Muscle Mass as a Biomarker for Health Status and Function in Pediatric Individuals with Neuromuscular Disabilities: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
- Average participant age is ≤21 years; studies with individual participants over the age of 21 were included as long as the average age remained below. This was to ensure that studies represented individuals across all stages of puberty and post-puberty; only one study [30] had participants over 21 (range was 18–22 years old, with average age of 19);
- The study focused on individuals with neuromuscular disabilities; studies with typically developing children were included to help emphasize differences between children with neuromuscular disabilities and those without;
- Primary examination included assessment of relationship(s) between MM (measured by LBM or FFM) and functional outcome measures, including but not limited to gait, mobility, bone, cardiovascular health, and metabolic health.
2.3. Data Synthesis
3. Results
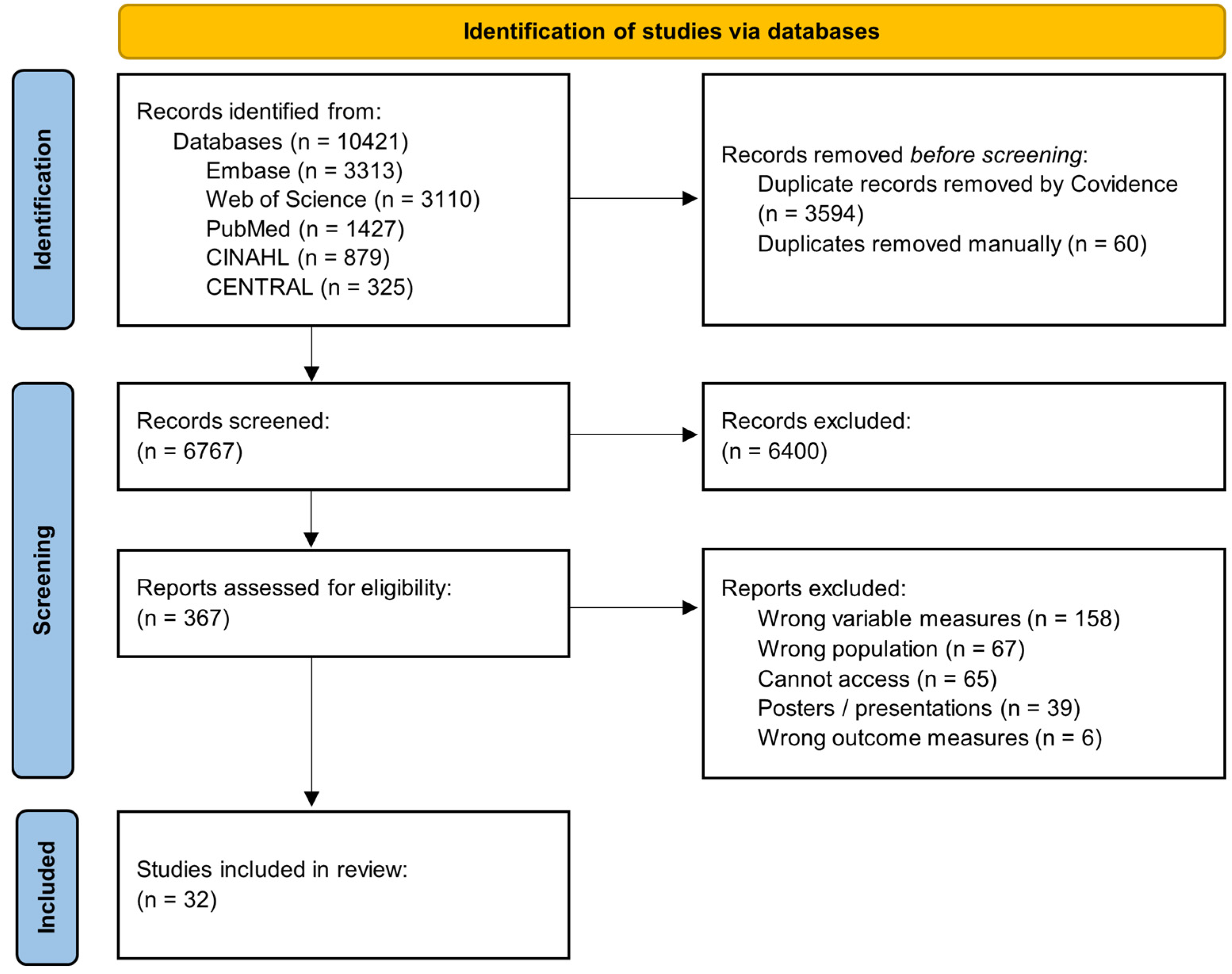
3.1. Study Screening and Inclusion
3.2. Study Characteristics
3.2.1. Location
3.2.2. Design
3.2.3. Size
3.2.4. Demographics
3.2.5. Muscle Mass Measurements Techniques
3.2.6. Outcome Measures
3.2.7. Participant Conditions
4. Discussion
4.1. Effect of Muscle Mass on Mobility and Functional Outcomes
4.2. Effect of Muscle Mass on Bone Outcomes
4.3. Effect of Muscle Mass on General Health and Disease Outcomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in: Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Gilligan, L.A.; Towbin, A.J.; Dillman, J.R.; Somasundaram, E.; Trout, A.T. Quantification of skeletal muscle mass: Sarcopenia as a marker of overall health in children and adults. Pediatr. Radiol. 2020, 50, 455–464. [Google Scholar] [CrossRef]
- Zembura, M.; Matusik, P. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022, 13, 914740. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.P. The epidemiology of neuromuscular disorders. Neurol. Clin. 2016, 34, 999–1021. [Google Scholar] [CrossRef]
- Rubin, M. How Common Are Neuromuscular Disorders? Relias Media. Published 1 March 2015. Available online: https://www.reliasmedia.com/articles/134780-how-common-are-neuromuscular-disorders (accessed on 14 March 2024).
- National Center on Birth Defects and Developmental Disabilities. What Is Cerebral Palsy? Centers for Disease Control and Prevention. Published 2 May 2022. Available online: https://www.cdc.gov/ncbddd/cp/facts.html (accessed on 14 March 2024).
- National Center on Birth Defects and Developmental Disabilities. Data and Statistics for Cerebral Palsy. Centers for Disease Control and Prevention. Published 2 May 2022. Available online: https://www.cdc.gov/ncbddd/cp/data.html (accessed on 14 March 2024).
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers. 2021, 7, 13. [Google Scholar] [CrossRef]
- Quijano-Roy, P.S. Congenital Muscular Dystrophy. OrphaNet. Last Updated September 2009. Available online: https://www.orpha.net/en/disease/detail/97242#menu (accessed on 14 March 2024).
