Macrodactyly
Abstract
1. Introduction
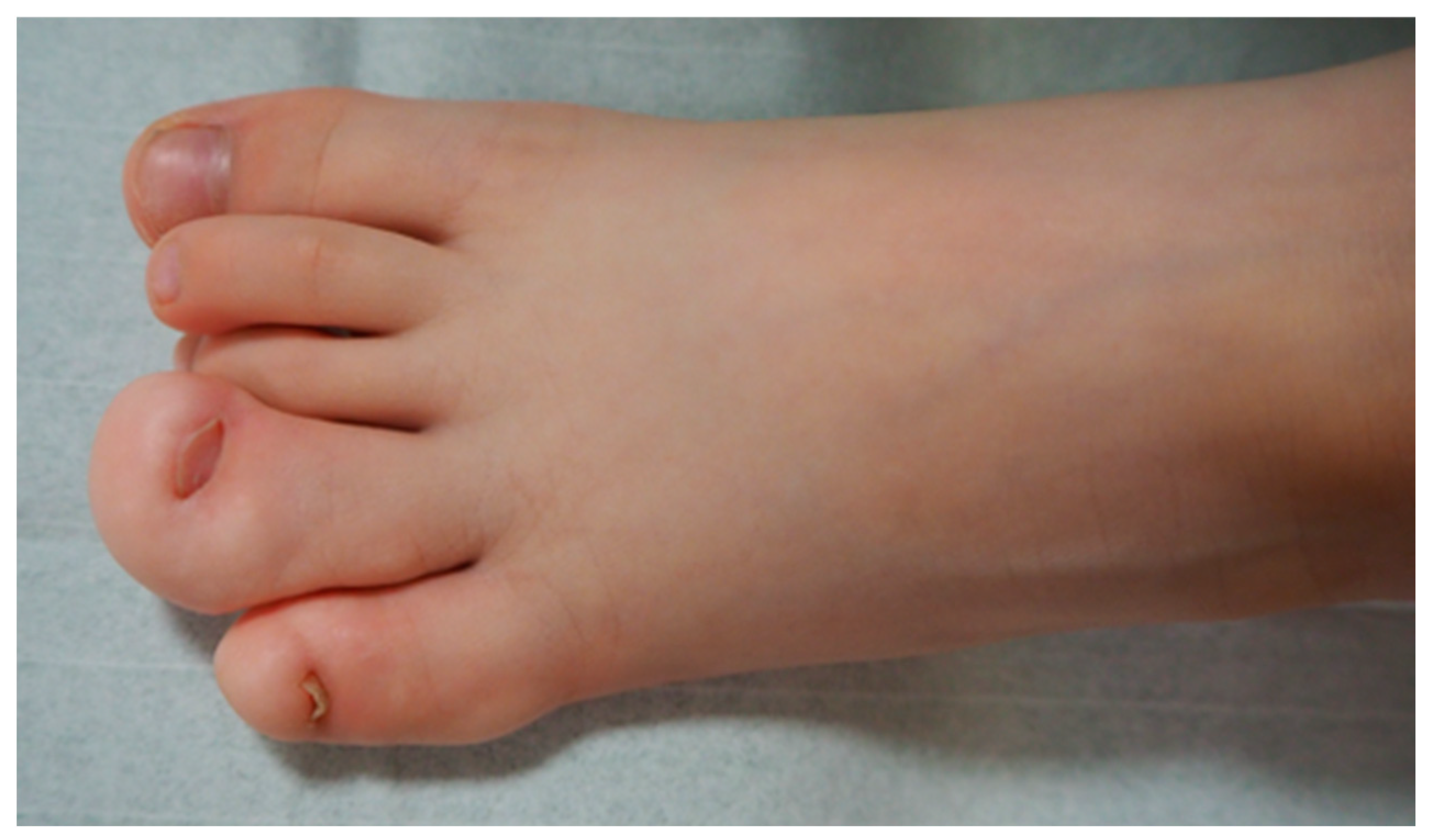
2. Clinical Picture and Classification
3. Epidemiology
4. Pathophysiology and Genetics
5. Diagnostics
6. Differential Diagnosis
- Congenital or early childhood onset
- Two or more spectrum features
- -
- Overgrowth: adipose, muscle, nerve, skeletal
- -
- Epidermal nevus
- -
- Vascular malformations: capillary, venous, arteriovenous, lymphatic
OR any one isolated feature- -
- Large, isolated lymphatic malformation
- -
- Truncal adipose overgrowth
- -
- Benign lichenoid keratoses
- -
- Seborrheic keratoses
- -
- HME (bilateral)/DMEG/focal cortical dysplasia type ll
- -
- Isolated macrodactyly or overgrown, splayed feet/hands, overgrown limbs
- -
- Epidermal nevus
- Sporadic and mosaic overgrowth
- Presence of a somatic PIK3CA mutation
7. Overgrowth Syndromes Associated with Neoplasms
8. Interdisciplinary Approach
9. Decision-Making in Macrodactyly
10. Surgical Treatment
11. Nonsurgical Treatment
12. Long-Term Outcomes of Macrodactyly
13. Future Studies
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, X.F.; Gasteratos, K.; Spyropoulou, G.A.; Yin, F.; Rui, Y.J. Congenital difference of the hand and foot: Pediatric macrodactyly. JPRAS 2022, 75, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Stor, M.L.; Lokhorst, M.M.; Horbach, S.E.; van der Horst, C.M. The long-term progression of macrodactyly. JPRAS Open 2022, 31, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tian, G.; Ji, Y.; Higgins, J.P.; Lee, W.A. Clinical characteristics of 90 macrodactyly cases. J. Hand Surg. Am. 2020, 45, 982.e1–982.e5. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, J.; Lester, R. Macrodactyly. In Congenital Anomalies of the Upper Extremity, 1st ed.; Laub, D.R., Jr., Ed.; Springer: Boston, MA, USA, 2014; Volume 1, pp. 275–293. [Google Scholar]
