Utilization of Indocyanine Green (ICG) Fluorescence in Patients with Pediatric Colorectal Diseases: The Current Applications and Reported Outcomes
Abstract
:1. Introduction
2. Materials and Methods
3. Background
4. ICG in General Pediatric Surgery
5. ICG in Pediatric Colorectal Surgery
5.1. Hirschsprung Disease
5.2. Anorectal Malformations
5.2.1. Anorectoplasty
5.2.2. Colostomy Closure
5.3. Cloaca
5.4. Vaginal Replacement
6. Potential Applications of ICG for Bowel and Bladder Management
6.1. Antegrade Continence Enemas
6.2. Urinary Reconstruction
7. Protocol for ICG Use in Pediatric Colorectal Surgery
7.1. Dose
7.2. Timing
7.3. Number of Doses per Procedure
7.4. Route
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urbanavičius, L.; Pattyn, P.; Putte, D.V.; Venskutonis, D. How to Assess Intestinal Viability during Surgery: A Review of Techniques. World J. Gastrointest. Surg. 2011, 3, 59. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Cherrick, G.R.; Stein, S.W.; Leevy, C.M.; Davidson, C.S. Indocyanine Green: Observations on Its Physical Properties, Plasma Decay, and Hepatic Extraction. J. Clin. Investig. 1960, 39, 592–600. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse Reactions Due to Indocyanine Green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef]
- Breuking, E.A.; Varsseveld, O.C.; Harms, M.; Tytgat, S.; Hulscher, J.B.F.; Ruiterkamp, J. Safety and Feasibility of Indocyanine Green Fluorescence Angiography in Pediatric Gastrointestinal Surgery: A Systematic Review. J. Pediatr. Surg. 2023, 58, 1534–1542. [Google Scholar] [CrossRef]
- Goldstein, S.D.; Heaton, T.E.; Bondoc, A. Evolving Applications of Fluorescence Guided Surgery in Pediatric Surgical Oncology: A Practical Guide for Surgeons. J. Pediatr. Surg. 2021, 56, 215–223. [Google Scholar] [CrossRef]
- Chung, P.H.; Chok, K.S.; Wong, K.K. Indocyanine Green Fluorescence-Assisted Laparoscopic Hepatectomy for Hepatocellular Carcinoma in a Pre-Adolescent Girl: A Case Report. Hong Kong Med. J. 2020, 26, 342–344. [Google Scholar] [CrossRef]
- Hirayama, Y.; Iinuma, Y.; Yokoyama, N.; Otani, T.; Masui, D.; Komatsuzaki, N.; Higashidate, N.; Tsuruhisa, S.; Iida, H.; Nakaya, K.; et al. Near-Infrared Fluorescence Cholangiography with Indocyanine Green for Biliary Atresia. Real-Time Imaging during the Kasai Procedure: A Pilot Study. Pediatr. Surg. Int. 2015, 31, 1177–1182. [Google Scholar] [CrossRef]
- Iinuma, Y.; Hirayama, Y.; Yokoyama, N.; Otani, T.; Nitta, K.; Hashidate, H.; Yoshida, M.; Iida, H.; Masui, D.; Manabe, S. Intraoperative Near-Infrared Indocyanine Green Fluorescence Angiography (NIR-ICG AG) Can Predict Delayed Small Bowel Stricture after Ischemic Intestinal Injury: Report of a Case. J. Pediatr. Surg. 2013, 48, 1123–1128. [Google Scholar] [CrossRef]
- Jeremiasse, B.; Van Scheltinga, C.E.J.T.; Smeele, L.E.; Tolboom, N.; Wijnen, M.H.W.A.; Van Der Steeg, A.F.W. Sentinel Lymph Node Procedure in Pediatric Patients with Melanoma, Squamous Cell Carcinoma, or Sarcoma Using Near-Infrared Fluorescence Imaging with Indocyanine Green: A Feasibility Trial. Ann. Surg. Oncol. 2023, 30, 2391–2398. [Google Scholar] [CrossRef]
