Contribution of Treatment with Ear Popper for Hearing in Children with Middle Ear Effusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instrument
2.3. Procedure
- Part 1 was the six-week follow-up.
- Part 2 was tympanometry before and immediately after using the EarPopper.
- Part 3 was the length of the effect and an observational note.
2.4. Statistical Analysis
3. Results
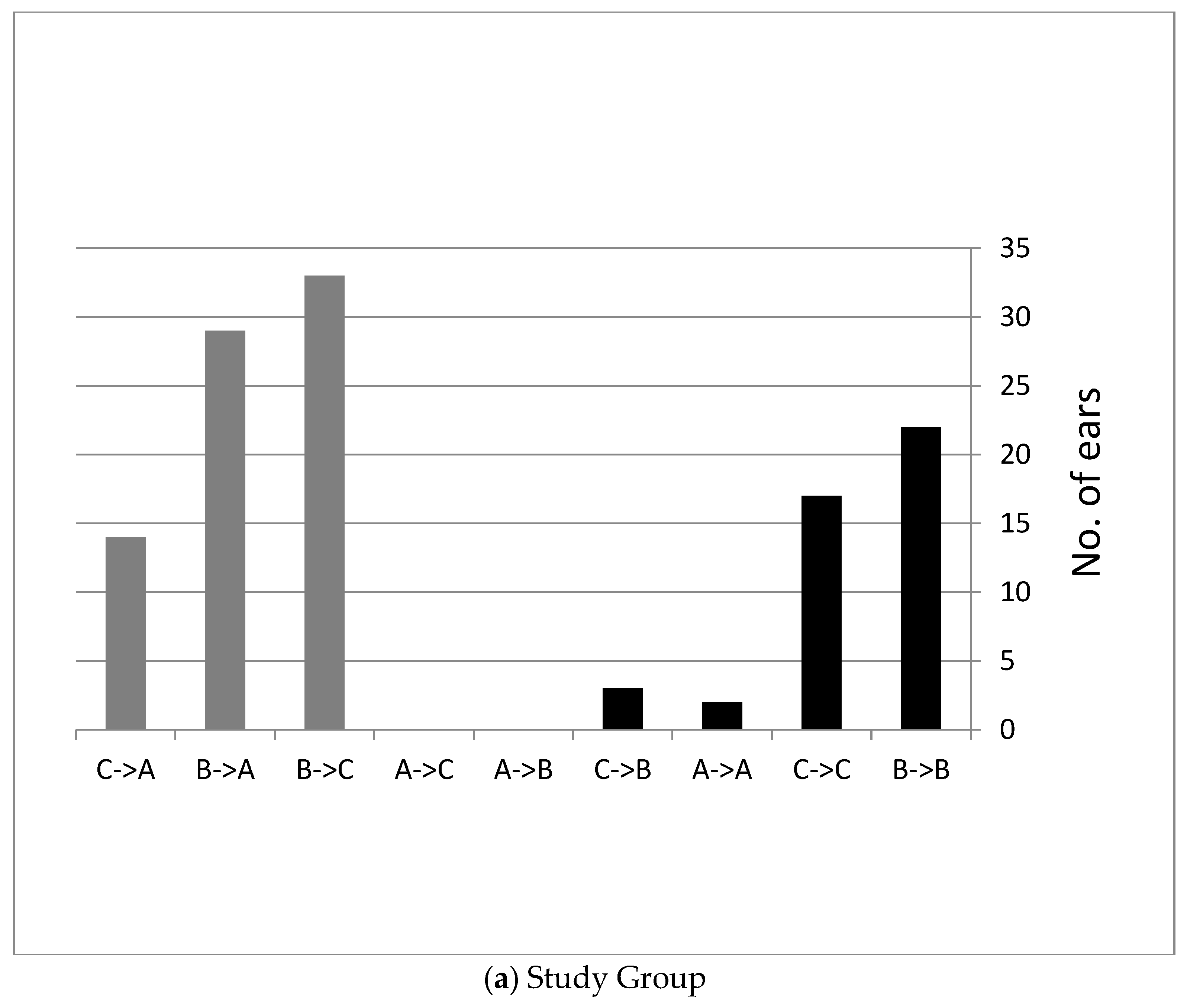
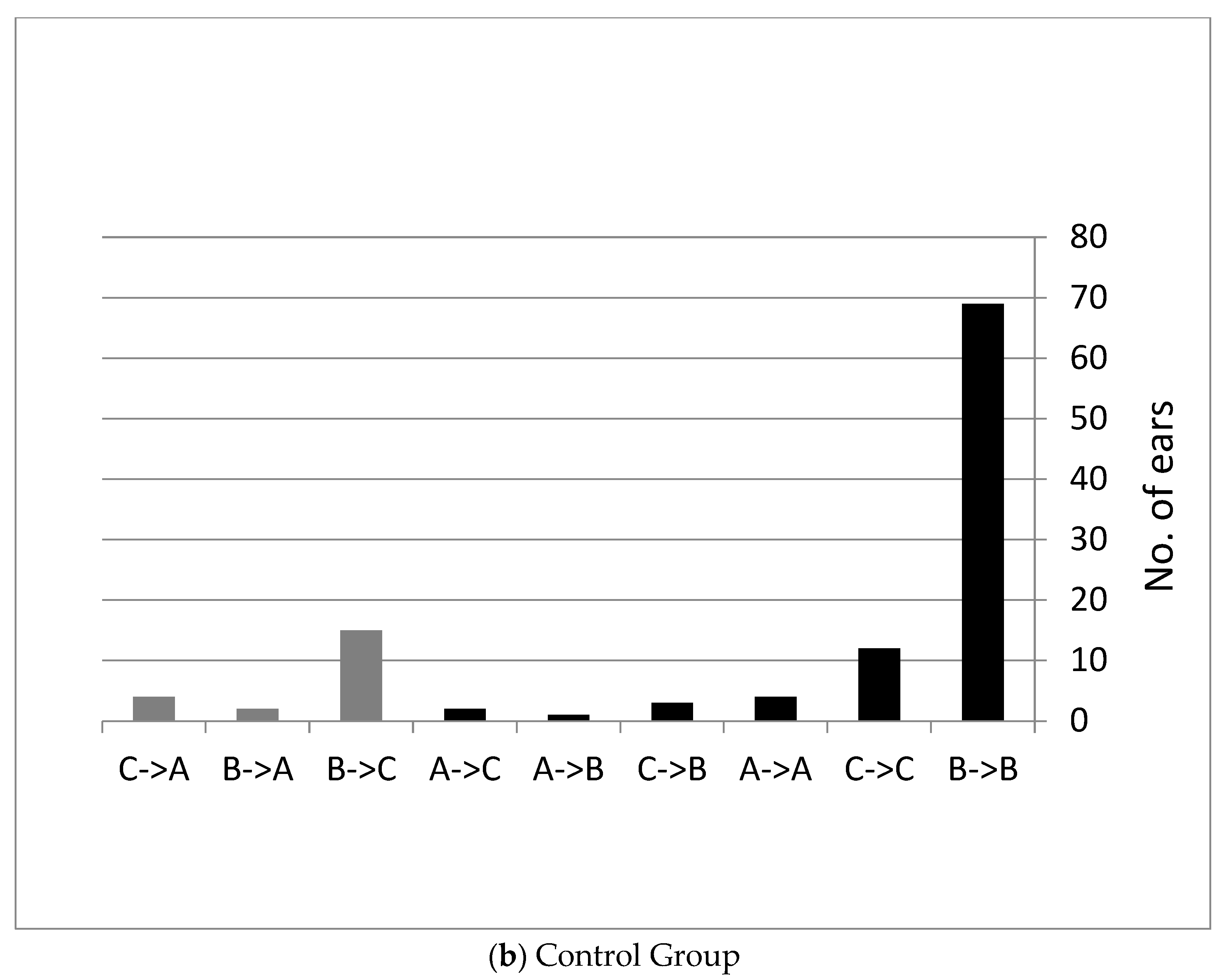
3.1. Follow Up Six Weeks after Using the Device Twice a Day
3.1.1. Difficulty in Using the Device over Time
3.1.2. Participants’ Age
Hearing Measurements
3.2. Tympanometry before and Immediately after Using EarPopper (Tympanometry during the First Use of the Device)
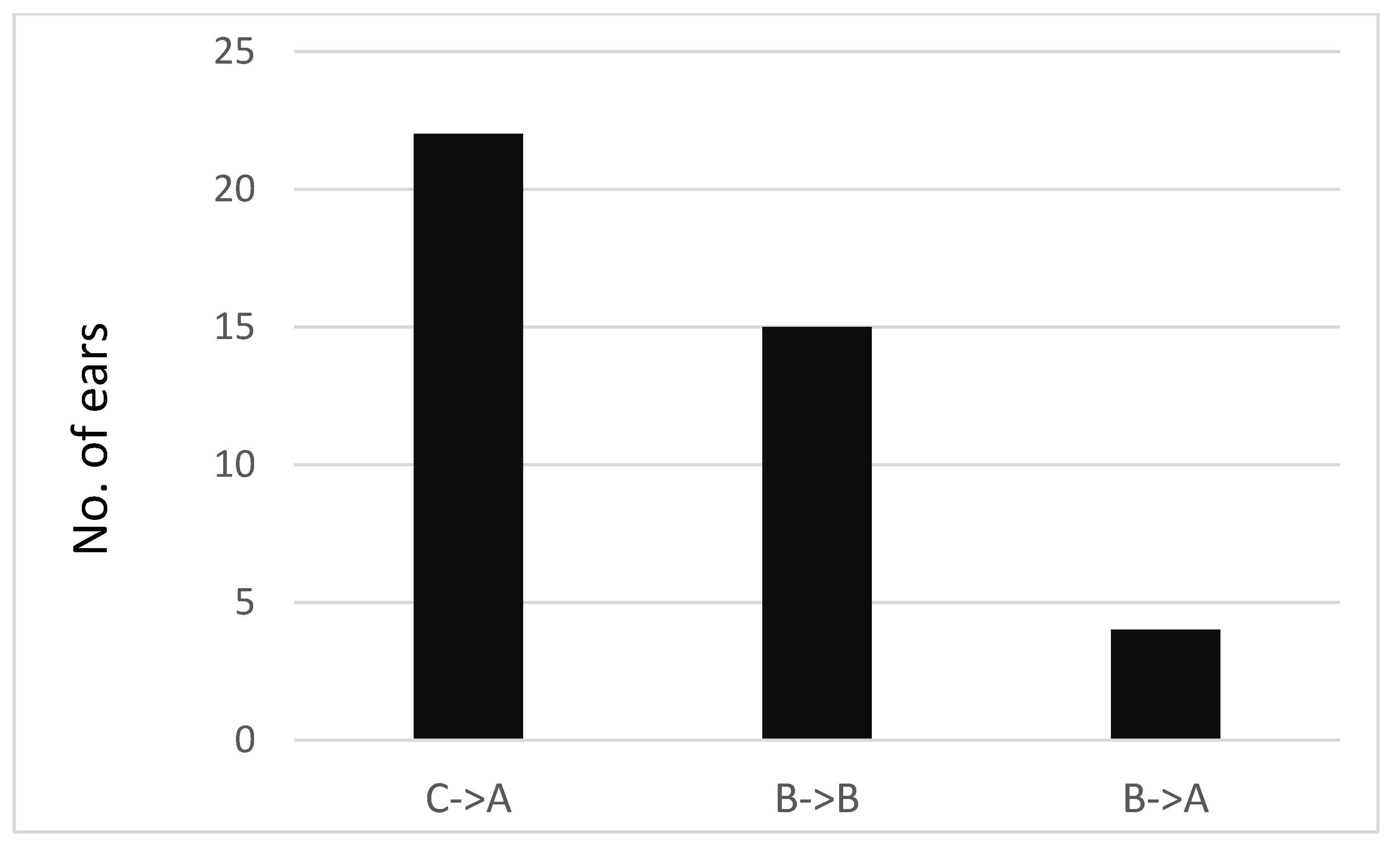
3.3. Length of Effect
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bluestone, C.D.; Doyle, W.J. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J. Allergy Clin. Immunol. 1988, 81, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Honjo, I.; Takahashi, H.; Sudo, M.; Ishijima, K.; Tanabe, M. Pathophysiological and therapeutic considerations of otitis media with effusion from viewpoint of middle ear ventilation. Int. J. Pediatr. Otorhinolaryngol. 1998, 43, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Paradise, J.L.; Dollaghan, C.A.; Campbell, T.F. Otitis media and tympanostomy tube insertion during the first three years of life: Developmental outcomes at the age of four years. Pediatrics 2003, 112, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.K.; Hutchinson, K.M.; Moravec, J. The use of tympanometry and pneumatic otoscopy for predicting middle ear disease. Am. J. Audiol. 2005, 14, 3–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arick, D.S.; Silman, S. Nonsurgical home treatment of middle ear effusion and associated hearing loss in children. Part I Clin. Trial Ear Nose Throat J. 2005, 84, 567–568, 570–574. [Google Scholar] [CrossRef][Green Version]