- Iolascon, G.; Paoletta, M.; Liguori, S.; Curci, C.; Moretti, A. Neuromuscular diseases and bone. Front. Endocrinol. 2019, 10, 794. [Google Scholar] [CrossRef]
- Sizoo, D.; de Heide, L.J.M.; Emous, M.; van Zutphen, T.; Navis, G.; van Beek, A.P. Measuring muscle mass and strength in obesity: A review of various methods. Obes. Surg. 2021, 31, 384–393. [Google Scholar] [CrossRef]
- Chaves, L.G.C.M.; Gonçalves, T.J.M.; Bitencourt, A.G.V.; Rstom, R.A.; Pereira, T.R.; Velludo, S.F. Assessment of body composition by whole-body densitometry: What radiologists should know. Radiol. Bras. 2022, 55, 305–311. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (BIA) and dual energy x-ray absorptiometry (DXA): A critical overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Fielding, R.A. Assessment of analytical methods used to measure changes in body composition in the elderly and recommendations for their use in phase II clinical trials. J. Nutr. Health Aging 2011, 15, 368–375. [Google Scholar] [CrossRef]
- Sadowsky, C.L. Targeting sarcopenia as an objective clinical outcome in the care of children with spinal cord-related paralysis: A clinician’s view. Children 2023, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Szkoda, L.; Szopa, A.; Kwiecien-Czerwieniec, I.; Siwiec, A.; Domagalska-Szopa, M. Body composition in outpatient children with cerebral palsy: A case-control study. Int. J. Gen. Med. 2023, 16, 281–291. [Google Scholar] [CrossRef]
- Kawakami, R.; Tanisawa, K.; Ito, T.; Usui, C.; Miyachi, M.; Torii, S.; Midorikawa, T.; Ishii, K.; Muraoka, I.; Suzuki, K.; et al. Fat-free mass index as a surrogate marker of appendicular skeletal muscle mass index for low muscle mass screening in sarcopenia. J. Am. Med. Dir. Assoc. 2022, 23, 1955–1961. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Alish, C.; Pereira, S.L.; Deutz, N.E.; Heyland, D.K.; Goodpaster, B.H.; Tappenden, K.A.; Heymsfield, S.B. Implications of low muscle mass across the continuum of care: A narrative review. Ann. Med. 2018, 50, 675–693. [Google Scholar] [CrossRef]
- Orsso, C.E.; Tibaes, J.R.B.; Oliveira, C.L.P.; Rubin, D.A.; Field, C.J.; Heymsfield, S.B.; Prado, C.M.; Hagg, A.M. Low muscle masss and strength in pediatrics patients: Why should we care? Clin. Nutr. 2019, 38, 2002–2015. [Google Scholar] [CrossRef]
- Lee, J.; Hong, Y.; Shin, H.J.; Lee, W. Associations of Sarcopenia and Sarcopenic Obesity with Metabolic Syndrome Considering Both Muscle Mass and Muscle Strength. J. Prev. Med. Public Health 2016, 49, 35–44. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Kibirige, M.S.; Fordham, J.N.; Banks, L.M.; Muntoni, F.; Chinn, D.; Boivin, C.M.; Shaw, N.J. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone 2004, 35, 965–972. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; Tian, Q.; Papasian, C.J.; Hu, T.; Deng, H.W. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos. Int. 2016, 27, 473–482. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, Y.; Yu, X.; Liu, J.; Long, Q.; Zeng, Y.; Lu, S. An analysis and systematic review of sarcopenia increasing osteopenia risk. PLoS ONE 2021, 16, e0250437. [Google Scholar] [CrossRef] [PubMed]