- Kaneshi, Y.; Shibasaki, J.; Aida, N.; Shimokaze, T.; Toyoshima, K. Indocyanine Green Lymphography for Congenital Lymphatic Dysplasia with Tuberous Sclerosis Complex: A Case Report. Pediatr. Int. 2020, 62, 234–236. [Google Scholar] [CrossRef]
- Kisaoglu, A.; Demiryilmaz, I.; Dandin, O.; Ozkan, O.; Aydinli, B. Management of Reperfusion Deficiency with Indocyanine Green Fluorescence Imaging during Deceased Donor Liver Transplantation in a Pediatric Recipient. HPB 2020, 22, 633. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Murphy, A.J.; Talbot, L.; Prajapati, H.; Maller, V.; Pappo, A.; Singhal, S.; Krasin, M.J.; Davidoff, A.M.; Abdelhafeez, A. Alternative Approaches to Retroperitoneal Lymph Node Dissection for Paratesticular Rhabdomyosarcoma. J. Pediatr. Surg. 2020, 55, 2677–2681. [Google Scholar] [CrossRef]
- Masuya, R.; Nakame, K.; Tahira, K.; Kai, K.; Hamada, T.; Yano, K.; Imamura, N.; Hiyoshi, M.; Nanashima, A.; Ieiri, S. Laparoscopic Dome Resection for Pediatric Nonparasitic Huge Splenic Cyst Safely Performed Using Indocyanine Green Fluorescence and Percutaneous Needle Grasper. Asian J. Endosc. Surg. 2022, 15, 693–696. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Murphy, A.J.; Brennan, R.; Santiago, T.C.; Lu, Z.; Krasin, M.J.; Bissler, J.J.; Gleason, J.M.; Davidoff, A.M. Indocyanine Green–Guided Nephron-Sparing Surgery for Pediatric Renal Tumors. J. Pediatr. Surg. 2022, 57, 174–178. [Google Scholar] [CrossRef]
- Bryant, M.K.; Marulanda, K.; Phillips, M.R. Laparoscopic Double Cholecystectomy in a Pediatric Patient for Gallbladder Duplication: An Unusual Case of Biliary Anatomy. Am. Surg. 2020, 86, 1531–1534. [Google Scholar] [CrossRef]
- Esposito, C.; Corcione, F.; Settimi, A.; Farina, A.; Centonze, A.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Twenty-Five Year Experience with Laparoscopic Cholecystectomy in the Pediatric Population—From 10 Mm Clips to Indocyanine Green Fluorescence Technology: Long-Term Results and Technical Considerations. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 1185–1191. [Google Scholar] [CrossRef]
- Esposito, C.; Del Conte, F.; Cerulo, M.; Gargiulo, F.; Izzo, S.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Clinical Application and Technical Standardization of Indocyanine Green (ICG) Fluorescence Imaging in Pediatric Minimally Invasive Surgery. Pediatr. Surg. Int. 2019, 35, 1043–1050. [Google Scholar] [CrossRef]
- Esposito, C.; Turrà, F.; Del Conte, F.; Izzo, S.; Gargiulo, F.; Farina, A.; Severino, G.; Cerulo, M.; Escolino, M. Indocyanine Green Fluorescence Lymphography: A New Technique to Perform Lymphatic Sparing Laparoscopic Palomo Varicocelectomy in Children. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 564–567. [Google Scholar] [CrossRef]
- Esposito, C.; Coppola, V.; Del Conte, F.; Cerulo, M.; Esposito, G.; Farina, A.; Crocetto, F.; Castagnetti, M.; Settimi, A.; Escolino, M. Near-Infrared Fluorescence Imaging Using Indocyanine Green (ICG): Emerging Applications in Pediatric Urology. J. Pediatr. Urol. 2020, 16, 700–707. [Google Scholar] [CrossRef]
- Fung, C.; Lau, C.; Wong, K.K. Indocyanine Green Fluorescence-Guided Pulmonary Wedge Resection in a Child: A Case Report. Hong Kong Med. J. 2020, 26, 345–347. [Google Scholar] [CrossRef]