- Paden, E.P.; Matthies, M.L.; Novak, M.A. Recovery from OME-related phonologic delay following tube placement. J. Speech Hear. Disord. 1989, 54, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.R.; Kharel, S.; Giri, S.; Ghimire, A.; Prabhu, P. Impact of Otitis Media with Effusion in Early Age on Auditory Processing Abilities in Children: A Systematic Review and Meta-Analysis. Ear Nose Throat J. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silman, S.; Arick, D. Efficacy of a modified politzer apparatus in management of eustachian tube dysfunction in adults. J. Am. Acad. Audiol. 1999, 10, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.P.; Reuchlin, A.G.; Kummer, E.E. Laser myringotomy versus ventilation tubes in children with otitis media with effusion: A randomized trial. Laryngoscope 2004, 114, 844–849. [Google Scholar] [CrossRef]
- Neff, J. American Academy of Pediatrics; American Academy of Family Physicians. AAP, AAFP release guideline on diagnosis and management of acute otitis media. Am. Fam. Physician 2004, 69, 2713–2715. [Google Scholar]
- Liming, B.J.; Carter, J.; Cheng, A. International Pediatric Otolaryngology Group (IPOG) consensus recommendations: Hearing loss in the pediatric patient. Int. J. Pediatr. Otorhinolaryngol. 