A Systematic Review of Telehealth Utilization for Bowel Management Programs in Pediatric Colorectal Surgery
Abstract
1. Introduction
2. Telehealth Applications in Pediatric Surgery
2.1. Outcomes of Telehealth in Pediatric Surgery
2.2. Challenges Associated with Telehealth in Pediatric Surgery
3. Bowel Management: Understanding the Basics
4. Systematic Analysis of Telemedical Bowel Management Efficacy and Outcomes
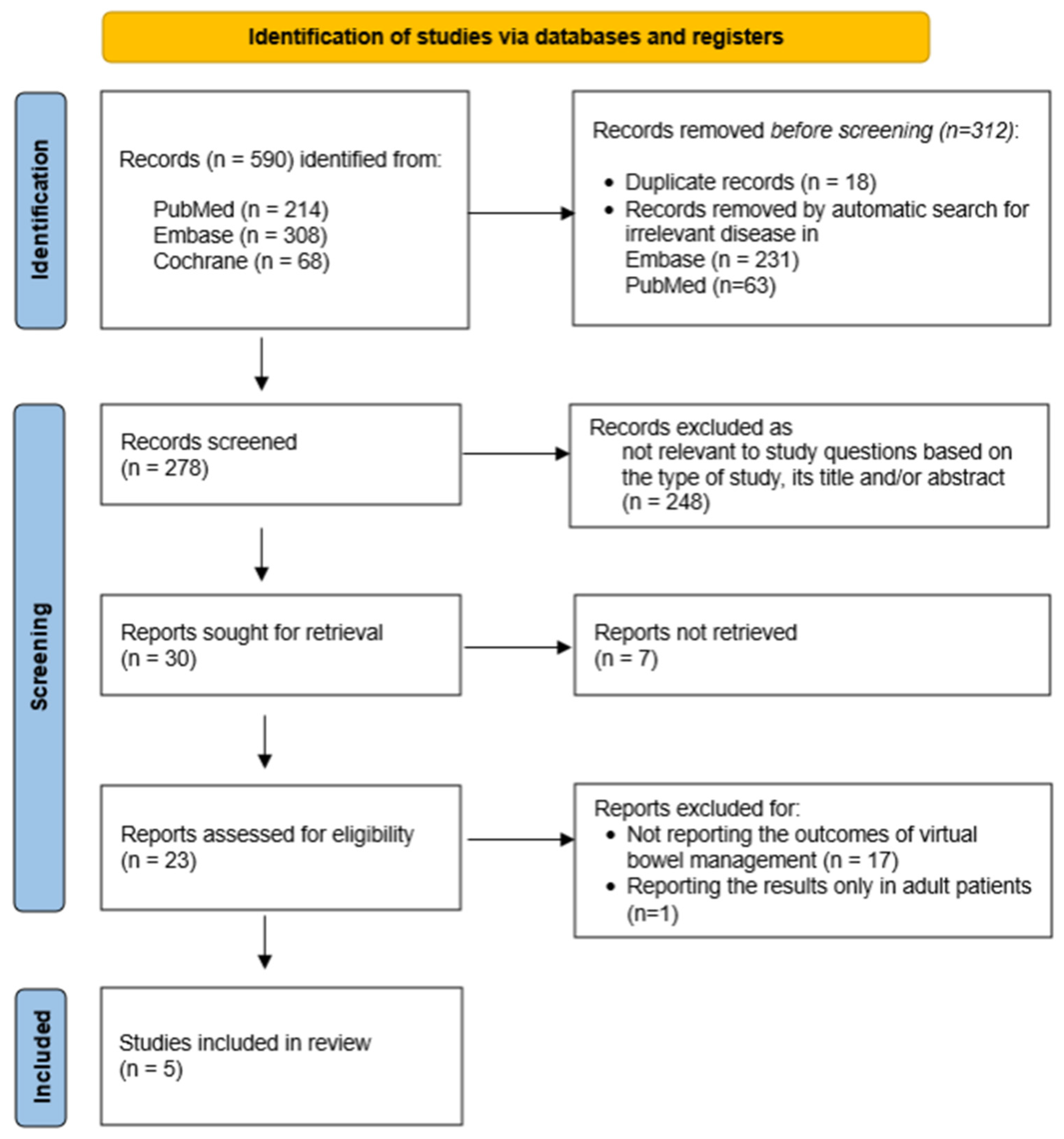
4.1. Study Search
4.2. Study Selection
- (1)
- Articles that exclusively addressed outcomes in adult populations, patients with colorectal cancer, or bowel management in children with inflammatory bowel disease (IBD).
