Application of Interferon-γ Release Assay in the Assessment of T-Cell Immunity to SARS-CoV-2 Antigens in the Cohort of Pediatric Patients with Juvenile Idiopathic Arthritis
Abstract
:1. Introduction
1.1. Current State of the Pandemic
1.2. Humoral Immunity
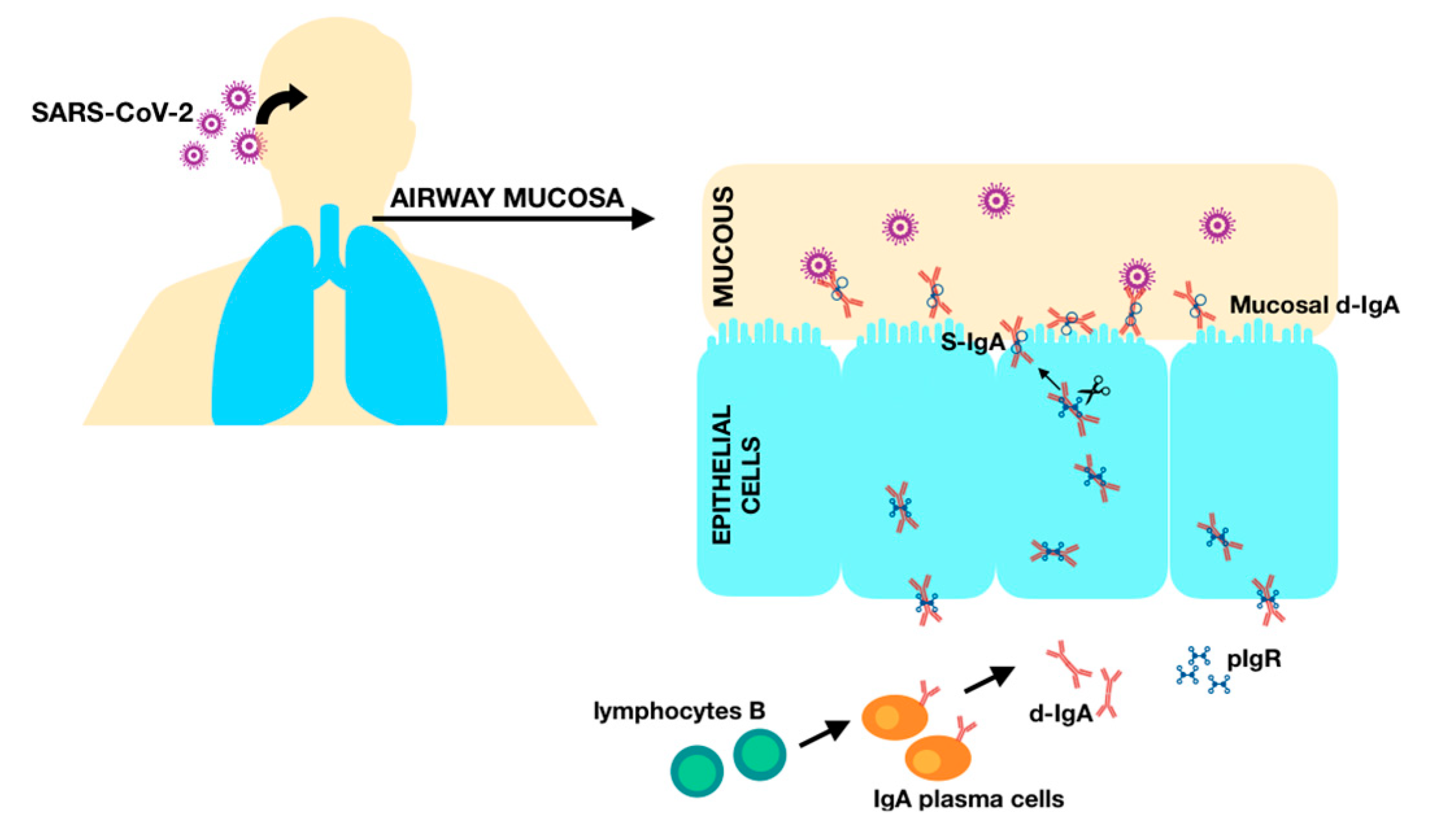
1.3. Cellular Immunity
1.4. Interferon-γ Release Assay (IGRA)
2. Materials and Methods
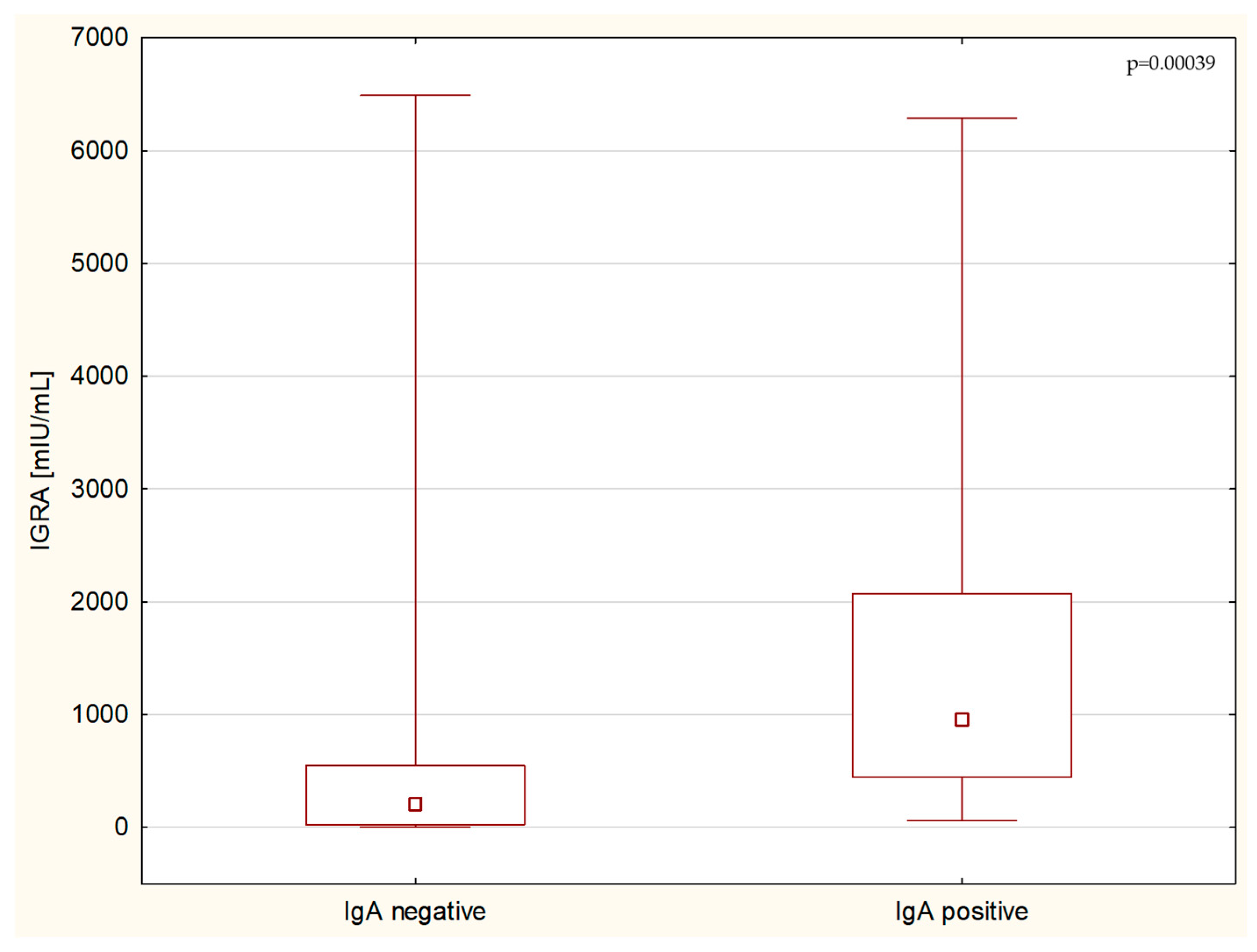
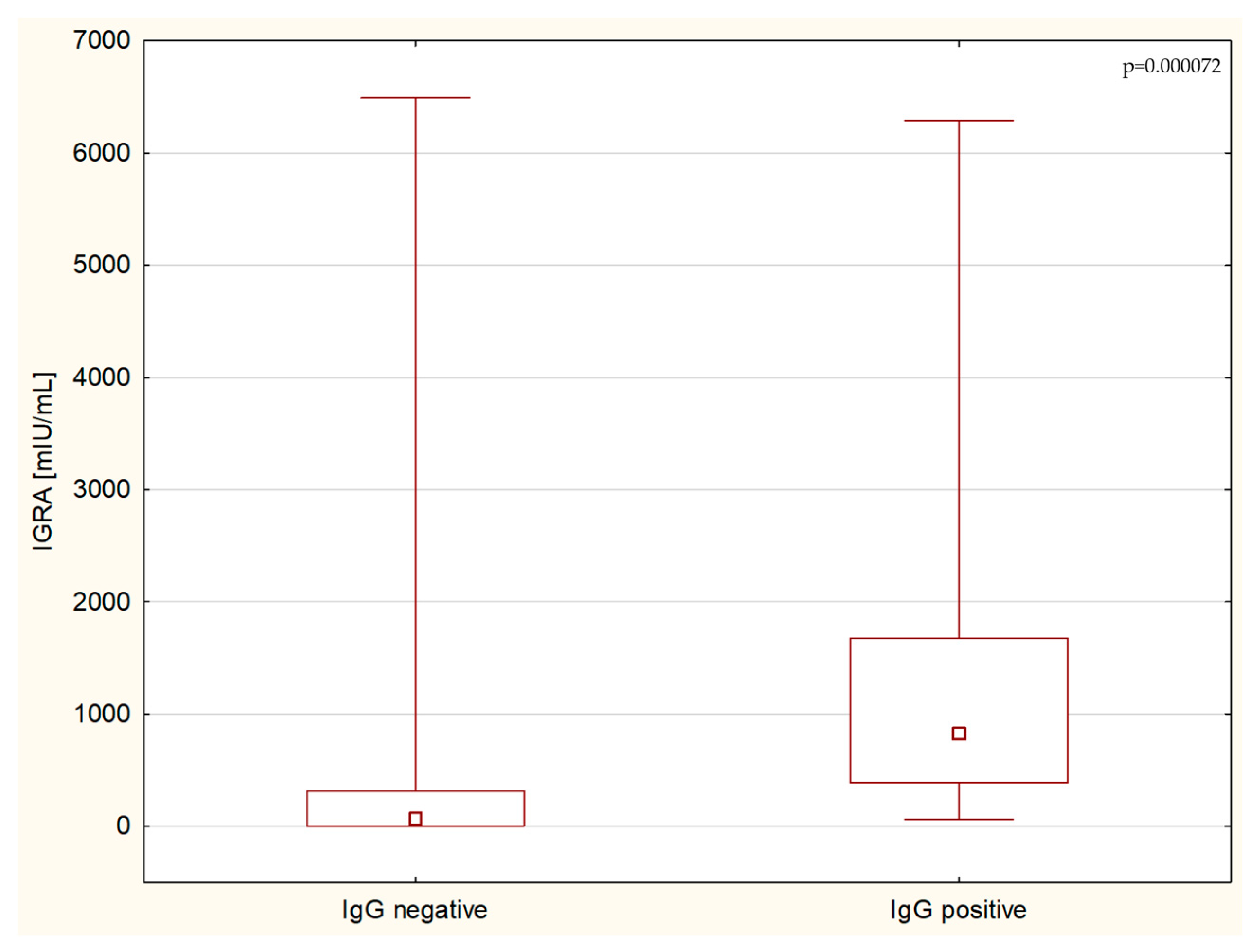
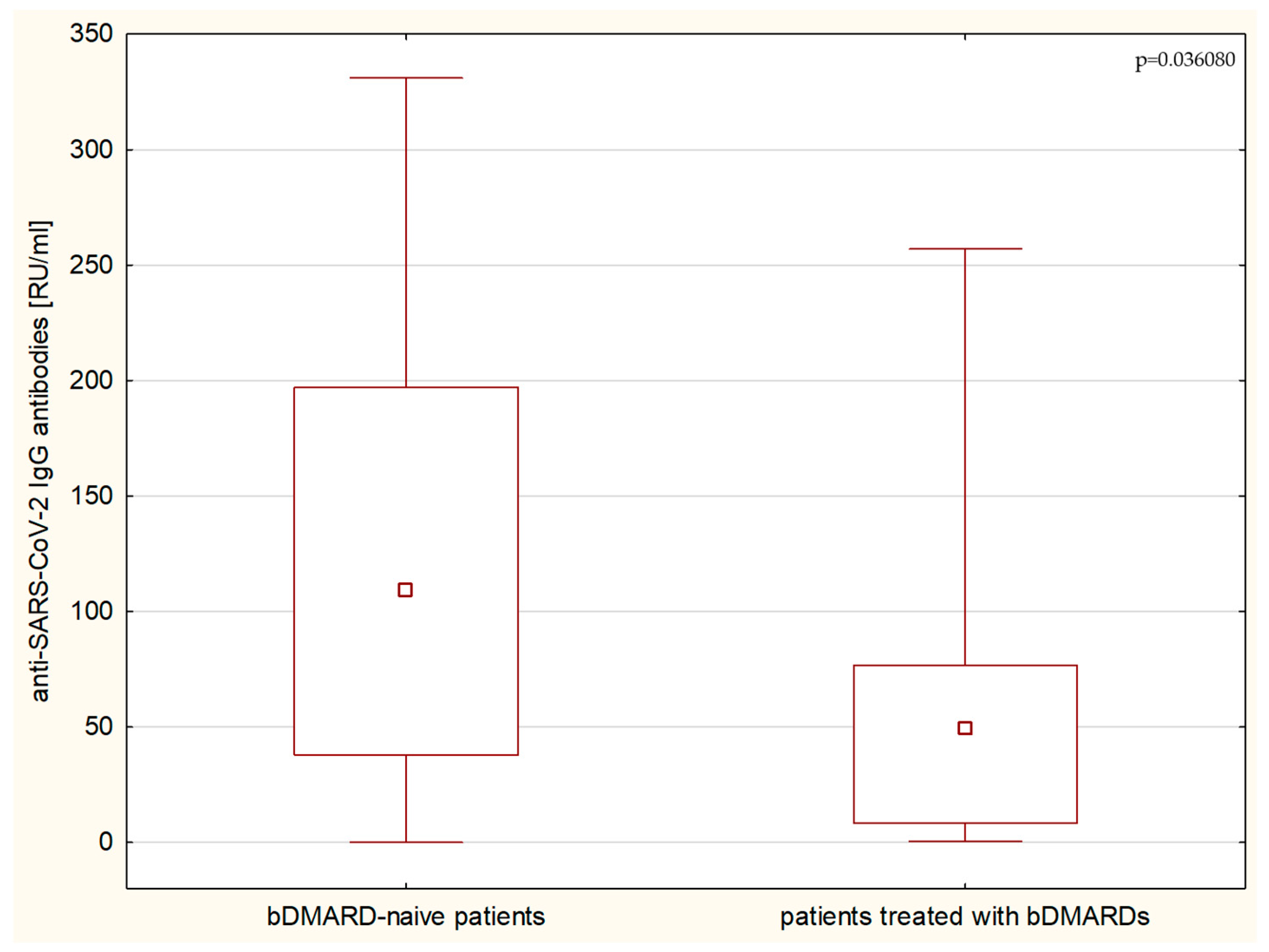
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Arduini, A.; Laprise, F.; Liang, C. SARS-CoV-2 ORF8: A Rapidly Evolving Immune and Viral Modulator in COVID-19. Viruses 2023, 15, 871. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; Moustafa Abo El-Ella, D.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540. [Google Scholar] [CrossRef]
- Primorac, D.; Vrdoljak, K.; Brlek, P.; Pavelić, E.; Molnar, V.; Matišić, V.; Erceg Ivkošić, I.; Parčina, M. Adaptive Immune Responses and Immunity to SARS-CoV-2. Front. Immunol. 2022, 13, 848582. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.S.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef]
- Hashem, A.M.; Algaissi, A.; Almahboub, S.A.; Alfaleh, M.A.; Abujamel, T.S.; Alamri, S.S.; Alluhaybi, K.A.; Hobani, H.I.; AlHarbi, R.H.; Alsulaiman, R.M.; et al. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses 2020, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Oh, J.E. Humoral Immunity against SARS-CoV-2 and the Impact on COVID-19 Pathogenesis. Mol. Cells 2021, 44, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Islamoglu, M.S.; Cengiz, M.; Uysal, B.B.; Ikitimur, H.; Ozdemir, Z.; Karamehmetoglu, A.; Akbulut, A.E.; Bakdur, A.N.; Ozdemir, A.; Kayıkcıoglu, H.; et al. Relationship between Antibody Levels and SARS-CoV-2 Reinfection. Ann. Clin. Lab. Sci. 2021, 51, 750–755. [Google Scholar] [PubMed]