- Herz, D.; DaJusta, D.; Ching, C.; McLeod, D. Segmental Arterial Mapping during Pediatric Robot-Assisted Laparoscopic Heminephrectomy: A Descriptive Series. J. Pediatr. Urol. 2016, 12, 266.e1–266.e6. [Google Scholar] [CrossRef]
- Pavel, M.-C.; Boira, M.A.; Bashir, Y.; Memba, R.; Llácer, E.; Estalella, L.; Julià, E.; Conlon, K.C.; Jorba, R. Near Infrared Indocyanine Green Fluorescent Cholangiography versus Intraoperative Cholangiography to Improve Safety in Laparoscopic Cholecystectomy for Gallstone Disease-a Systematic Review Protocol. Syst. Rev. 2022, 11, 36. [Google Scholar] [CrossRef]
- Numanoglu, A.; Millar, A.J.W. Necrotizing Enterocolitis: Early Conventional and Fluorescein Laparoscopic Assessment. J. Pediatr. Surg. 2011, 46, 348–351. [Google Scholar] [CrossRef]
- Onishi, S.; Muto, M.; Yamada, K.; Murakami, M.; Kedoin, C.; Nagano, A.; Matsui, M.; Sugita, K.; Yano, K.; Harumatsu, T.; et al. Feasibility of Delayed Anastomosis for Long Gap Esophageal Atresia in the Neonatal Period Using Internal Traction and Indocyanine Green-guided Near-infrared Fluorescence. Asian J. Endosc. Surg. 2022, 15, 877–881. [Google Scholar] [CrossRef]
- Boni, L.; David, G.; Dionigi, G.; Rausei, S.; Cassinotti, E.; Fingerhut, A. Indocyanine Green-Enhanced Fluorescence to Assess Bowel Perfusion during Laparoscopic Colorectal Resection. Surg. Endosc. 2016, 30, 2736–2742. [Google Scholar] [CrossRef]
- Sutton, P.A.; van Dam, M.A.; Cahill, R.A.; Mieog, S.; Polom, K.; Vahrmeijer, A.L.; van der Vorst, J. Fluorescence-Guided Surgery: Comprehensive Review. BJS Open 2023, 7, zrad049. [Google Scholar] [CrossRef]
- Figueroa, R.; Golse, N.; Alvarez, F.A.; Ciacio, O.; Pittau, G.; Sa Cunha, A.; Cherqui, D.; Adam, R.; Vibert, E. Indocyanine Green Fluorescence Imaging to Evaluate Graft Perfusion during Liver Transplantation. HPB 2019, 21, 387–392. [Google Scholar] [CrossRef]
- Degett, T.H.; Andersen, H.S.; Gögenur, I. Indocyanine Green Fluorescence Angiography for Intraoperative Assessment of Gastrointestinal Anastomotic Perfusion: A Systematic Review of Clinical Trials. Langenbecks Arch. Surg. 2016, 401, 767–775. [Google Scholar] [CrossRef]
- Bédat, B.; Triponez, F.; Sadowski, S.M.; Ellenberger, C.; Licker, M.; Karenovics, W. Impact of Near-Infrared Angiography on the Quality of Anatomical Resection during Video-Assisted Thoracic Surgery Segmentectomy. J. Thorac. Dis. 2018, 10, S1229–S1234. [Google Scholar] [CrossRef]
- Sincavage, J.; Gulack, B.C.; Zamora, I.J. Indocyanine Green (ICG) Fluorescence-Enhanced Applications in Pediatric Surgery. Semin. Pediatr. Surg. 2024, 33, 151384. [Google Scholar] [CrossRef]
- Esposito, C.; Soria-Gondek, A.; Castagnetti, M.; Cerulo, M.; Del Conte, F.; Esposito, G.; Pecoraro, C.; Cicala, D.; Farina, A.; Escolino, M. Laparoscopic or Robotic Deroofing Guided by Indocyanine Green Fluorescence and Perirenal Fat Tissue Wadding Technique of Pediatric Simple Renal Cysts. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 471–476. [Google Scholar] [CrossRef]
- Le-Nguyen, A.; Bourque, C.J.; Trudeau, M.O.; Ducruet, T.; Faure, C.; Piché, N. Indocyanine Green Fluorescence Angiography in Pediatric Intestinal Resections: A First Prospective Mixed Methods Clinical Trial. J. Pediatr. Surg. 2023, 58, 82–88. [Google Scholar] [CrossRef]