- (2)
- Review articles, book chapters, guidelines, preprints, and trial studies.
- (3)
- Studies where virtual communication was used solely for survey data collection rather than routine clinical follow-ups.
- (4)
- Manuscripts not available in full text in English.
5. Results
5.1. Protocols for Telemedical Bowel Management
- (1)
- (2)
- (3)
- Absence of acute issues that may necessitate immediate admission to a healthcare facility [23].
5.2. Outcomes of Virtual Bowel Management
5.2.1. Bowel and Bladder Function
5.2.2. Satisfaction Rates and Resource Utilization
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, R.J.; Vilanova-Sanchez, A.; El-Gohary, Y.; Ahmad, H.; Halleran, D.R.; Reck-Burneo, C.A.; Rentea, R.; Sebastiao, Y.; Nash, O.; Booth, K. One-Year Impact of a Bowel Management Program in Treating Fecal Incontinence in Patients with Anorectal Malformations. J. Pediatr. Surg. 2021, 56, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, H.; Wu, Q.; Zheng, H.; Liu, G.; Wen, Z.; Lan, M.; Yu, J.; Zhu, D.; Liang, J.; et al. Bowel Management Program for Pediatric Postoperative Fecal Incontinence in China: A Surgeon’s Experience. Medicine 2017, 96, e7078. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, S.; Oyania, F.; Bingana, C.; Nuwagaba, I.; Obermeyer, M.; Odongo, C.; Kotagal, M.; Situma, M. Pilot bowel management program at Mbarara Hospital, Uganda. Pediatr. Surg. Int. 2023, 39, 292. [Google Scholar] [CrossRef]
- Russell, K.W.; Barnhart, D.C.; Zobell, S.; Scaife, E.R.; Rollins, M.D. Effectiveness of an Organized Bowel Management Program in the Management of Severe Chronic Constipation in Children. J. Pediatr. Surg. 2015, 50, 444–447. [Google Scholar] [CrossRef]
- Colares, J.H.; Purcaru, M.; Silva, G.P.; Frota, M.A.; Silva, C.A.; Melo-Filho, A.A. Impact of the Bowel Management Program on the Quality of Life in Children with Fecal Incontinence. Pediatr. Surg. Int. 2016, 32, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Vilanova-Sanchez, A.; Gasior, A.C.; Toocheck, N.; Weaver, L.; Wood, R.J.; Reck, C.A.; Wagner, A.; Hoover, E.; Gagnon, R.; Jaggers, J.; et al. Are Senna Based Laxatives Safe When Used as Long Term Treatment for Constipation in Children? J. Pediatr. Surg. 2018, 53, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Lee, H.-C.; Yeung, C.-Y.; Chan, W.-T.; Jiang, C.-B.; Sheu, J.-C.; Wang, N.-L.; Lin, J.-R. Constipation Is a Major Complication after Posterior Sagittal Anorectoplasty for Anorectal Malformations in Children. Pediatr. Neonatol. 2012, 53, 252–256. [Google Scholar] [CrossRef]
- Divya, G.; Kundal, V.K.; Debnath, P.R.; Addagatla, R.; Garbhapu, A.K.; Sen, A.; Saha, A.K.; Meena, A.K.; Shah, S.; Syal, S.; et al. Delayed Presentation of Anorectal Malformations in a Tertiary Care Hospital in India. Pediatr. Surg. Int. 2021, 37, 451–456. [Google Scholar] [CrossRef]
- Wiebe, M.E.; Shawyer, A.C. Impact of Distance on Postoperative Follow-up in Patients of Pediatric Surgery: A Retrospective Review. World J. Pediatr. Surg. 2020, 3, e000195. [Google Scholar] [CrossRef]
- Cotache-Condor, C.F.; Moody, K.; Concepcion, T.; Mohamed, M.; Dahir, S.; Adan Ismail, E.; Cook, J.; Will, J.; Rice, H.E.; Smith, E.R. Geospatial Analysis of Pediatric Surgical Need and Geographical Access to Care in Somaliland: A Cross-Sectional Study. BMJ Open 2021, 11, e042969. [Google Scholar] [CrossRef]