2016, 90, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, H.; Tuomilehto, H.; Qvarnberg, Y.; Nuutinen, J. A 14-year prospective follow-up study of children treated early in life with tympanostomy tubes: Part 1: Clinical outcomes. Arch. Otolaryngol. Head. Neck Surg. 2005, 131, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Barfoed, C.; Rosborg, J. Secretory otitis media. Long-term observations after treatment with grommets. Arch. Otolaryngol. 1980, 106, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Iwaki, E.; Kohno, Y.; Ohtsubo, T.; Noda, I.; Mori, S.; Yamamoto, T.; Shibamori, Y.; Saito, H. Prevention of persistent ear drum perforation after long-term ventilation tube treatment for otitis media with effusion in children. Int. J. Pediatr. Otorhinolaryngol. 1996, 38, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, R.N.E.; Henningsson, R.N. Visiting the Operating Theatre Before Surgery Did Not Reduce the Anxiety in Children and Their Attendant Parent. J. Pediatr. Nurs. 2018, 38, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Blanshard, J.D.; Maw, A.R.; Bawden, R. Conservative treatment of otitis media with effusion by autoinflation of the middle ear. Clin. Otolaryngol. Allied Sci. 1993, 18, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Tham, T.; Rahman, L.; Costantino, P. Efficacy of a non-invasive middle ear aeration device in children with recurrent otitis media: A randomized controlled trial protocol. Contemp. Clin. Trials Commun. 2018, 12, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Hidir, Y.; Ulus, S.; Karahatay, S.; Satar, B. A comparative study on efficiency of middle ear pressure equalization techniques in healthy volunteers. Auris Nasus Larynx. 2011, 38, 450–455. [Google Scholar] [CrossRef]
- Goto, F.; Yabe, H.; Goto, K. “EarPopper®”, new device to treat otitis media with effusion. Oto-Rhino-Laryngol. Tokyo 2007, 50, 330–332. [Google Scholar]