- Menon, R.; Saxena, R.; Sinha, A.; Nayak, S.; Jadhav, A.; Rathod, K.; Pathak, M. Retrospective Analysis of Laparoscopically Managed Pediatric Patients with Hirschsprung Disease. J. Pediatr. Endosc. Surg. 2022, 4, 149–155. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yokota, K.; Uchida, H.; Hinoki, A.; Shirota, C.; Tainaka, T.; Sumida, W.; Makita, S.; Amano, H.; Takimoto, A.; et al. Laparoscopic Restorative Proctocolectomy with Ileal-J-Pouch Anal Canal Anastomosis without Diverting Ileostomy for Total Colonic and Extensive Aganglionosis Is Safe and Feasible with Combined Lugol’s Iodine Staining Technique and Indocyanine Green Fluorescence Angiography. Front. Pediatr. 2023, 10, 1090336. [Google Scholar] [CrossRef]
- Shafy, S.Z.; Hakim, M.; Lynch, S.; Chen, L.; Tobias, J.D. Fluorescence Imaging Using Indocyanine Green Dye in the Pediatric Population. J. Pediatr. Pharmacol. Ther. 2020, 25, 309–313. [Google Scholar] [CrossRef]
- Rentea, R.M.; Halleran, D.R.; Ahmad, H.; Sanchez, A.V.; Gasior, A.C.; McCracken, K.; Hewitt, G.D.; Alexander, V.; Smith, C.; Weaver, L.; et al. Preliminary Use of Indocyanine Green Fluorescence Angiography and Value in Predicting the Vascular Supply of Tissues Needed to Perform Cloacal, Anorectal Malformation, and Hirschsprung Reconstructions. Eur. J. Pediatr. Surg. 2020, 30, 505–511. [Google Scholar] [CrossRef]
- Muto, M.; Onishi, S.; Murakami, M.; Yano, K.; Harumatsu, T.; Ieiri, S. Transanal Mesenteric Resection in Hirschsprung’s Disease Using ICG under Concept of NOTES Technique. Eur. J. Pediatr. Surg. Rep. 2022, 10, e115–e117. [Google Scholar] [CrossRef]
- Shirota, C.; Hiroo Uchida, H.; Tanaka, Y.; Tainaka, T.; Yokota, K.; Makita, S.; Oshima, K.; Chiba, K.; Hinoki, A. S055 Endoscopic Navigation Surgery with Indocyanine Green Fluorescence in Pediatric Patients. The 28th Annual Congress for Endosurgery in Children March 20–22, 2019, Santiago, Chile. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, A-1–A-72. [Google Scholar] [CrossRef]
- Paraboschi, I.; Privitera, L.; Loukogeorgakis, S.; Giuliani, S. Indocyanine Green-Based Fluorescence-Guided Surgery in a Male Infant with Anorectal Malformation. Eur. J. Pediatr. Surg. Rep. 2022, 10, e122–e125. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Zhang, Y.; Zhao, J.; Zhao, Y.; Liao, J.; Li, S.; Huang, J. Indocyanine Green Fluorescence Imaging Localization: A Helpful Addition to Laparoscopic Dissection and Division of Rectourethral Fistulae. Photodiagn. Photodyn. Ther. 2023, 42, 103335. [Google Scholar] [CrossRef]
- Yada, K.; Migita, M.; Nakamura, R.; Abe, S.; Matsufuji, H. Indocyanine Green Fluorescence during Pediatric Stoma Closure. J. Pediatr. Surg. Case Rep. 2020, 61, 101595. [Google Scholar] [CrossRef]
- Fontoura Oliveira, A.; Ferreira, H. Neovagina Creation in Congenital Vaginal Agenesis: New Mini-Laparoscopic Approach Applying Intraoperative Indocyanine Green Fluorescence. Surg. Innov. 2021, 28, 24–32. [Google Scholar] [CrossRef]
- Saxena, R.; Agarwal, T.; Pathak, M.; Sinha, A. Novel Use of Indocyanine Green Fluorescence in Total Laparoscopic Sigmoid Colon Vaginoplasty. J. Pediatr. Endosc. Surg. 2022, 4, 181–184. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hyakudomi, R.; Takai, K.; Taniura, T.; Uchida, Y.; Ishitobi, K.; Hirahara, N.; Tajima, Y. Altemeier Perineal Rectosigmoidectomy with Indocyanine Green Fluorescence Imaging for a Female Adolescent with Complete Rectal Prolapse: A Case Report. World J. Clin. Cases 2021, 9, 847–853. [Google Scholar] [CrossRef]