- Metzger, G.A.; Cooper, J.; Lutz, C.; Jatana, K.R.; Nishimura, L.; Deans, K.J.; Minneci, P.C.; Halaweish, I. The Value of Telemedicine for the Pediatric Surgery Patient in the Time of COVID-19 and Beyond. J. Pediatr. Surg. 2021, 56, 1305–1311. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Daboos, M.; Gouda, S.; Othman, A.; Abdelmaboud, M.; Hussein, M.E.; Akl, M. Telemedicine (Virtual Clinic) Effectively Delivers the Required Healthcare Service for Pediatric Ambulatory Surgical Patients during the Current Era of COVID-19 Pandemic: A Mixed Descriptive Study. J. Pediatr. Surg. 2022, 57, 630–636. [Google Scholar] [CrossRef]
- Knaus, M.E.; Ahmad, H.; Metzger, G.A.; Beyene, T.J.; Thomas, J.L.; Weaver, L.J.; Gasior, A.C.; Wood, R.J.; Halaweish, I. Outcomes of a Telemedicine Bowel Management Program during COVID-19. J. Pediatr. Surg. 2022, 57, 80–85. [Google Scholar] [CrossRef]
- Metzger, G.; Jatana, K.; Apfeld, J.; Deans, K.J.; Minneci, P.C.; Halaweish, I. State of Telemedicine Use in Pediatric Surgery in the USA-Where We Stand and What We Can Gain from the COVID-19 Pandemic: A Scoping Review. World J. Pediatr. Surg. 2021, 4, e000257. [Google Scholar] [CrossRef]
- Metzger, G.A.; Cooper, J.; Lutz, C.; Jatana, K.R.; Nishimura, L.; Patterson, K.N.; Deans, K.J.; Minneci, P.C.; Halaweish, I. Examining the Utility of Preoperative Telemedicine Care Across Multiple Pediatric Surgery Disciplines. J. Surg. Res. 2022, 277, 138–147. [Google Scholar] [CrossRef]
- Metzger, G.A.; Cooper, J.; Lutz, C.; Jatana, K.R.; Nishimura, L.; Deans, K.J.; Minneci, P.C.; Halaweish, I. Recognizing the Benefit of Telemedicine Before and After COVID-19: A Survey of Pediatric Surgery Providers. J. Surg. Res. 2021, 267, 274–283. [Google Scholar] [CrossRef]
- Dean, P.; O’Donnell, M.; Zhou, L.; Skarsgard, E.D. Improving Value and Access to Specialty Medical Care for Families: A Pediatric Surgery Telehealth Program. Can. J. Surg. 2019, 62, 436–441. [Google Scholar] [CrossRef]
- Abdulhai, S.; Glenn, I.C.; McNinch, N.L.; Craner, D.; Chou, E.; Ponsky, T.A. Public Perception of Telemedicine and Surgical Telementoring in the Pediatric Population: Results of a Parental Survey. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 215–217. [Google Scholar] [CrossRef]
- Parnell, K.; Kuhlenschmidt, K.; Madni, D.; Chernyakhovsky, C.; Donovan, I.; Garofalo, K.; Hambrick, S.; Scott, D.J.; Oltmann, S.C.; Luk, S. Using Telemedicine on an Acute Care Surgery Service: Improving Clinic Efficiency and Access to Care. Surg. Endosc. 2021, 35, 5760–5765. [Google Scholar] [CrossRef]
- Young, K.; Gupta, A.; Palacios, R. Impact of Telemedicine in Pediatric Postoperative Care. Telemed. e-Health 2019, 25, 1083–1089. [Google Scholar] [CrossRef]
- Macwilliam, J.; Hennessey, I.; Cleary, G. Telemedicine: Improving Clinical Care and Medical Education in Paediatrics. Paediatr. Child Health 2021, 31, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.E.; Falcone, R.A.J.; Fallat, M.E. Rural Health, Telemedicine and Access for Pediatric Surgery. Curr. Opin. Pediatr. 2019, 31, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Svetanoff, W.J.; Rosen, J.M.; Carrasco, A.; Rentea, R.M. Leveraging Collaboration in Pediatric Multidisciplinary Colorectal Care Using a Telehealth Platform. Am. Surg. 2022, 88, 2320–2326. [Google Scholar] [CrossRef]
- Hovaguimian, A.; Joshi, A.; Onorato, S.; Schwartz, A.W.; Frankl, S. Twelve Tips for Clinical Teaching with Telemedicine Visits. Med. Teach. 2022, 44, 19–25. [Google Scholar] [CrossRef]
