Meconium Aspiration Syndrome, Hypoxic-Ischemic Encephalopathy and Therapeutic Hypothermia—A Recipe for Severe Pulmonary Hypertension?
Abstract
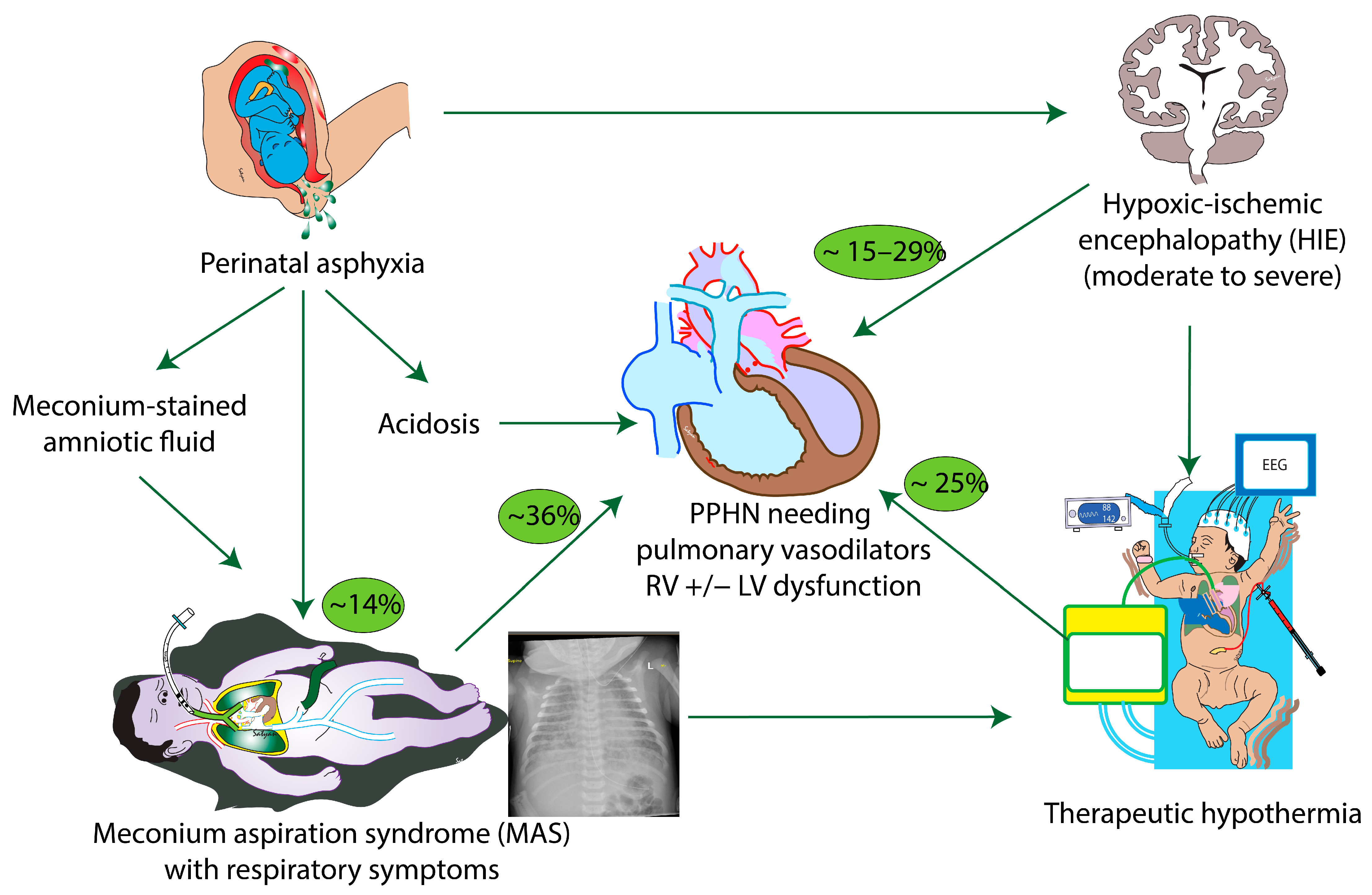
:1. Introduction
2. Meconium Aspiration Syndrome and PPHN
3. Hypoxic-Ischemic Encephalopathy and PPHN
3.1. Mechanisms Contributing to PPHN and Cardiac Dysfunction in HIE
3.1.1. Disrupted Fetal to Neonatal Cardiopulmonary Transition
3.1.2. Myocardial Dysfunction
4. MAS, HIE and TH
4.1. MAS and HIE—A Precarious Combination
4.2. Cardiovascular Effects of TH
4.3. Cardiorespiratory Effects of TH
5. Optimal Therapeutic Strategies for PPHN in the Setting of MAS, HIE and TH
5.1. Oxygen Saturation Targets
5.2. Ventilation Strategies, Surfactant and iNO
5.2.1. Surfactant and Inhaled Nitric Oxide
5.2.2. Targets for pH and PaCO2
5.2.3. Sodium Bicarbonate
5.3. Systemically Administered Pulmonary Vasodilators
5.3.1. Sildenafil
5.3.2. Milrinone
5.3.3. Prostaglandin I2 (PGI2)
5.3.4. Bosentan
5.3.5. Riociguat
5.4. Management of Systemic Hypotension in PPHN in MAS and HIE during TH (Figure 6)

5.5. Extracorporeal Membrane Oxygenation
6. Long-Term Outcomes
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74 (Suppl. S1), 50–72. [Google Scholar] [CrossRef] [PubMed]
- Walsh-Sukys, M.C.; Tyson, J.E.; Wright, L.L.; Bauer, C.R.; Korones, S.B.; Stevenson, D.K.; Verter, J.; Stoll, B.J.; Lemons, J.A.; Papile, L.A.; et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics 2000, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Keszler, M. Persistent Pulmonary Hypertension of the Newborn. NeoReviews 2015, 16, e680–e692. [Google Scholar] [CrossRef] [PubMed]
- Steurer, M.A.; Jelliffe-Pawlowski, L.L.; Baer, R.J.; Partridge, J.C.; Rogers, E.E.; Keller, R.L. Persistent Pulmonary Hypertension of the Newborn in Late Preterm and Term Infants in California. Pediatrics 2017, 139, e20161165. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Shankaran, S.; Laptook, A.; McDonald, S.; Keszler, M.; Van Meurs, K.; Guillet, R.; Chawla, S.; Sood, B.G.; Bonifacio, S.; et al. Pulmonary Hypertension Associated with Hypoxic-Ischemic Encephalopathy-Antecedent Characteristics and Comorbidities. J. Pediatr. 2018, 196, 45–51.e43. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Laptook, A.R.; Pappas, A.; McDonald, S.A.; Das, A.; Tyson, J.E.; Poindexter, B.B.; Schibler, K.; Bell, E.F.; Heyne, R.J.; et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: A randomized clinical trial. JAMA 2014, 312, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Leegwater, A.; Vadlaputi, P.; Garlapati, P.; Chawla, S.; Kalra, V. Approach to non-vigorous infants born through meconium-stained amniotic fluid—Differences between randomized and observational studies. J. Perinatol. 2023, 43, 129–130. [Google Scholar] [CrossRef]
- Chiruvolu, A.; Miklis, K.K.; Chen, E.; Petrey, B.; Desai, S. Delivery room management of meconium-stained newborns and respiratory support. Pediatrics 2018, 142, e20181485. [Google Scholar] [CrossRef] [PubMed]