- Ezaki, M.; Beckwith, T.; Oishi, S.N. Macrodactyly: Decision-making and surgery timing. J. Hand Surg. 2019, 44, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, C.A.; Ezaki, M.; Wall, L.B.; Lam, W.L.; Oberg, K.C. The Oberg-Manske-Tonkin (OMT) classification of congenital upper extremities: Update for 2020. J. Hand Surg. Am. 2020, 45, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Vuillermin, C.; Canizares, M.F.; Bauer, A.S.; Miller, P.E.; Goldfarb, C.A.; Bae, D.S.; Waters, P.M.; Wall, L.B.; Roberts, S. Congenital upper limb differences registry (CoULD): Registry inclusion effect. J. Hand Surg. Am. 2021, 46, 515.e1–515.e11. [Google Scholar] [CrossRef] [PubMed]
- Dell, P.C. Macrodactyly. Hand Clin. 1985, 1, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Paria, N.; Burns, D.K.; Israel, B.A.; Cornelia, R.; Wise, C.A.; Ezaki, M. Somatic gain-of-function mutations in PIK3CA in patients with macrodactyly. Hum. Mol. Genet. 2013, 22, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Lindhurst, M.J.; Parker, V.E.; Payne, F.; Sapp, J.C.; Rudge, S.; Harris, J.; Witkowski, A.M.; Zhang, Q.; Groeneveld, M.P.; Scott, C.E.; et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat. Genet. 2012, 44, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, M. Insights into the pathogenesis of macrodactyly. J. Hand Surg. 2019, 44, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fuster, A.I.; Serra, P.C.; Riera-Mestre, A. PIK3CA-related overgrowth spectrum (PROS): New insight in known diseases. Med. Clin. 2021, 157, 483–488. [Google Scholar]
- Keppler-Noreuil, K.M.; Rios, J.J.; Parker, V.E.; Semple, R.K.; Lindhurst, M.J.; Sapp, J.C.; Alomari, A.; Ezaki, M.; Dobyns, W.; Biesecker, L.G. PiK3CA-related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am. J. Med. Genet. A 2015, 167, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tian, W.; Tian, G.; Sumner, K.; Hutchinson, D.T.; Ji, Y. An investigation of PIK3CA mutations in isolated macrodactyly. J. Hand Surg. 2018, 43, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.R.; Vanhaesebroeck, B.; Semple, R.K. Cancer-associated PIK3CA mutations in overgrowth disorders. Trends Mol. Med. 2018, 24, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Mirzaa, G.; Graham, J.M., Jr.; Keppler-Noreuil, K. PIK3CA-Related Overgrowth Spectrum. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 15 August 2013. [Google Scholar]