- Bator, E.X.; Gleason, J.M.; Lorenzo, A.J.; Kanaroglou, N.; Farhat, W.A.; Bägli, D.J.; Koyle, M.A. The Burden of Attending a Pediatric Surgical Clinic and Family Preference toward Telemedicine. J. Pediatr. Surg. 2015, 50, 1776–1782. [Google Scholar] [CrossRef]
- Jungbauer, W.N.; Gudipudi, R.; Brennan, E.; Melvin, C.L.; Pecha, P.P. The Cost Impact of Telehealth Interventions in Pediatric Surgical Specialties: A Systematic Review. J. Pediatr. Surg. 2023, 58, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Yousef, Y.; Lee, A.; Ayele, F.; Poenaru, D. Delayed Access to Care and Unmet Burden of Pediatric Surgical Disease in Resource-Constrained African Countries. J. Pediatr. Surg. 2019, 54, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Kancherla, V.; Sundar, M.; Tandaki, L.; Lux, A.; Bakker, M.K.; Bergman, J.E.; Bermejo-Sánchez, E.; Canfield, M.A.; Dastgiri, S.; Feldkamp, M.L.; et al. Prevalence and Mortality among Children with Anorectal Malformation: A multi-country Analysis. Birth Defects Res. 2023, 115, 390–404. [Google Scholar] [CrossRef]
- Reddy, M.; Tank, N.; Bawa, M.; Kanojia, R.P.; Samujh, R. Anorectal Malformations: The Earlier the Diagnosis, the Better the Outcome. Indian J. Pediatr. 2022, 89, 536–540. [Google Scholar] [CrossRef]
- Kayima, P.; Kitya, D.; Punchak, M.; Anderson, G.A.; Situma, M. Patterns and Treatment Outcomes of Anorectal Malformations in Mbarara Regional Referral Hospital, Uganda. J. Pediatr. Surg. 2019, 54, 838–844. [Google Scholar] [CrossRef]
- Lane, V.A.; Skerritt, C.; Wood, R.J.; Reck, C.; Hewitt, G.D.; McCracken, K.A.; Jayanthi, V.R.; DaJusta, D.; Ching, C.; Deans, K.J.; et al. A Standardized Approach for the Assessment and Treatment of Internationally Adopted Children with a Previously Repaired Anorectal Malformation (ARM). J. Pediatr. Surg. 2016, 51, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Knaus, M.E.; Kersey, K.; Ahmad, H.; Weaver, L.; Thomas, J.L.; Metzger, G.A.; Wood, R.J.; Gasior, A.C. Both Sides of the Screen: Provider and Patient Perspective on Telemedicine in Pediatric Surgery. J. Pediatr. Surg. 2022, 57, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, E.; Skerritt, C.; Fitzpatrick, G.; Hooker, E.; Lander, A.; Gee, O.; Jester, I. Children with Congenital Colorectal Malformations during the UK SARS-CoV-2 Pandemic Lockdown: An Assessment of Telemedicine and Impact on Health. Pediatr. Surg. Int. 2021, 37, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Skertich, N.J.; Sullivan, G.A.; Silverberg, J.T.; Gadepalli, S.; Raval, M.V.; Gulack, B.C. The Utilization of Telehealth during the COVID-19 Pandemic: An American Pediatric Surgical Association Survey. J. Pediatr. Surg. 2022, 57, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Levitt, M.A.; Peña, A. Bowel Management for the Treatment of Pediatric Fecal Incontinence. Pediatr. Surg. Int. 2009, 25, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Stenström, P.; Kockum, C.C.; Emblem, R.; Arnbjörnsson, E.; Bjørnland, K. Bowel Symptoms in Children with Anorectal Malformation—A Follow-up with a Gender and Age Perspective. J. Pediatr. Surg. 2014, 49, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kaneyama, K.; Okazaki, T.; Lane, G.J.; Kato, Y.; Kobayashi, H.; Yamataka, A. A Comparative Study of Laparoscopy-Assisted Pull-through and Open Pull-through for Hirschsprung’s Disease with Special Reference to