- Haakonsen Lindenskov, P.H.; Castellheim, A.; Saugstad, O.D.; Mollnes, T.E. Meconium aspiration syndrome: Possible pathophysiological mechanisms and future potential therapies. Neonatology 2015, 107, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Shaul, P.W.; Yuhanna, I.S.; German, Z.; Chen, Z.; Steinhorn, R.H.; Morin, F.C. 3rd. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1997, 272, L1005–L1012. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.T.; Zaher, F.M.; Svinarich, D.M.; Konduri, G.G. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr. Res. 1998, 44, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.J.; Eis, A.; Alexander, M.; Michalkiewicz, T.; Teng, R.-J.; Lakshminrusimha, S.; Konduri, G.G. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L40–L49. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, R.; Rios, D.R.; McNamara, P.J.; Levy, P.T. Asphyxia, Therapeutic Hypothermia, and Pulmonary Hypertension. Clin. Perinatol. 2024, 51, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Pappas, A.; Laptook, A.R.; McDonald, S.A.; Ehrenkranz, R.A.; Tyson, J.E.; Walsh, M.; Goldberg, R.N.; Higgins, R.D.; Das, A. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics 2008, 122, e791–e798. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S. The pulmonary circulation in neonatal respiratory failure. Clin. Perinatol. 2012, 39, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, D.W.; Hall, F.K.; Sheehan, M.B.; Hall, R.T. Postasphyxial lung disease in newborn infants with severe pennatal acidosis. Am. J. Obstet. Gynecol. 1984, 150, 393–399. [Google Scholar] [CrossRef]
- Peeters, L.L.; Sheldon, R.E.; Jones Jr, M.D.; Makowski, E.L.; Meschia, G. Blood flow to fetal organs as a function of arterial oxygen content. Am. J. Obstet. Gynecol. 1979, 135, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, D.; Lane, E.C.A.; Valdez, R.; Lesneski, A.L.; Lakshminrusimha, S. Role of Volume Replacement during Neonatal Resuscitation in the Delivery Room. Children 2022, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.R.; Lapointe, A.; Schmolzer, G.M.; Mohammad, K.; VanMeurs, K.P.; Keller, R.L.; Sehgal, A.; Lakshminrusimha, S.; Giesinger, R.E. Hemodynamic optimization for neonates with neonatal encephalopathy caused by a hypoxic ischemic event: Physiological and therapeutic considerations. Semin. Fetal Neonatal Med. 2021, 26, 101277. [Google Scholar] [CrossRef] [PubMed]
- Giesinger, R.E.; Levy, P.T.; Ruoss, J.L.; El Dib, M.; Mohammad, K.; Wintermark, P.; McNamara, P.J. Cardiovascular management following hypoxic–ischemic encephalopathy in North America: Need for physiologic consideration. Pediatr. Res. 2021, 90, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Koestenberger, M.; Sallmon, H.; Avian, A.; Cantinotti, M.; Gamillscheg, A.; Kurath-Koller, S.; Schweintzger, S.; Hansmann, G. Ventricular–ventricular interaction variables correlate with surrogate variables of clinical outcome in children with pulmonary hypertension. Pulm. Circ. 2019, 9, 2045894019854074. [Google Scholar] [CrossRef]