- Sullivan, A.; Alfego, D.; Hu, P.; Gillim, L.; Grover, A.; Garcia, C.; Cohen, O.; Letovsky, S. Antibody titer levels and the effect on subsequent SARS-CoV-2 infection in a large US-based cohort. Heliyon 2023, 9, e13103. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild COVID-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef]
- Błaszczuk, A.; Michalski, A.; Sikora, D.; Malm, M.; Drop, B.; Polz-Dacewicz, M. Antibody Response after SARS-CoV-2 Infection with the Delta and Omicron Variant. Vaccines 2022, 10, 1728. [Google Scholar] [CrossRef]
- Ng, O.W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, H.; Ye, B.; Zhao, M.; Zhan, J.; Dong, S.; Guo, Y.; Zhao, Y.; Li, M.; Liu, S.; et al. One-Year Sustained Cellular and Humoral Immunities in Coronavirus Disease 2019 (COVID-19) Convalescents. Clin. Infect. Dis. 2022, 75, e1072–e1081. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, R.; Woon, S.T.; Steele, R.; Lehnert, K.; Leung, E.; Brooks, A.E.S. Critical role of diagnostic SARS-CoV-2 T cell assays for immunodeficient patients. J. Clin. Pathol. 2022, 75, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Schwarz, T.; Corman, V.M.; Gebert, L.; Kleinschmidt, M.C.; Wald, A.; Gläser, S.; Kruse, J.M.; Zickler, D.; Peric, A.; et al. SARS-CoV-2 T Cell Response in Severe and Fatal COVID-19 in Primary Antibody Deficiency Patients without Specific Humoral Immunity. Front. Immunol. 2022, 13, 840126. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.M.; Saleh, R.; Sasidharan Nair, V.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021, 162, 30–43. [Google Scholar] [CrossRef]
- Heitmann, J.S.; Bilich, T.; Tandler, C.; Nelde, A.; Maringer, Y.; Marconato, M.; Reusch, J.; Jäger, S.; Denk, M.; Richter, M.; et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 2022, 601, 617–622. [Google Scholar] [CrossRef]
- Sester, M.; Sotgiu, G.; Lange, C.; Giehl, C.; Girardi, E.; Migliori, G.B.; Bossink, A.; Dheda, K.; Diel, R.; Dominguez, J.; et al. Interferon-γ release assays for the diagnosis of active tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 100–111. [Google Scholar] [CrossRef]