- Aworanti, O.; Hung, J.; McDowell, D.; Martin, I.; Quinn, F. Are Routine Dilatations Necessary Post Pull-through Surgery for Hirschsprung Disease? Eur. J. Pediatr. Surg. 2013, 23, 383–388. [Google Scholar] [CrossRef]
- Hoff, N.; Wester, T.; Granström, A.L. Classification of Short-Term Complications after Transanal Endorectal Pullthrough for Hirschsprung’s Disease Using the Clavien–Dindo-Grading System. Pediatr. Surg. Int. 2019, 35, 1239–1243. [Google Scholar] [CrossRef]
- Lin, P.H.; Chaikof, E.L. Embryology, anatomy, and surgical exposure of the great abdominal vessels. Surg. Clin. N. Am. 2000, 80, 417–433. [Google Scholar] [CrossRef]
- Momin, A.A.; Chaubal, N.G.; Saifi, S.G.A.; Kazi, Z.N. Sonographic Diagnosis of Inferior Mesenteric Artery Aneurysm and Marginal Artery of Drummond. J. Clin. Ultrasound 2008, 36, 42–44. [Google Scholar] [CrossRef]
- Mann, M.R.; Kawzowicz, M.; Komosa, A.J.; Sherer, Y.M.; Łazarz, D.P.; Loukas, M.; Tubbs, R.S.; Pasternak, A. The Marginal Artery of Drummond Revisited: A Systematic Review. Transl. Res. Anat. 2021, 24, 100118. [Google Scholar] [CrossRef]
- Karatay, E.; Javadov, M. The Importance of the Moskowitz Artery as a Lesser-Known Collateral Pathway in the Medial Laparoscopic Approach to Splenic Flexure Mobilisation and Its Evaluation with Preoperative Computed Tomography. Wideochir. Inne. Tech. Maloinwazyjne 2021, 16, 305–311. [Google Scholar] [CrossRef]
- Drummond, H. The Arterial Supply of the Rectum and Pelvic Colon. Br. J. Surg. 2006, 1, 677–685. [Google Scholar] [CrossRef]
- Moskowitz, M.; Zimmerman, H.; Felson, B. The meandering mesenteric artery of the colon. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1964, 92, 1088–1099. [Google Scholar]
- van Tonder, J.J.; Boon, J.M.; Becker, J.H.R.; van Schoor, A.-N. Anatomical Considerations on Sudeck’s Critical Point and Its Relevance to Colorectal Surgery. Clin. Anat. 2007, 20, 424–427. [Google Scholar] [CrossRef]
- Watanabe, J.; Ota, M.; Suwa, Y.; Suzuki, S.; Suwa, H.; Momiyama, M.; Ishibe, A.; Watanabe, K.; Masui, H.; Nagahori, K.; et al. Evaluation of the Intestinal Blood Flow near the Rectosigmoid Junction Using the Indocyanine Green Fluorescence Method in a Colorectal Cancer Surgery. Int. J. Color. Dis. 2015, 30, 329–335. [Google Scholar] [CrossRef]
- Sinkeet, S.; Mwachaka, P.; Muthoka, J.; Saidi, H. Branching Pattern of Inferior Mesenteric Artery in a Black African Population: A Dissection Study. Int. Sch. Res. Not. 2012, 2013, e962904. [Google Scholar] [CrossRef]
- Michels, N.A.; Siddharth, P.; Kornblith, P.L.; Parke, W.W. The Variant Blood Supply to the Descending Colon, Rectosigmoid and Rectum Based on 400 Dissections. Its Importance in Regional Resections: A Review of Medical Literature. Dis. Colon Rectum 1965, 8, 251. [Google Scholar] [CrossRef]
- Ventemiglia, R.; Khalil, K.G.; Frazier, O.H.; Mountain, C.F. The Role of Preoperative Mesenteric Arteriography in Colon Interposition. J. Thorac. Cardiovasc. Surg. 1977, 74, 98–104. [Google Scholar] [CrossRef]