- Lapointe, A.; Barrington, K.J. Pulmonary hypertension and the asphyxiated newborn. J. Pediatr. 2011, 158, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Devi, U.; Pullattayil, A.K.; Chandrasekaran, M. Hypocarbia is associated with adverse outcomes in hypoxic ischaemic encephalopathy (HIE). Acta Paediatr. 2023, 112, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Committee on Fetus and Newborn; Papile, L.A.; Baley, J.E.; Benitz, W.; Cummings, J.; Carlo, W.A.; Eichenwald, E.; Kumar, P.; Polin, R.A.; Tan, R.C.; et al. Hypothermia and neonatal encephalopathy. Pediatrics 2014, 133, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, M.; Whitelaw, A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics 2000, 106, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Benumof, J.L.; Wahrenbrock, E.A. Dependency of hypoxic pulmonary vasoconstriction on temperature. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.S. Hypothermia: A systematic review and meta-analysis of clinical trials. Semin. Fetal Neonatal Med. 2010, 15, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Joanna, R.G.V.; Lopriore, E.; Te Pas, A.B.; Rijken, M.; van Zwet, E.W.; de Bruine, F.T.; Steggerda, S.J. Persistent pulmonary hypertension in neonates with perinatal asphyxia and therapeutic hypothermia: A frequent and perilous combination. J. Matern. Fetal Neonatal Med. 2022, 35, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.M.; Bischoff, A.R.; Giesinger, R.E.; McNamara, P.J. Impact of therapeutic hypothermia on echocardiography indices of pulmonary hemodynamics. J. Perinatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Thayyil, S.; Pant, S.; Montaldo, P.; Shukla, D.; Oliveira, V.; Ivain, P.; Bassett, P.; Swamy, R.; Mendoza, J.; Moreno-Morales, M.; et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): A randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 2021, 9, e1273–e1285. [Google Scholar] [CrossRef] [PubMed]
- Aker, K.; Stoen, R.; Eikenes, L.; Martinez-Biarge, M.; Nakken, I.; Haberg, A.K.; Gibikote, S.; Thomas, N. Therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy in India (THIN study): A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Tanigasalam, V.; Bhat, V.; Adhisivam, B.; Sridhar, M.G. Does therapeutic hypothermia reduce acute kidney injury among term neonates with perinatal asphyxia?—A randomized controlled trial. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2016, 29, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Ovali, F. Hemodynamic changes and evaluation during hypoxic-ischemic encephalopathy and therapeutic hypothermia. Early Hum. Dev. 2022, 167, 105563. [Google Scholar] [CrossRef] [PubMed]
- Hochwald, O.; Jabr, M.; Osiovich, H.; Miller, S.P.; McNamara, P.J.; Lavoie, P.M. Preferential cephalic redistribution of left ventricular cardiac output during therapeutic hypothermia for perinatal hypoxic-ischemic encephalopathy. J. Pediatr. 2014, 164, 999–1004.e1001. [Google Scholar] [CrossRef] [PubMed]