- Banigo, A.; Hunt, A.; Rourke, T.; Whiteside, O.; Aldren, C. Does the EarPopper(®) device improve hearing outcomes in children with persistent otitis media with effusion? A randomised single-blinded controlled trial. Clin. Otolaryngol. 2016, 41, 59–65. [Google Scholar] [CrossRef]
- Ovesen, T.; Børglum, J.D. New aspects of secretory otitis media, eustachian tube function and middle ear gas. Ear Nose Throat J. 1998, 77, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Sadé, J.; Luntz, M.; Levy, D. Middle ear gas composition and middle ear aeration. Ann. Otol. Rhinol. Laryngol. 1995, 104, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Williamson, I.; Vennik, J.; Harnden, A.; Voysey, M.; Perera, R.; Kelly, S.; Yao, G.; Raftery, J.; Mant, D.; Little, P. Effect of nasal balloon autoinflation in children with otitis media with effusion in primary care: An open randomized controlled trial. CMAJ 2015, 187, 961–969. [Google Scholar] [CrossRef] [PubMed]
| Study Group (N = 57) | Right Ear | Frequency | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz |
| MTB * (SD) | 32.9 (11.4) | 31.4 (12.2) | 27.0 (11.9) | 18.5 (11.8) | 24.2 (13.3) | ||
| MTA ** (SD) | 19.2 (10.8) | 17.4 (9.0) | 13.5 (8.0) | 8.4 (7.6) | 11.5 (8.2) | ||
| Left ear | Frequency | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | |
| MTB * (SD) | 34.0 (11.9) | 31.8 (11.2) | 27.1 (11.4) | 18.5 (12.0) | 23.6 (13.8) | ||
| MTA ** (SD) | 20.8 (8.6) | 18.6 (7.8) | 14.7 (9.0) | 9.4 (7.7) | 12.4 (8.9) | ||
| Control Group (N = 52) | Right ear | Frequency | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz |
| MTB * (SD) | 30.0 (9.1) | 28.7 (9.7) | 26.1 (12.0) | 18.9 (10.6) | 23.6 (12.3) | ||
| MTA ** (SD) | 30.2 (12.2) | 29.6 (13.6) | 25.8 (13.7) | 18.0 (12.0) | 23.4 (14.1) | ||