- Bonnard, A.; de Lagausie, P.; Leclair, M.D.; Marwan, K.; Languepin, J.; Bruneau, B.; Berribi, D.; Aigrain, Y. Definitive Treatment of Extended Hirschsprung’s Disease or Total Colonic Form. Surg. Endosc. 2001, 15, 1301–1304. [Google Scholar] [CrossRef]
- Jouvin, I.; Pocard, M.; Najah, H. Deloyers Procedure. J. Visc. Surg. 2018, 155, 493–501. [Google Scholar] [CrossRef]
- Sciuto, A.; Grifasi, C.; Pirozzi, F.; Leon, P.; Pirozzi, R.E.M.; Corcione, F. Laparoscopic Deloyers Procedure for Tension-Free Anastomosis after Extended Left Colectomy: Technique and Results. Tech. Coloproctol. 2016, 20, 865–869. [Google Scholar] [CrossRef]
- Kontovounisios, C.; Baloyiannis, Y.; Kinross, J.; Tan, E.; Rasheed, S.; Tekkis, P. Modified Right Colon Inversion Technique as a Salvage Procedure for Colorectal or Coloanal Anastomosis. Color. Dis. 2014, 16, 971–975. [Google Scholar] [CrossRef]
- Chu, D.I.; Dozois, E.J. Pearls for the Small Bowel and Colon That Will Not Reach. In Gastrointestinal Surgery; Pawlik, T.M., Maithel, S.K., Merchant, N.B., Eds.; Springer: New York, NY, USA, 2015; pp. 329–340. ISBN 978-1-4939-2222-2. [Google Scholar]
- Manceau, G.; Karoui, M.; Breton, S.; Blanchet, A.-S.; Rousseau, G.; Savier, E.; Siksik, J.-M.; Vaillant, J.-C.; Hannoun, L. Right Colon to Rectal Anastomosis (Deloyers Procedure) as a Salvage Technique for Low Colorectal or Coloanal Anastomosis: Postoperative and Long-Term Outcomes. Dis. Colon Rectum 2012, 55, 363–368. [Google Scholar] [CrossRef]
- Rentea, R.M.; Halleran, D.R.; Vilanova-Sanchez, A.; Lane, V.A.; Reck, C.A.; Weaver, L.; Booth, K.; DaJusta, D.; Ching, C.; Fuchs, M.E.; et al. Diagnosis and Management of a Remnant of the Original Fistula (ROOF) in Males Following Surgery for Anorectal Malformations. J. Pediatr. Surg. 2019, 54, 1988–1992. [Google Scholar] [CrossRef]
- Lane, V.A.; Calisto, J.I.; Calkins, C.M.; Samuk, I.; Avansino, J. Assessing the Previously Repaired Patient with an Anorectal Malformation Who Is Not Doing Well. Semin. Pediatr. Surg. 2020, 29, 150995. [Google Scholar] [CrossRef]
- Levitt, M.A.; Dickie, B.; Peña, A. The Hirschsprungs Patient Who Is Soiling after What Was Considered a “Successful” Pull-Through. Semin. Pediatr. Surg. 2012, 21, 344–353. [Google Scholar] [CrossRef]
- Malone, P.S.J. The Antegrade Continence Enema Procedure. BJU Int. 2004, 93, 248–249. [Google Scholar] [CrossRef]
- Shandling, B.; Chait, P.G.; Richards, H.F. Percutaneous Cecostomy: A New Technique in the Management of Fecal Incontinence. J. Pediatr. Surg. 1996, 31, 534–537. [Google Scholar] [CrossRef]
- Chatoorgoon, K.; Pena, A.; Lawal, T.; Hamrick, M.; Louden, E.; Levitt, M.A. Neoappendicostomy in the Management of Pediatric Fecal Incontinence. J. Pediatr. Surg. 2011, 46, 1243–1249. [Google Scholar] [CrossRef]
- Fuchs, M.E.; Halleran, D.R.; Bourgeois, T.; Sebastião, Y.; Weaver, L.; Farrell, N.; Vilanova-Sánchez, A.; Gasior, A.; Halaweish, I.; Jayanthi, V.R.; et al. Correlation of Anorectal Malformation Complexity and Associated Urologic Abnormalities. J. Pediatr. Surg. 2021, 56, 1988–1992. [Google Scholar] [CrossRef]
- Johnston, A.W.; Wiener, J.S.; Todd Purves, J. Pediatric Neurogenic Bladder and Bowel Dysfunction: Will My Child Ever Be out of Diapers? Eur. Urol. Focus 2020, 6, 838–867. [Google Scholar] [CrossRef] [PubMed]