- Kim, S.H. Interferon-γ Release Assay for Cytomegalovirus (IGRA-CMV) for Risk Stratification of Posttransplant CMV Infection: Is It Time to Apply IGRA-CMV in Routine Clinical Practice? Clin. Infect. Dis. 2020, 71, 2386–2388. [Google Scholar] [CrossRef]
- Terada, K.; Itoh, Y.; Fujii, A.; Kitagawa, S.; Ogita, S.; Ouchi, K. Varicella-zoster virus-specific, cell-mediated immunity with interferon-gamma release assay after vaccination of college students with no or intermediate IgG antibody response. J. Med. Virol. 2015, 87, 350–356. [Google Scholar] [CrossRef]
- Huzly, D.; Panning, M.; Smely, F.; Enders, M.; Komp, J.; Falcone, V.; Steinmann, D. Accuracy and real life performance of a novel interferon-γ release assay for the detection of SARS-CoV2 specific T cell response. J. Clin. Virol. 2022, 148, 105098. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, P.; Sanz, J.C.; San Román, J.; Pérez-Abeledo, M.; Carretero, M.; Megías, G.; Viñuela-Prieto, J.M.; Ramos, B.; Canora, J.; Martínez-Peromingo, F.J.; et al. A Pilot Study for the Evaluation of an Interferon Gamma Release Assay (IGRA) To Measure T-Cell Immune Responses after SARS-CoV-2 Infection or Vaccination in a Unique Cloistered Cohort. J. Clin. Microbiol. 2022, 60, e0219921. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.Y.; Lee, N.Y.; Hwang, N.; Song, K.E. Diagnostic Performance of Interferon Gamma Release Assay (IGRA) for Cell-Mediated Immune Responses to SARS-CoV-2. Clin. Lab. 2023, 69, 604. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.L.; Lilleri, D.; Zelini, P.; Gregorini, M.; Morosini, M.; Pattonieri, E.F.; Grignano, M.A.; Libetta, C.; Sepe, V.; Di Toro, A.; et al. SARS-CoV-2-specific IgG and NCP in vulnerable patients without symptoms. New Microbiol. 2022, 45, 213–218. [Google Scholar] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Chia, W.N.; Tham, C.Y.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.A.; Shankar, N.; et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef] [PubMed]