- Morin, G.; Degrugillier-Chopinet, C.; Vincent, M.; Fraissenon, A.; Aubert, H.; Chapelle, C.; Hoguin, C.; Dubos, F.; Catteau, B.; Petit, F.; et al. Treatment of two infants with PIK3CA-related overgrowth spectrum by alpelisib. J. Exp. Med. 2022, 219, e20212148. [Google Scholar] [CrossRef] [PubMed]
- Kuentz, P.; St-Onge, J.; Duffourd, Y.; Courcet, J.B.; Carmignac, V.; Jouan, T.; Sorlin, A.; Abasq-Thomas, C.; Albuisson, J.; Amiel, J.; et al. Molecular diagnosis of PIK3CA-related overgrowth spectrum (PROS) in 162 patients and recommendations for genetic testing. Genet. Med. 2017, 19, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, S.M.; Adam, L.; Atef, H.; Häberli, D.; Bramer, W.M.; Minder, B.; Döring, Y.; Laine, J.E.; Muka, T.; Rössler, J.; et al. A systematic review of the safety and efficacy of currently used treatment modalities in the treatment of patients with PIK3CA-related overgrowth spectrum. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, A.; Yaǧmur, H.; Kural, B.S. Prenatal diagnosis of isolated macrodactyly. Ultrasound Obstet. Gynecol. 2009, 33, 360–362. [Google Scholar] [CrossRef] [PubMed]
- AbuMoussa, S.; Roshan, M.P.; Souza, F.F.; Daley, D.; Rosenberg, A.; Pretell, J.; Fullerton, N.; Subhawong, T. Soft tissue masses of the hand: A review of clinical presentation and imaging features. Curr. Oncol. 2023, 30, 2032–2048. [Google Scholar] [CrossRef] [PubMed]
- El Abiad, J.M.; Robbins, S.M.; Cohen, B.; Levin, A.S.; Valle, D.L.; Morris, C.D.; de Macena Sobreira, N.L. Natural history of Ollier disease and Maffucci syndrome: Patient survey and review of clinical literature. Am. J. Med. Genet. A 2020, 182, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- PROSSpectrum. Available online: https://www.prosspectrum.com/ (accessed on 15 December 2023).