- VanderBrink, B.A.; Cain, M.P.; Kaefer, M.; Meldrum, K.K.; Misseri, R.; Rink, R.C. Split-Appendix Technique for Simultaneous Appendicovesicostomy and Appendicocecostomy. J. Pediatr. Surg. 2011, 46, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Zann, A.; Sebastiao, Y.; Ching, C.C.; Fuchs, M.; Jayanthi, V.R.; Wood, R.J.; Levitt, M.A.; DaJusta, D. Split Appendix Mitrofanoffs Have Higher Risk of Complication than Intact Appendix or Monti Channels. J. Pediatr. Urol. 2021, 17, 700.e1–700.e6. [Google Scholar] [CrossRef] [PubMed]
- Halleran, D.R.; Wood, R.J.; Vilanova-Sanchez, A.; Rentea, R.M.; Brown, C.; Fuchs, M.; Jayanthi, V.R.; Ching, C.; Ahmad, H.; Gasior, A.C.; et al. Simultaneous Robotic-Assisted Laparoscopy for Bladder and Bowel Reconstruction. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Petrut, B.; Bujoreanu, C.E.; Porav-Hodade, D.; Hardo, V.V.; Coste, B.O.; Maghiar, T.T.; Achimas-Cadariu, P.; Vlad, C. Indocyanine Green Use in Urology. J. Buon 2021, 26, 266–274. [Google Scholar] [PubMed]
- Chu, W.; Chennamsetty, A.; Toroussian, R.; Lau, C. Anaphylactic Shock After Intravenous Administration of Indocyanine Green During Robotic Partial Nephrectomy. Urol. Case Rep. 2017, 12, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, S.; Park, J.C.; Jang, D.-M.; Ha, S.I.; Kim, J.-U.; Ahn, J.S.; Park, W. Anaphylactic Shock After Indocyanine Green Video Angiography During Cerebrovascular Surgery. World Neurosurg. 2020, 133, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Die, X.; Cui, M.; Feng, W.; Hou, J.; Chen, P.; Liu, W.; Wu, F.; Guo, Z. Applications of Indocyanine Greenenhanced Fluorescence in the Laparoscopic Treatment of Colonic Stricture after Necrotizing Enterocolitis. BMC Pediatr. 2023, 23, 635. [Google Scholar] [CrossRef]
| Diagnosis | Procedure | Reference | Study Type | n | Route | Dose | Time to Sufficient Fluorescence, (Seconds, s) | Comments |
|---|---|---|---|---|---|---|---|---|
| HD | Pull-Through | Le-Nguyen et al. [33] | Prospective single-institution clinical trial | 8 | IV | Per bolus: 0.25 mg/kg–2.5 mg 1 | 32 | If fluorescence was insufficient after the initial bolus, another bolus was injected. |
| Menon et al. [34] | Retrospective single-institution study | N/S | IV | N/S | N/S | ICG was used in “some” of the reported 28 patients. | ||
| Nakagawa et al. [35] | 10 | IV | 0.01 mg/kg | 60 | J-pouch in children with TCHD. ICG-FA combined with Lugol’s iodine staining to visualize the anal canal. | |||
| Shafy et al. [36] | N/S | IV | N/S | N/S | ||||
| Rentea et al. [37] | 3 | IV | 0.1–0.3 mg/kg | N/S | Swenson pull-through. | |||
| Muto et al. [38] | Case report | 1 | IV | 1 mL (0.5 mg/kg) | N/S | Soave pull-through. | ||
| Shirota et al. [39] | 1 | IV | 0.01–0.1 mg/kg | N/S | ||||
| ARM | PSARP | Paraboschi et al. [40] | Case report | 1 | IV | 1 mg (0.2 mg/kg) | 60 2 | |
| Rentea et al. [37] | Retrospective single-institution study | 1 | IV | 0.1–0.3 mg/kg | N/S | |||
| LAARP | Li et al. [41] | 4 | Enteral 3 | 1.25 mg | - | The timing was not reported as the goal was not to assess blood supply but to identify the rectourethral fistula before its ligation. | ||