- Kapten, K.; Orczyk, K.; Smolewska, E. Immunity in SARS-CoV-2 Infection: Clarity or Mystery? A Broader Perspective in the Third Year of a Worldwide Pandemic. Arch. Immunol. Ther. Exp. 2023, 71, 7. [Google Scholar] [CrossRef] [PubMed]
- Brand, I.; Gilberg, L.; Bruger, J.; Garí, M.; Wieser, A.; Eser, T.M.; Frese, J.; Ahmed, M.I.; Rubio-Acero, R.; Guggenbuehl Noller, J.M.; et al. Broad T Cell Targeting of Structural Proteins After SARS-CoV-2 Infection: High Throughput Assessment of T Cell Reactivity Using an Automated Interferon Gamma Release Assay. Front. Immunol. 2021, 12, 688436. [Google Scholar] [CrossRef]
- Björkander, S.; Du, L.; Zuo, F.; Ekström, S.; Wang, Y.; Wan, H.; Sherina, N.; Schoutens, L.; Andréll, J.; Andersson, N.; et al. BAMSE COVID-19 study group. SARS-CoV-2-specific B- and T-cell immunity in a population-based study of young Swedish adults. J. Allergy Clin. Immunol. 2022, 149, 65–75. [Google Scholar] [CrossRef]
- Oja, A.E.; Saris, A.; Ghandour, C.A.; Kragten, N.A.; Hogema, B.M.; Nossent, E.J.; Heunks, L.M.; Cuvalay, S.; Slot, E.; Linty, F.; et al. Divergent SARS-CoV-2-specific T- and B-cell responses in severe but not mild COVID-19 patients. Eur. J. Immunol. 2020, 50, 1998–2012. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zhang, X.; Lin, Y.; Liu, D.; Xun, J.; Wang, Z.; Gu, L.; Li, Q.; Yin, D.; et al. Decline in neutralising antibody responses, but sustained T-cell immunity, in COVID-19 patients at 7 months post-infection. Clin. Transl. Immunol. 2021, 10, e1319. [Google Scholar] [CrossRef] [PubMed]
- Schnizer, C.; Andreas, N.; Vivas, W.; Kamradt, T.; Baier, M.; Kiehntopf, M.; Glöckner, S.; Scherag, A.; Löffler, B.; Kolanos, S.; et al. Persistent humoral and CD4+ TH cell immunity after mild SARS-CoV-2 infection—The CoNAN long-term study. Front. Immunol. 2023, 13, 1095129. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, G.; Wang, Y.; Zhang, Q.; Ren, L.; Gu, X.; Huang, T.; Zhong, J.; Wang, Y.; Wang, X.; et al. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: A longitudinal cohort study. Lancet Microbe 2022, 3, e348–e356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Mei, X.; Zhou, Y.; Tang, Z.; Li, G.; Zhong, J.; Yu, M.; Huang, M.; Su, X.; et al. SARS-CoV-2-specific CD4+ T cells are associated with long-term persistence of neutralizing antibodies. Signal Transduct. Target. Ther. 2022, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gallais, F.; Velay, A.; Nazon, C.; Wendling, M.J.; Partisani, M.; Sibilia, J.; Candon, S.; Fafi-Kremer, S. Intrafamilial Exposure to SARS-CoV-2 Associated with Cellular Immune Response without Seroconversion, France. Emerg. Infect. Dis. 2021, 27, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Swadling, L.; Gibbons, J.M.; Pade, C.; Jensen, M.P.; Diniz, M.O.; Schmidt, N.M.; Butler, D.K.; Amin, O.E.; Bailey, S.N.; et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci. Immunol. 2020, 5, eabf3698. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.G.; Nganou-Makamdop, K.; Tenbusch, M.; El Kenz, B.; Maier, C.; Lapuente, D.; Überla, K.; Spriewald, B.; Bergmann, S.; Harrer, E.G.; et al. SARS-CoV-2-Seronegative Subjects Target CTL Epitopes in the SARS-CoV-2 Nucleoprotein Cross-Reactive to Common Cold Coronaviruses. Front. Immunol. 2021, 12, 627568. [Google Scholar] [CrossRef] [PubMed]
- Quinti, I.; Mortari, E.P.; Fernandez Salinas, A.; Milito, C.; Carsetti, R. IgA Antibodies and IgA Deficiency in SARS-CoV-2 Infection. Front. Cell. Infect. Microbiol. 2021, 11, 655896. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Zervou, F.N.; Louie, P.; Stachel, A.; Zacharioudakis, I.M.; Ortiz-Mendez, Y.; Thomas, K.; Aguero-Rosenfeld, M.E. SARS-CoV-2 antibodies: IgA correlates with severity of disease in early COVID-19 infection. J. Med. Virol. 2021, 93, 5409–5415. [Google Scholar] [CrossRef]