Postoperative Fecal Continence. J. Pediatr. Surg. 2007, 42, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Tomuschat, C.; Puri, P. Long-Term Results of Transanal Pull-through for Hirschsprung’s Disease: A Meta-Analysis. Pediatr. Surg. Int. 2016, 32, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Vilanova-Sanchez, A.; Halleran, D.R.; Reck-Burneo, C.A.; Gasior, A.C.; Weaver, L.; Fisher, M.; Wagner, A.; Nash, O.; Booth, K.; Peters, K.; et al. A Descriptive Model for a Multidisciplinary Unit for Colorectal and Pelvic Malformations. J. Pediatr. Surg. 2019, 54, 479–485. [Google Scholar] [CrossRef]
- Vilanova-Sánchez, A.; Choueiki, J.; Smith, C.A.; Callicot, S.; Frischer, J.S.; Levitt, M.A. Creating a Collaborative Program for the Care of Children with Colorectal and Pelvic Problems. Semin. Pediatr. Surg. 2020, 29, 150985. [Google Scholar] [CrossRef]
- Style, C.C.; Hsu, D.M.; Verla, M.A.; Mittal, A.G.; Austin, P.; Seth, A.; Dietrich, J.E.; Adeyemi-Fowode, O.A.; Bercaw-Pratt, J.L.; Chiou, E.H.; et al. Development of a Multidisciplinary Colorectal and Pelvic Health Program: Program Implementation and Clinical Impact. J. Pediatr. Surg. 2020, 55, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Bealer, J.; Peña, A. Critical Analysis of Fecal Incontinence Scores. Pediatr. Surg. Int. 2016, 32, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Bokova, E.; Svetanoff, W.J.; Levitt, M.A.; Rentea, R.M. Pediatric Bowel Management Options and Organizational Aspects. Children 2023, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Rome IV Criteria. Available online: https://theromefoundation.org/rome-iv/rome-iv-criteria/ (accessed on 23 June 2024).
- Cushing, C.C.; Threlkeld, M.R.S.; Martinez-Leo, B.; Hall, J.; Hossain, M.; Dickie, B.H.; Rymeski, B.; Helmrath, M.; Zeller, M.H.; Frischer, J.S. Initial Development and Validation of a Fecal Incontinence-Specific Quality of Life Measure. J. Pediatr. Surg. 2018, 53, 1148–1153. [Google Scholar] [CrossRef]
- Kilpatrick, J.A.; Zobell, S.; Leeflang, E.J.; Cao, D.; Mammen, L.; Rollins, M.D. Intermediate and Long-Term Outcomes of a Bowel Management Program for Children with Severe Constipation or Fecal Incontinence. J. Pediatr. Surg. 2020, 55, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.I.P.; Cushing, C.C.; Jenkins, T.; Troutt, M.; Zeller, M.H.; Hossain, M.; Rymeski, B.; Helmrath, M.; Frischer, J.S. Prospective Quality of Life Outcomes in Pediatric Fecal Incontinence Following Bowel Management. J. Pediatr. Surg. 2021, 56, 1459–1464. [Google Scholar] [CrossRef]
- Desai, A.D.; Zhou, C.; Stanford, S.; Haaland, W.; Varni, J.W.; Mangione-Smith, R.M. Validity and Responsiveness of the Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales in the Pediatric Inpatient Setting. JAMA Pediatr. 2014, 168, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Burwinkle, T.M.; Lane, M.M. Health-Related Quality of Life Measurement in Pediatric Clinical Practice: An Appraisal and Precept for Future Research and Application. Health Qual. Life Outcomes 2005, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Pane, S.; Estrada, M.D. Health-Related Quality of Life Measurement in Children and Adolescents: A Systematic Review of Generic and Disease-Specific Instruments. Value Health 2008, 11, 742–764. [Google Scholar] [CrossRef]
- Peña, A.; Bischoff, A. Surgical Treatment of Colorectal Problems in Children; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar]