- Pryds, O.; Greisen, G.; Lou, H.; Friis-Hansen, B. Vasoparalysis associated with brain damage in asphyxiated term infants. J. Pediatr. 1990, 117, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.A. Regulation of cerebral blood flow after asphyxia in neonatal lambs. Stroke A J. Cereb. Circ. 1988, 19, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H.; Hansmann, G.; Archer, S.L.; Ivy, D.D.; Adatia, I.; Chung, W.K.; Hanna, B.D.; Rosenzweig, E.B.; Raj, J.U.; Cornfield, D.; et al. Pediatric Pulmonary Hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015, 132, 2037–2099. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, V.S.; Chalak, L.F.; DuPont, T.L.; Rollins, N.K.; Brion, L.P.; Wyckoff, M.H. Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 949–954. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Abman, S.H. Oxygen Targets in Neonatal Pulmonary Hypertension: Individualized, “Precision-Medicine” Approach. Clin. Perinatol. 2024, 51, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Bancalari, A.; Osorio, W.; Luco, M.; Gonzalez, A.; Perez, H.; Kattan, J. Early use of combined exogenous surfactant and inhaled nitric oxide reduces treatment failure in persistent pulmonary hypertension of the newborn: A randomized controlled trial. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.W.; Abman, S.H.; Kinsella, J.P.; Mandell, E.W. Pulmonary vasodilator strategies in neonates with acute hypoxemic respiratory failure and pulmonary hypertension. Semin. Fetal Neonatal Med. 2022, 27, 101367. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.W.; Kinsella, J.P. Inhaled Nitric Oxide in Neonatal Pulmonary Hypertension. Clin. Perinatol. 2024, 51, 95–111. [Google Scholar] [CrossRef]
- Konduri, G.; Sokol, G.; Van Meurs, K.; Singer, J.; Ambalavanan, N.; Lee, T.; Solimano, A. Impact of early surfactant and inhaled nitric oxide therapies on outcomes in term/late preterm neonates with moderate hypoxic respiratory failure. J. Perinatol. 2013, 33, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.M.; Yuan, S. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J. Clin. Investig. 1966, 45, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Aschner, J.L.; Poland, R.L. Sodium bicarbonate: Basically useless therapy. Pediatrics 2008, 122, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, M.H.; Sawyer, T.; Lakshminrusimha, S.; Collins, A.; Ohls, R.K.; Leone, T.A. Resuscitation 2020: Proceedings From the NeoHeart 2020 International Conference. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 77–88. [Google Scholar] [CrossRef]
- Lou, H.C.; Lassen, N.A.; Fris-Hansen, B. Decreased cerebral blood flow after administration of sodium bicarbonate in the distressed newborn infant. Acta Neurol. Scand. 1978, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chilakala, S.K.; Parfenova, H.; Pourcyrous, M. The effects of sodium bicarbonate infusion on cerebrovascular function in newborn pigs. Pediatr. Res. 2022, 92, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Fox, W.W.; Duara, S. Persistent pulmonary hypertension in the neonate: Diagnosis and management. J. Pediatr. 