- Jongmans, M.C.; Loeffen, J.L.; Waanders, E.; Hoogerbrugge, P.M.; Ligtenberg, M.J.; Kuiper, R.P.; Hoogerbrugge, N. Recognition of genetic predisposition in pediatric cancer patients: An easy-to-use selection tool. Eur. J. Med. Genet. 2016, 59, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Connolly, G.K.; Harris, R.D.; Shumate, C.; Rednam, S.P.; Canfield, M.A.; Plon, S.E.; Nguyen, J.; Schraw, J.M.; Lupo, P.J. Pediatric cancer incidence among individuals with overgrowth syndromes and overgrowth features: A population-based assessment in seven million children. Cancer 2024, 130, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Weksberg, R.; Shuman, C.; Beckwith, J. Beckwith–Wiedemann syndrome. Eur. J. Hum. Genet. 2010, 18, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Brioude, F.; Toutain, A.; Giabicani, E.; Cottereau, E.; Cormier-Daire, V.; Netchine, I. Overgrowth syndromes—Clinical and molecular aspects and tumour risk. Nat. Rev. Endocrinol. 2019, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Gracia Bouthelier, R.; Lapunzina, P. Follow-up and risk of tumors in overgrowth syndromes. J. Pediatr. Endocrinol. Metab. 2005, 18 (Suppl. 1), 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Laguna, L.; Davis, K.; Finger, M.; Aubel, D.; Vlamis, R.; Johnson, C. Mapping the PIK3CA-related overgrowth spectrum (PROS) patient and caregiver journey using a patient-centered approach. Orphanet J. Rare Dis. 2022, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Kobraei, E.M.; Dusch, M.N.; Meisel, E.M.; Stevanovic, M. A novel method of treatment of macrodactyly with digital nerve resection and nerve allograft. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2483. [Google Scholar] [CrossRef] [PubMed]
- Kaempf de Oliveira, R.; Farina Brunelli, J.P.; Soldado, F.; Vergara Amador, E. Quadrant Flap for Fingertip Reconstruction in Macrodactyly: Technique and Case Report. J. Hand Surg. Asian Pac. Vol. 2019, 27, 560–564. [Google Scholar] [CrossRef]
- Yushan, M.; Alike, Y.; Keremu, A.; Abulaiti, A.; Ren, P.; Yusufu, A. Precise resection of macrodactyly under assistance of three-dimensional reconstruction technology: A case report. J. Foot Ankle Surg. 2020, 59, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Cavadas, P.C.; Thione, A. Treatment of hand macrodactyly with resection and toe transfers. J. Hand Surg. Am. 2018, 43, 388.e1–388.e6. [Google Scholar] [CrossRef] [PubMed]
- Golyana, S.I.; Tikhonenko, T.I.; Govorov, A.V.; Natal’ya, V.Z. Microsurgical toe-to-hand transfer in children with macrodactyly of the hand. Pediatr. Traumatol. Orthop. Reconstr. Surg. 2018, 6, 32–39. [Google Scholar] [CrossRef][Green Version]
- Kozin, S.H. Pollicization: The concept, technical details, and outcome. Clin. Orthop. Surg. 2012, 4, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Enokido, Y.; Yamada, K.; Inaba, M.; Kuwata, K.; Hanada, N.; Morishita, T.; Mizuno, S.; Wakamatsu, N. The effect of rapamycin, NVP-BEZ235, aspirin, and metformin on PI3K/AKT/mTOR signaling pathway of PIK3CA-related overgrowth spectrum (PROS). Oncotarget 2017, 8, 45470–45483. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.; Ma, Y.; Baldrighi, C.; Oestreich, K.; Jester, A. Efficacy of mTOR inhibitors (sirolimus) in isolated limb overgrowth: A systematic review. J. Hand Surg. 2022, 47, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Sandbank, S.; Molho-Pessach, V.; Farkas, A.; Barzilai, A.; Greenberger, S. Oral and Topical Sirolimus for Vascular Anomalies: A Multicentre Study and Review. Acta Derm.-Venereol. 2019, 99, 990–996. [Google Scholar] [CrossRef] [PubMed]
- VIJOICE® (Alpelisib) Label. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed on 26 March 2024).
- Study of Miransertib. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03094832 (accessed on 24 March 2024).