| Shirota et al. [39] | Case series | 3 | IV | 0.01–0.1 mg/kg | N/S | |||
| Colostomy closure | Yada et al. [42] | 2 | IV | 0.3 mg/kg | 30 | |||
| Cloaca | PSARVUP | Rentea et al. [37] | Retrospective single-institution study | 8 | IV | 0.1–0.3 mg/kg | N/S | |
| MRKH Syndrome | Vaginal replacement | Fontoura Oliveira et al. [43] | Retrospective single-institution study | 4 | Intra-ureteral 2 | 25 mg | - | ICG was used to visualize the urinary system to prevent its injury during dissection and screen for associated urologic malformations. |
| Saxena et al. [44] | Case report | 1 | IV | 0.2 mg/kg | N/S | Total laparoscopic sigmoid colon vaginoplasty. | ||
| Rectal Prolapse | Perineal rectosigmoid-ectomy | Yamamoto et al. [45] | Case report | 1 | IV | 0.2 mg/kg | N/S | |
| Constipation/Fecal Incontinence | Antegrade continence enema procedure * (Malone/Neomalone) | |||||||
| Urinary Incontinence | Urinary continence channel creation * (Mitrofanoff/Monti) | |||||||
| Bladder augmentation * | ||||||||
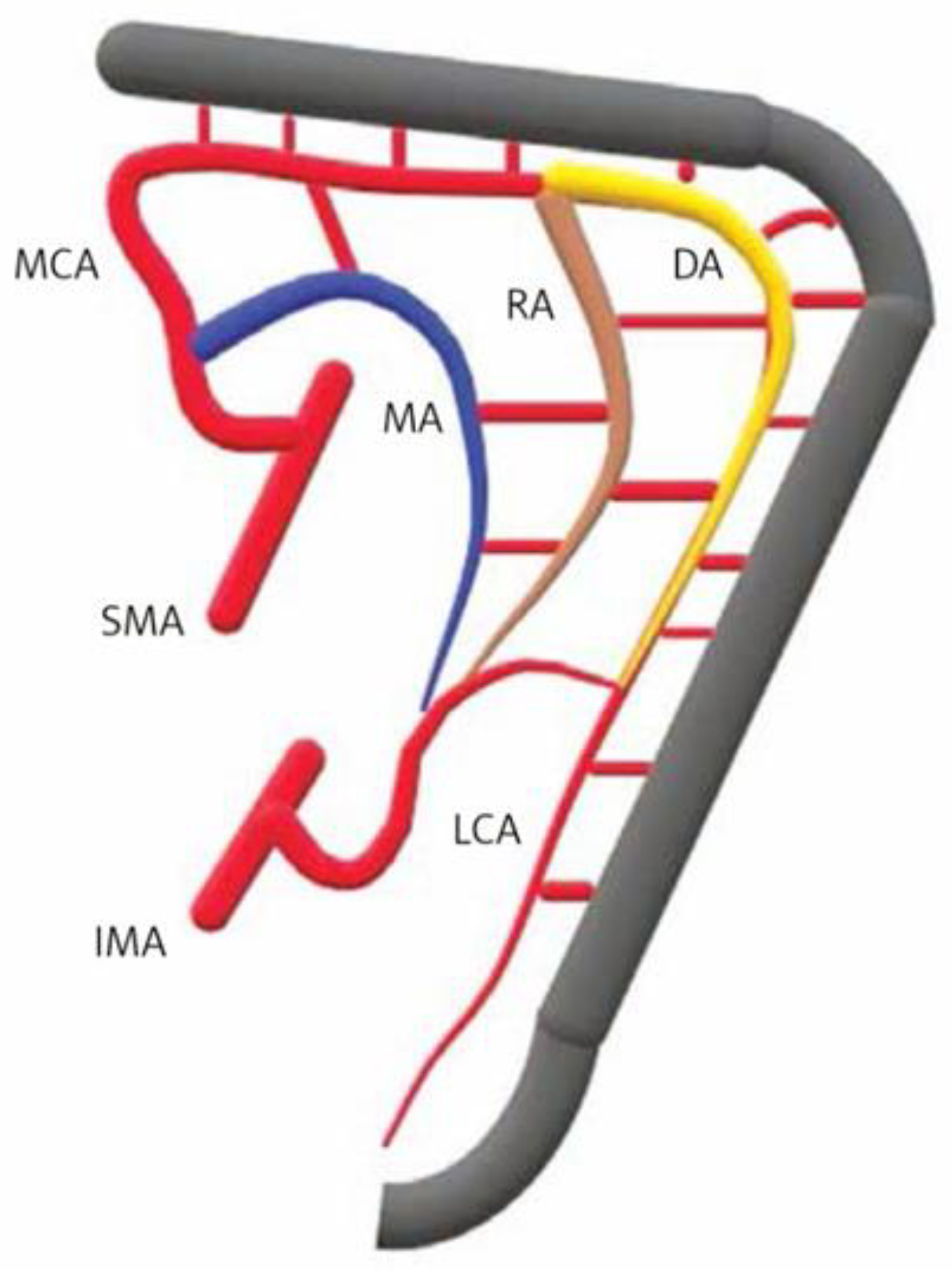
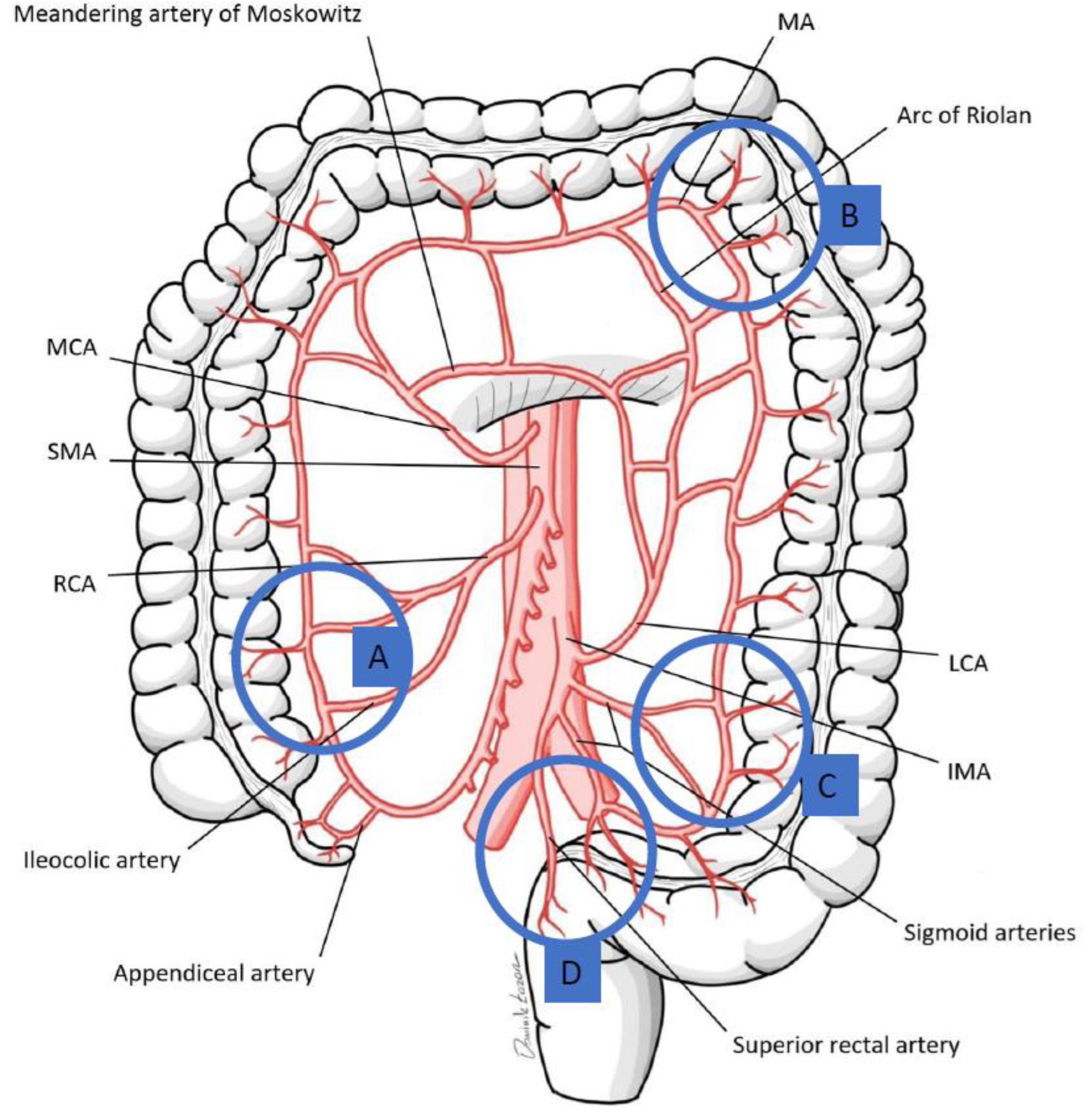
| Site | Watershed | |
|---|---|---|
| Rectosigmoid junction (Sudeck’s point) | Last sigmoid artery (IMA) | Superior rectal artery (IMA) |
| Sigmoid colon | Sigmoid arteries (IMA) | |
| Splenic flexure (Griffith’s point) | Middle colic artery (SMA) | Left colic artery (IMA) |
| Ileocolic region | Ileocolic artery (SMA) | Right colic artery (SMA) |
| Indications: | Evaluate bowel and vaginal tissue perfusion in pediatric colorectal surgeries. |
| Type of Procedure: | Colorectal resections, anastomoses, and assessment of tissue perfusion. |
| Administration Route: | Intravenous injection. |
| Dosage: | 0.1–0.3 mg/kg |
| Advantages: |
|
| Disadvantages: |
|
| Limitations and Precautions: |
|
| Comments: | Bowel (colon/small bowel) and vaginal tissue perfusion were clinically assessed firsthand and then measured using the Stryker system. The tissue perfusion is visualized within 1 to 2 min of intravenous injection [37]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokova, E.; Elhalaby, I.; Saylors, S.; Lim, I.I.P.; Rentea, R.M. Utilization of Indocyanine Green (ICG) Fluorescence in Patients with Pediatric Colorectal Diseases: The Current Applications and Reported Outcomes. Children 2024, 11, 665. https://doi.org/10.3390/children11060665
Bokova E, Elhalaby I, Saylors S, Lim IIP, Rentea RM. Utilization of Indocyanine Green (ICG) Fluorescence in Patients with Pediatric Colorectal Diseases: The Current Applications and Reported Outcomes. Children. 2024; 11(6):665. https://doi.org/10.3390/children11060665
Chicago/Turabian StyleBokova, Elizaveta, Ismael Elhalaby, Seth Saylors, Irene Isabel P. Lim, and Rebecca M. Rentea. 2024. "Utilization of Indocyanine Green (ICG) Fluorescence in Patients with Pediatric Colorectal Diseases: The Current Applications and Reported Outcomes" Children 11, no. 6: 665. https://doi.org/10.3390/children11060665
APA StyleBokova, E., Elhalaby, I., Saylors, S., Lim, I. I. P., & Rentea, R. M. (2024). Utilization of Indocyanine Green (ICG) Fluorescence in Patients with Pediatric Colorectal Diseases: The Current Applications and Reported Outcomes. Children, 11(6), 665. https://doi.org/10.3390/children11060665