- de Fays, C.; Carlier, F.M.; Gohy, S.; Pilette, C. Secretory Immunoglobulin A Immunity in Chronic Obstructive Respiratory Diseases. Cells 2022, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Manfredi, M.; Grossi, V.; Lari, B.; Fabbri, S.; Benucci, M.; Fortini, A.; Damiani, A.; Mobilia, E.M.; Panciroli, M.; et al. Closing the serological gap in the diagnostic testing for COVID-19: The value of anti-SARS-CoV-2 IgA antibodies. J. Med. Virol. 2021, 93, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Hennings, V.; Thörn, K.; Albinsson, S.; Lingblom, C.; Andersson, K.; Andersson, C.; Järbur, K.; Pullerits, R.; Idorn, M.; Paludan, S.R.; et al. The presence of serum anti-SARS-CoV-2 IgA appears to protect primary health care workers from COVID-19. Eur. J. Immunol. 2022, 52, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Lledó, A.; Retuerto, M.; Almendro-Vázquez, P.; Fernández-Ruiz, M.; Galindo, M.; Laguna-Goya, R.; Paz-Artal, E.; Lalueza, A.; Aguado, J.M.; Pablos, J.L. SARS-CoV-2-specific T-cell responses after COVID-19 recovery in patients with rheumatic diseases on immunosuppressive therapy. Semin. Arthritis Rheum. 2021, 51, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.; Ahmed, S.; Shanoj, K.C.; Shenoy, V.; Damodaran, D.; Menon, A.R.; Alias, B.; SanjoSaijan; Devakumar, D.; Babu, A.S. Antibody responses after documented COVID-19 disease in patients with autoimmune rheumatic disease. Clin. Rheumatol. 2021, 40, 4665–4670. [Google Scholar] [CrossRef] [PubMed]
- Deakin, C.T.; Cornish, G.H.; Ng, K.W.; Faulkner, N.; Bolland, W.; Hope, J.; Rosa, A.; Harvey, R.; Hussain, S.; Earl, C.; et al. Favorable antibody responses to human coronaviruses in children and adolescents with autoimmune rheumatic diseases. Med 2021, 2, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Tascilar, K.; Kleyer, A.; Fagni, F.; Krönke, G.; Meder, C.; Dietrich, P.; Orlemann, T.; Kliem, T.; Mößner, J.; et al. Impact of Cytokine Inhibitor Therapy on the Prevalence, Seroconversion Rate, and Longevity of the Humoral Immune Response against SARS-CoV-2 in an Unvaccinated Cohort. Arthritis Rheumatol. 