| Left ear | Frequency | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | |
| MTB * (SD) | 31.5 (10.0) | 30.0 (11.6) | 27.2 (11.9) | 19.0 (11.7) | 23.5 (11.8) | ||
| MTA ** (SD) | 32.3 (11.8) | 29.9 (10.4) | 26.1 (11.3) | 18.0 (11.7) | 24.3 (12.6) |
| Study Group (N = 60) | Right Ear | SRT | |
| MTB * (SD) | 22.5 | ||
| (11.3) | |||
| MTA ** (SD) | 9.7 | ||
| (6.1) | |||
| Left ear | SRT | ||
| MTB * (SD) | 22.2 | ||
| (9.5) | |||
| MTA ** (SD) | 10.7 | ||
| (7.0) | |||
| Control Group (N = 56) | Right ear | SRT | |
| MTB * (SD) | 22.7 | ||
| (11.2) | |||
| MTA ** (SD) | 21.9 | ||
| (11.5) | |||
| Left ear | SRT | ||
| MTB * (SD) | 22.6 | ||
| (10.9) | |||
| MTA ** (SD) | 21.6 (10.1) |
| Right Ear (daPa Pressure) | Left Ear (daPa Pressure) | |
|---|---|---|
| Before use | −400 | −124 |
| Immediately after use | +44 | +124 |
| 10 min after use | −116 | +44 |
| 1.5 h after use | −268 | −56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priner, R.; Ilan, O. Contribution of Treatment with Ear Popper for Hearing in Children with Middle Ear Effusion. Children 2024, 11, 744. https://doi.org/10.3390/children11060744
Priner R, Ilan O. Contribution of Treatment with Ear Popper for Hearing in Children with Middle Ear Effusion. Children. 2024; 11(6):744. https://doi.org/10.3390/children11060744
Chicago/Turabian StylePriner, Ronit, and Ophir Ilan. 2024. "Contribution of Treatment with Ear Popper for Hearing in Children with Middle Ear Effusion" Children 11, no. 6: 744. https://doi.org/10.3390/children11060744
APA StylePriner, R., & Ilan, O. (2024). Contribution of Treatment with Ear Popper for Hearing in Children with Middle Ear Effusion. Children, 11(6), 744. https://doi.org/10.3390/children11060744