1983, 103, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Munoz, K.D.; Walton, J.P. Hearing loss in infants with persistent fetal circulation. Pediatrics 1988, 81, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Buckley, E.M.; Naim, M.Y.; Lynch, J.M.; Goff, D.A.; Schwab, P.J.; Diaz, L.K.; Nicolson, S.C.; Montenegro, L.M.; Lavin, N.A.; Durduran, T.; et al. Sodium bicarbonate causes dose-dependent increases in cerebral blood flow in infants and children with single-ventricle physiology. Pediatr. Res. 2013, 73, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.; Khoja, Z.; Johnstone, A.; Dale, L.; Rampakakis, E.; Wintermark, P. Sildenafil Improves Brain Injury Recovery following Term Neonatal Hypoxia-Ischemia in Male Rat Pups. Dev. Neurosci. 2016, 38, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, P.; Lapointe, A.; Steinhorn, R.; Rampakakis, E.; Burhenne, J.; Meid, A.D.; Bajraktari-Sylejmani, G.; Khairy, M.; Altit, G.; Adamo, M.T.; et al. Feasibility and Safety of Sildenafil to Repair Brain Injury Secondary to Birth Asphyxia (SANE-01): A Randomized, Double-blind, Placebo-controlled Phase Ib Clinical Trial. J. Pediatr. 2024, 266, 113879. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.R.; Habib, S.; McNamara, P.J.; Giesinger, R.E. Hemodynamic response to milrinone for refractory hypoxemia during therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Berger-Caron, F.; Piedboeuf, B.; Morissette, G.; Simonyan, D.; Chétaille, P.; Pellerin, A.; Hébert, A. Inhaled epoprostenol for pulmonary hypertension treatment in neonates: A 12-year experience. Am. J. Perinatol. 2019, 36, 1142–1149. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Banales, J.; Henderson, C.L.; Ramos, S.E.; Brandt, K.M.; Powers, G.C. Intravenous epoprostenol improves oxygenation index in patients with persistent pulmonary hypertension of the newborn refractory to nitric oxide. J. Perinatol. 2018, 38, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, R.H.; Fineman, J.; Kusic-Pajic, A.; Cornelisse, P.; Gehin, M.; Nowbakht, P.; Pierce, C.M.; Beghetti, M. Bosentan as Adjunctive Therapy for Persistent Pulmonary Hypertension of the Newborn: Results of the Randomized Multicenter Placebo-Controlled Exploratory Trial. J. Pediatr. 2016, 177, 90–96.e93. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Callan, E.; Konduri, G.G. Pulmonary vasodilator therapy in persistent pulmonary hypertension of the newborn. Clin. Perinatol. 2022, 49, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Giesinger, R.E.; Stanford, A.H.; Thomas, B.; Abman, S.H.; McNamara, P.J. Safety and Feasibility of Riociguat Therapy for the Treatment of Chronic Pulmonary Arterial Hypertension in Infancy. J. Pediatr. 2023, 255, 224–229.e221. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; El-Khuffash, A.F.; van Herpen, C.H.; Resende, M.H.F.; Giesinger, R.E.; Weisz, D.; Mertens, L.; Jankov, R.P.; McNamara, P.J. Cardiac Function and Ventricular Interactions in Persistent Pulmonary Hypertension of the Newborn. Pediatr. Crit. Care Med. 2021, 22, e145–e157. [Google Scholar] [CrossRef] [PubMed]