- Tolerton, S.K.; Tonkin, M.A. Keloid formation after syndactyly release in patients with associated macrodactyly: Management with methotrexate therapy. J. Hand Surg. 2011, 36, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Huang, Y.; Sun, L.; Guo, Y.; Zhao, S.; Lin, M.; Dong, X.; Zhong, W.; Yin, Y.; Chen, Z.; et al. Phenotypic and genetic spectrum of isolated macrodactyly: Somatic mosaicism of PIK3CA and AKT1 oncogenic variants. Orphanet J. Rare Dis. 2020, 15, 288. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, J.; Agaimy, A.; Fried, F.; Stoehr, R.; Janka, R.; Arkudas, A.; Horch, R.E. PIK3CA mutation testing as a valuable molecular surrogate for lipomatosis of the median nerve: Clinicopathological and molecular analysis of six cases. Virchows Arch. 2023, 483, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jiang, Y.; Cui, H.; Fang, X.; Han, G.; Dai, X.; Zhou, S.; Mao, H.; Wang, B. Activating PIK3CA mutation promotes adipogenesis of adipose-derived stem cells in macrodactyly via up-regulation of E2F1. Cell Death Dis. 2020, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Han, G.; Sun, B.; Fang, X.; Dai, X.; Zhou, S.; Mao, H.; Wang, B. Activating PIK3CA mutation promotes osteogenesis of bone marrow mesenchymal stem cells in macrodactyly. Cell Death Dis. 2020, 11, 505. [Google Scholar] [CrossRef] [PubMed]





| Characteristic | Classification | |
|---|---|---|
| Growth | Static | Progressive |
| Associations | Isolated | Associated syndrome or anomalies |
| Structure | Lipomatous nerve territory orientated | Hyperostotic vascular malformation |
| Soft Tissue Tumors of the Hand |
|---|
| Ganglion cyst |
| Tenosynovial Giant Cell Tumor |
| Lipoma |
| Schwannoma |
| Glomus Tumor |
| Vascular Tumors and Malformations |
| Superficial Fibromatoses |
| Synovial Chondromatosis |
| Soft Tissue Sarcomas, e.g., Fibrosarcoma, Rhabdomyosarcoma |
| Most Common PIK3CA-Related Overgrowth Spectrum (PROS) Conditions |
|---|
| KTS (Klippel–Trenaunay Syndrome) |
| CLOVES syndrome (Congenital Lipomatous Overgrowth, Vascular malformations, Epidermal nevi, Scoliosis/skeletal and spinal) |
| ILM (Isolated Lymphatic Malformation) |
| MCAP or M-CM (Megalencephaly-Capillary Malformation) |
| HME (HemiMegalEncephaly)/DMEG (Dysplastic MEGalencephaly)/Focal cortical dysplasia type II |
| HHML (HemiHyperplasia-Multiple Lipomatosis) |
| FIL (Facial Infiltrating Lipomatosis) |
| FAVA (FibroAdipose Vascular Anomaly) |
| Macrodactyly |
| Muscular HH (HemiHyperplasia) |
| FAO (FibroAdipose hyperplasia or Overgrowth) |
| CLAPO syndrome (Capillary malformation of the lower lip, Lymphatic malformation of the face and neck, Asymmetry of the face and limbs, and Partial or generalized Overgrowth) |
| Epidermal nevus, benign lichenoid keratosis, or seborrheic keratosis |
| Generalized overgrowth |
| Beckwith–Wiedemann syndrome |
| Sotos syndrome |
| Simpson–Golabi–Behmel syndrome |
| Perlman syndrome |
| Weaver syndrome |
| Segmental overgrowth |
| PTEN hamartoma tumor syndrome |
| Bannayan–Riley–Ruvalcaba Syndrome |
| Macrocephaly cutis marmorata telangiectatica |
| Costello syndrome |
| Neurofibromatosis type 1 |
| Gorlin syndrome |
| MEN 2B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giżewska-Kacprzak, K.; Śliwiński, M.; Nicieja, K.; Babiak-Choroszczak, L.; Walaszek, I. Macrodactyly. Children 2024, 11, 753. https://doi.org/10.3390/children11070753
Giżewska-Kacprzak K, Śliwiński M, Nicieja K, Babiak-Choroszczak L, Walaszek I. Macrodactyly. Children. 2024; 11(7):753. https://doi.org/10.3390/children11070753
Chicago/Turabian StyleGiżewska-Kacprzak, Kaja, Maximilian Śliwiński, Karol Nicieja, Lidia Babiak-Choroszczak, and Ireneusz Walaszek. 2024. "Macrodactyly" Children 11, no. 7: 753. https://doi.org/10.3390/children11070753
APA StyleGiżewska-Kacprzak, K., Śliwiński, M., Nicieja, K., Babiak-Choroszczak, L., & Walaszek, I. (2024). Macrodactyly. Children, 11(7), 753. https://doi.org/10.3390/children11070753