- Gabr, A.A.; Gad, M.A.; Shalaby, A. Quality of Life in Children with Pseudoincontinence after Implementing a Bowel Management Program in Egypt. J. Pediatr. Surg. 2020, 55, 261–264. [Google Scholar] [CrossRef]
- Halleran, D.R.; Lane, V.A.; Leonhart, K.L.; Fischer, B.; Sebastião, Y.V.; Chisolm, D.J.; Levitt, M.A.; Wood, R.J.; Minneci, P.C.; Deans, K.J. Development of a Patient-Reported Experience and Outcome Measures in Pediatric Patients Undergoing Bowel Management for Constipation and Fecal Incontinence. J. Pediatr. Gastroenterol. Nutr. 2019, 69, e34. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, E.; Hearnshaw, H.; Van Royen, P.; Denekens, J. Patient Adherence to Treatment: Three Decades of Research. A Comprehensive Review. J. Clin. Pharm. Ther. 2001, 26, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Ausili, E.; Marte, A.; Brisighelli, G.; Midrio, P.; Mosiello, G.; Pergola, E.; Lombardi, L.; Iacobelli, B.D.; Caponcelli, E.; Meroni, M.; et al. Short versus Mid-Long-Term Outcome of Transanal Irrigation in Children with Spina Bifida and Anorectal Malformations. Childs Nerv. Syst. 2018, 34, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Modin, L.; Walsted, A.-M.; Rittig, C.S.; Hansen, A.V.; Jakobsen, M.S. Follow-up in Childhood Functional Constipation: A Randomized, Controlled Clinical Trial. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 594–599. [Google Scholar] [CrossRef]
- Finup, J.L.; Bhatia, V.P.; Perry, D.M.; Truscott, S.J.; Cannon, S.T.; O’Kelly, F.; Farhat, W.A. Personalized Pre-Clinic Nursing Telemedicine Visit: An Efficient and Efficacious Approach for Bowel and Bladder Dysfunction in Children. Urology 2023, 179, 158–163. [Google Scholar] [CrossRef]

| Barriers to TH | Examples |
|---|---|
| Hardware malfunction |
| Poor internet connection | |
| Digital literacy issues | |
| Distractions and interruptions |
| Building provider-patient communication |
| Communication etiquette | |
| Miscommunication via email or texts | |
| Coordination issues | |
| Information misinterpretation due to multitasking | |
| Limited access to devices |
| TH reimbursement issues | |
| Need for specific software | |
| State-specific differences in TH regulations |
| Necessity for the provider to be licensed in each state they practice in | |
| Privacy concerns | |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokova, E.; Elhalaby, I.; Saylors, S.; Lim, I.I.P.; Rentea, R.M. A Systematic Review of Telehealth Utilization for Bowel Management Programs in Pediatric Colorectal Surgery. Children 2024, 11, 786. https://doi.org/10.3390/children11070786
Bokova E, Elhalaby I, Saylors S, Lim IIP, Rentea RM. A Systematic Review of Telehealth Utilization for Bowel Management Programs in Pediatric Colorectal Surgery. Children. 2024; 11(7):786. https://doi.org/10.3390/children11070786
Chicago/Turabian StyleBokova, Elizaveta, Ismael Elhalaby, Seth Saylors, Irene Isabel P. Lim, and Rebecca M. Rentea. 2024. "A Systematic Review of Telehealth Utilization for Bowel Management Programs in Pediatric Colorectal Surgery" Children 11, no. 7: 786. https://doi.org/10.3390/children11070786
APA StyleBokova, E., Elhalaby, I., Saylors, S., Lim, I. I. P., & Rentea, R. M. (2024). A Systematic Review of Telehealth Utilization for Bowel Management Programs in Pediatric Colorectal Surgery. Children, 11(7), 786. https://doi.org/10.3390/children11070786