- Lesneski, A.L.; Vali, P.; Hardie, M.E.; Lakshminrusimha, S.; Sankaran, D. Randomized Trial of Oxygen Saturation Targets during and after Resuscitation and Reversal of Ductal Flow in an Ovine Model of Meconium Aspiration and Pulmonary Hypertension. Children 2021, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Mydam, J.; Zidan, M.; Chouthai, N.S. A comprehensive study of clinical biomarkers, use of inotropic medications and fluid resuscitation in newborns with persistent pulmonary hypertension. Pediatr. Cardiol. 2015, 36, 233–239. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Giesinger, R.E.; Lakshminrusimha, S. Dopamine and Neonatal Pulmonary Hypertension-Pressing Need for a Better Pressor? J. Pediatr. 2022, 246, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.L.; Stensvold, H.J.; Risnes, K.; Guthe, H.J.; Astrup, H.; Nordhov, S.M.; Selberg, T.R.; Rønnestad, A.; Lang, A.M. Inotropic Therapy in Newborns, A Population-Based National Registry Study. Pediatr. Crit. Care Med. 2016, 17, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.D.; Morton, M.J.; Reid, D.L.; Thornburg, K.L. Fetal lamb ventricles respond differently to filling and arterial pressures and to in utero ventilation. Pediatr. Res. 1987, 22, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Feltes, T.F.; Hansen, T.N.; Martin, C.G.; Leblanc, A.L.; Smith, S.; Giesler, M.E. The effects of dopamine infusion on regional blood flow in newborn lambs. Pediatr. Res. 1987, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.Y.; Barrington, K.J.; Pearson, R.J.; Bigam, D.L.; Finer, N.N.; Van Aerde, J.E. Systemic, pulmonary and mesenteric perfusion and oxygenation effects of dopamine and epinephrine. Am. J. Respir. Crit. Care Med. 1997, 155, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, N.; Bigam, D.L.; Churchill, T.; Rayner, D.; Joynt, C.; Cheung, P.Y. A comparison of combination dopamine and epinephrine treatment with high-dose dopamine alone in asphyxiated newborn piglets after resuscitation. Pediatr. Res. 2013, 73, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Tourneux, P.; Rakza, T.; Bouissou, A.; Krim, G.; Storme, L. Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J. Pediatr. 2008, 153, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Golden, P.J.; Kajiura, L.N.; Murata, L.A.; Uyehara, C.F. Vasopressin decreases pulmonary-to-systemic vascular resistance ratio in a porcine model of severe hemorrhagic shock. Shock 2015, 43, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.Y.; Barrington, K.J. The effects of dopamine and epinephrine on hemodynamics and oxygen metabolism in hypoxic anesthetized piglets. Crit. Care 2001, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Nasef, N.; Shah, V.; McNamara, P.J. Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates: Case series. Pediatr. Crit. Care Med. 2014, 15, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Kiyomoto, K.; He, H.; Tomohiro, A.; Nishiyama, A.; Aki, Y.; Kimura, S.; Abe, Y. Vasodilation induced by vasopressin V2 receptor stimulation in afferent arterioles. Kidney Int. 1996, 49, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Evora, P.R.; Pearson, P.J.; Schaff, H.V. Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. V1-receptor-mediated production of nitric oxide. Chest 1993, 103, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Sai, Y.; Okamura, T.; Amakata, Y.; Toda, N. Comparison of responses of canine pulmonary artery and vein to angiotensin II, bradykinin and vasopressin. Eur. J. Pharmacol. 1995, 282, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.K.; Chen, Y.F.; Yang, R.H.; McKenna, T.M.; Jackson, R.M.; Oparil, S. Vasopressin lowers pulmonary artery pressure in hypoxic rats by releasing atrial natriuretic peptide. Am. J. Med. Sci. 1989, 298, 227–236. [Google Scholar] [CrossRef]
