128 |
| NEC, n (%) | 1 (2.0) | 1 (11.1) | 0.159 | 2 (3.3) | 1 (2.8) | 0.880 |
| ROP, n (%) | 39 (76.5) | 3 (33.3) | 0.009 | 42 (70.0) | 20 (55.6) | 0.152 |
| PVL, n (%) | 1 (2.0) | 1 (11.1) | 0.159 | 2 (3.3) | 3 (8.3) | 0.286 |
| Pulmonary air leak syndrome, n (%) | 2 (3.9) | 0 (0.0) | 0.546 | 2 (3.3) | 0 (0.0) | 0.268 |
| Post-hemorrhagic hydrocephalus, n (%) | 1 (2.0) | 0 (0.0) | 0.672 | 1 (1.7) | 2 (5.6) | 0.289 |
| Factors | Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RC | Beta Value | R, R2 | 95% CI | p Value | RC | Beta Value | R, R2 | 95% CI | p Value | |||
| Lower | Upper | Lower | Upper | |||||||||
| Birth body weight | −0.402 | −0.001 | 0.402, 0.161 | −0.001 | 0.000 | <0.001 | 0.081 | 0.000 | 0.539, 0.291 | 0.000 | 0.001 | 0.608 |
| Gestational age | −0.486 | −0.104 | 0.486, 0.237 | −0.143 | −0.066 | <0.001 | −0.403 | −0.087 | −0.154 | −0.020 | 0.012 | |
| 1st minute Apgar score | −0.313 | −0.078 | 0.313, 0.098 | −0.127 | −0.030 | 0.002 | −0.100 | −0.025 | −0.108 | 0.058 | 0.550 | |
| 5th minute Apgar score | −0.311 | −0.068 | 0.311, 0.097 | −0.111 | −0.025 | 0.002 | −0.105 | −0.023 | −0.095 | 0.049 | 0.529 | |
| Furosemide exposure | 0.349 | 0.437 | 0.349, 0.122 | 0.197 | 0.678 | <0.001 | 0.182 | 0.228 | −0.024 | 0.480 | 0.076 | |
| PDA | 0.155 | 0.150 | 0.155, 0.024 | −0.045 | 0.345 | 0.131 | ||||||
| Male | 0.125 | 0.117 | 0.125, 0.016 | −0.074 | 0.308 | 0.226 | ||||||
| SGA | 0.101 | 0.330 | 0.101, 0.010 | −0.337 | 0.997 | 0.329 | ||||||
| LGA | −0.079 | −0.185 | 0.079, 0.006 | −0.662 | 0.293 | 0.444 | ||||||
| Antenatal steroid exposure | −0.052 | −0.067 | 0.052, 0.003 | −0.330 | 0.197 | 0.616 | ||||||
| Intubation at birth | 0.143 | 0.135 | 0.143, 0.021 | −0.056 | 0.325 | 0.163 | ||||||
| Factors | Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RC | Beta Value | R, R2 | 95% CI | p Value | RC | Beta Value | R, R2 | 95% CI | p Value | |||
| Lower | Upper | Lower | Upper | |||||||||
| Birth body weight | −0.524 | −0.001 | 0.524, 0.275 | −0.001 | −0.001 | <0.001 | −0.115 | 0.000 | 0.602, 0.363 | −0.001 | 0.000 | 0.429 |
| Gestational age | −0.599 | −0.131 | 0.599, 0.358 | −0.167 | −0.095 | <0.001 | −0.518 | −0.114 | −0.177 | −0.050 | 0.001 | |
| Furosemide exposure | 0.253 | 0.325 | 0.253, 0.064 | 0.071 | 0.579 | 0.013 | −0.030 | −0.038 | −0.278 | 0.201 | 0.751 | |
| 1st minute Apgar score | −0.180 | −0.046 | 0.180, 0.032 | −0.098 | 0.006 | 0.079 | ||||||
| 5th minute Apgar score | −0.115 | −0.026 | 0.115, 0.013 | −0.071 | 0.020 | 0.266 | ||||||
| PDA | 0.146 | 0.144 | 0.146, 0.021 | −0.056 | 0.345 | 0.155 | ||||||
| Male | −0.069 | −0.066 | 0.069, 0.005 | −0.263 | 0.130 | 0.503 | ||||||
| SGA | −0.044 | −0.149 | 0.044, 0.002 | −0.834 | 0.536 | 0.667 | ||||||
| LGA | 0.045 | 0.109 | 0.045, 0.002 | −0.381 | 0.598 | 0.660 | ||||||
| Antenatal steroid exposure | −0.079 | −0.104 | 0.079, 0.006 | −0.373 | 0.165 | 0.446 | ||||||
| Intubation at birth | 0.157 | 0.150 | 0.157, 0.025 | −0.044 | 0.344 | 0.127 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-M.; Su, P.-C.; Yang, S.-T.; Chung, H.-W.; Chen, H.-L. Furosemide and Ductus Arteriosus Closure in Very-Low-Birth-Weight Preterm Infants: A Comprehensive Retrospective Study. Children 2024, 11, 610. https://doi.org/10.3390/children11050610
Kuo C-M, Su P-C, Yang S-T, Chung H-W, Chen H-L. Furosemide and Ductus Arteriosus Closure in Very-Low-Birth-Weight Preterm Infants: A Comprehensive Retrospective Study. Children. 2024; 11(5):610. https://doi.org/10.3390/children11050610
Chicago/Turabian StyleKuo, Chi-Mei, Pin-Chun Su, Shu-Ting Yang, Hao-Wei Chung, and Hsiu-Lin Chen. 2024. "Furosemide and Ductus Arteriosus Closure in Very-Low-Birth-Weight Preterm Infants: A Comprehensive Retrospective Study" Children 11, no. 5: 610. https://doi.org/10.3390/children11050610
APA StyleKuo, C.-M., Su, P.-C., Yang, S.-T., Chung, H.-W., & Chen, H.-L. (2024). Furosemide and Ductus Arteriosus Closure in Very-Low-Birth-Weight Preterm Infants: A Comprehensive Retrospective Study. Children, 11(5), 610. https://doi.org/10.3390/children11050610