2022, 74, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, G.; Minoia, F.; Carbogno, S.; Costi, S.; Romano, M.; Cimaz, R. Pediatric Rheumatology Group of the Milan Area. Absence of Severe Complications from SARS-CoV-2 Infection in Children with Rheumatic Diseases Treated with Biologic Drugs. J. Rheumatol. 2021, 48, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Haslak, F.; Yildiz, M.; Adrovic, A.; Sahin, S.; Koker, O.; Aliyeva, A.; Barut, K.; Kasapcopur, O. Management of childhood-onset autoinflammatory diseases during the COVID-19 pandemic. Rheumatol. Int. 2020, 40, 1423–1431. [Google Scholar] [CrossRef]
- Santos, C.S.; Férnandez, X.C.; Morales, C.M.; Álvarez, E.D.; Castro, C.Á.; Robles, A.L.; Sandoval, T.P. Biological agents for rheumatic diseases in the outbreak of COVID-19: Friend or foe? RMD Open 2021, 7, e001439. [Google Scholar] [CrossRef] [PubMed]
- Ceribelli, A.; Motta, F.; De Santis, M.; Ansari, A.A.; Ridgway, W.M.; Gershwin, M.E.; Selmi, C. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J. Autoimmun. 2020, 109, 102442. [Google Scholar] [CrossRef] [PubMed]
- Esatoglu, S.N.; Tascilar, K.; Babaoğlu, H.; Bes, C.; Yurttas, B.; Akar, S.; Pehlivan, O.; Akleylek, C.; Tecer, D.; Seyahi, E.; et al. Turkish Society for Rheumatology COVID-19 Registry Investigators. COVID-19 among Patients with Inflammatory Rheumatic Diseases. Front. Immunol. 2021, 12, 651715. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, A.; Carrara, G.; Landolfi, G.; Rozza, D.; Chighizola, C.B.; Alunno, A.; Andreoli, L.; Caporali, R.; Gerli, R.; Sebastiani, G.D.; et al. CONTROL-19 study by the Italian Society for Rheumatology. Increased COVID-19 mortality in patients with rheumatic diseases: Results from the CONTROL-19 study by the Italian Society for Rheumatology. Clin. Exp. Rheumatol. 2022, 40, 2038–2043. [Google Scholar]
- Grainger, R.; Kim, A.H.J.; Conway, R.; Yazdany, J.; Robinson, P.C. COVID-19 in people with rheumatic diseases: Risks, outcomes, treatment considerations. Nat. Rev. Rheumatol. 2022, 18, 191–204. [Google Scholar] [CrossRef]