- Walker, B.R.; Haynes, J.J.; Wang, H.L.; Voelkel, N.F. Vasopressin-induced pulmonary vasodilation in rats. Am. J. Physiol.-Heart Circ. Physiol. 1989, 257, H415–H422. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, M.R.; Walker, B.R. Enhanced pulmonary arterial dilation to arginine vasopressin in chronically hypoxic rats. Am. J. Physiol. 1994, 267, H2413–H2419. [Google Scholar] [CrossRef]
- Siefkes, H.M.; Lakshminrusimha, S. Management of systemic hypotension in term infants with persistent pulmonary hypertension of the newborn: An illustrated review. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Alsaleem, M.; Malik, A.; Lakshminrusimha, S.; Kumar, V.H. Hydrocortisone Improves Oxygenation Index and Systolic Blood Pressure in Term Infants With Persistent Pulmonary Hypertension. Clin. Med. Insights Pediatr. 2019, 13, 1179556519888918. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Boonstra, P.S.; MacLaren, G.; Paden, M.; Brodie, D.; Anders, M.; Hoskote, A.; Ramanathan, K.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal Life Support Organization Registry International Report 2022: 100,000 Survivors. Asaio J. 2024, 70, 131–143. [Google Scholar] [CrossRef]
- Stieren, E.S.; Sankaran, D.; Lakshminrusimha, S.; Rottkamp, C.A. Comorbidities and Late Outcomes in Neonatal Pulmonary Hypertension. Clin. Perinatol. 2024, 51, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.A.; Lee, N.R.; Vaver, K.N.; Werner, D.; Fashaw, L.; Hale, K.; Waas, N. School-age outcomes of newborns treated for persistent pulmonary hypertension. J. Perinatol. 2010, 30, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Timberline, S.; Bhatt, A.; Sunderji, S.; Tancredi, D.J.; Lakshminrusimha, S.; Siefkes, H. Novel scoring tool of hypoxemic respiratory failure and pulmonary hypertension for defining severity of persistent pulmonary hypertension of newborn. J. Perinatol. 2023, 43, 1281–1287. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankaran, D.; Li, J.R.A.; Lakshminrusimha, S. Meconium Aspiration Syndrome, Hypoxic-Ischemic Encephalopathy and Therapeutic Hypothermia—A Recipe for Severe Pulmonary Hypertension? Children 2024, 11, 673. https://doi.org/10.3390/children11060673
Sankaran D, Li JRA, Lakshminrusimha S. Meconium Aspiration Syndrome, Hypoxic-Ischemic Encephalopathy and Therapeutic Hypothermia—A Recipe for Severe Pulmonary Hypertension? Children. 2024; 11(6):673. https://doi.org/10.3390/children11060673
Chicago/Turabian StyleSankaran, Deepika, Jessa Rose A. Li, and Satyan Lakshminrusimha. 2024. "Meconium Aspiration Syndrome, Hypoxic-Ischemic Encephalopathy and Therapeutic Hypothermia—A Recipe for Severe Pulmonary Hypertension?" Children 11, no. 6: 673. https://doi.org/10.3390/children11060673