- Liguoro, I.; Pilotto, C.; Bonanni, M.; Ferrari, M.E.; Pusiol, A.; Nocerino, A.; Vidal, E.; Cogo, P. SARS-CoV-2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020, 179, 1029–1046, Erratum in: Eur. J. Pediatr. 2021, 180, 2343. [Google Scholar] [CrossRef] [PubMed]
| n = 55 | |
|---|---|
| Male/Female | 14/41 |
| Age on examination (years) | 10.31 ± 4.16 |
| Positive IgA antibodies | 33 |
| Positive IgG antibodies | 40 |
| Positive IgG NCP antibodies | 23 |
| Positive IgM antibodies | 6 |
| Positive IGRA (>200 mlU/mL) | 41 |
| History of confirmed SARS-CoV-2 infection | 8 |
| Received SARS-CoV-2 vaccination | 8 * |
| COVID-19-like symptoms in the history | 32 |
| Treatment protocol: | |
| Biological agents: | 22 |
| adalimumab | 13 |
| tocilizumab | 6 |
| etanercept | 2 |
| baricitinib | 1 |
| Methotrexate | 32 |
| Sulfasalazine | 8 |
| Hydroxychloroquine | 7 |
| Cyclosporine | 3 |
| Azathioprine | 1 |
| Glucocorticoids | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapten, K.; Orczyk, K.; Smolewska, E. Application of Interferon-γ Release Assay in the Assessment of T-Cell Immunity to SARS-CoV-2 Antigens in the Cohort of Pediatric Patients with Juvenile Idiopathic Arthritis. Children 2024, 11, 736. https://doi.org/10.3390/children11060736
Kapten K, Orczyk K, Smolewska E. Application of Interferon-γ Release Assay in the Assessment of T-Cell Immunity to SARS-CoV-2 Antigens in the Cohort of Pediatric Patients with Juvenile Idiopathic Arthritis. Children. 2024; 11(6):736. https://doi.org/10.3390/children11060736
Chicago/Turabian StyleKapten, Katarzyna, Krzysztof Orczyk, and Elzbieta Smolewska. 2024. "Application of Interferon-γ Release Assay in the Assessment of T-Cell Immunity to SARS-CoV-2 Antigens in the Cohort of Pediatric Patients with Juvenile Idiopathic Arthritis" Children 11, no. 6: 736. https://doi.org/10.3390/children11060736