- Zembura, M.; Czepczor-Bernat, K.; Dolibog, P.; Dolibog, P.T.; Matusik, P. Skeletal muscle mass, muscle strength, and physical performance in children and adolescents with obesity. Front. Endocrinol. 2023, 14, 1252853. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.; Hager, A.; Gilmour, S. Challenges and physiological implications of sarcopenia in children and youth in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 528–533. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 22 November 2022).
- Tajaldeen, A.; Alghamdi, S.S.; Aljondi, R.; Awan, Z.; Helmi, N.; Lingawi, K.; Mujalad, A.; Alzahrani, W. Associations between body mass index, body composition, and bone density in young adults: Findings from Saudi cohort. J. Radiat. Res. Appl. Sci. 2022, 15, 268–274. [Google Scholar] [CrossRef]
- Palmieri, G.M.; Bertorini, T.E.; Griffin, J.W.; Igarashi, M.; Karas, J.G. Assessment of whole-body composition with dual energy X-ray absorptiometry in Duchenne muscular dystrophy: Correlation of lean body mass with muscle mass. Muscle Nerve 1996, 19, 777–779. [Google Scholar] [CrossRef]
- Campanozzi, A.; Capano, G.; Miele, E.; Romano, A.; Scuccimarra, G.; Del Giudice, E.; Strisciuglio, C.; Militerni, R.; Staiano, A. Impact of malnutrition on gastrointestinal disorders and gross motor abilities in children with cerebral palsy. Brain Dev. 2007, 29, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Skalsky, A.J.; Han, J.J.; Abresch, R.T.; Shin, C.S.; McDonald, C.M. Assessment of regional body composition with dual-energy X-ray absorptiometry in Duchenne muscular dystrophy: Correlation of regional lean mass and quantitative strength. Muscle Nerve 2009, 39, 647–651. [Google Scholar] [CrossRef]
- Hazell, T.J.; Sharma, A.K.; Vanstone, C.A.; Gagnon, I.; Pham, T.T.; Finch, S.L.; Weiler, H.A.; Rodd, C.J. Normative data and predictors of leg muscle function and postural control in children. Med. Sci. Sports Exerc. 2014, 46, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Bell, K.L.; Stevenson, R.D.; Weir, K.A.; Boyd, R.N.; Davies, P.S.W. Differences in body composition according to functional ability in preschool-aged children with cerebral palsy. Clin. Nutr. 2015, 34, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Finbraten, A.K.; Martins, C.; Andersen, G.L.; Skranes, J.; Brannsether, B.; Júlíusson, P.B.; Syversen, U.; Stevenson, R.D.; Vik, T. Assessment of body composition in children with cerebral palsy: A cross-sectional study in Norway. Dev. Med. Child Neurol. 2015, 57, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Pucillo, E.M.; Dibella, D.L.; Hung, M.; Bs, J.B.; Crockett, B.; Dixon, M.; Butterfield, R.J.; Campbell, C.; Johnson, N.E. Physical function and mobility in children with congenital myotonic dystrophy. Muscle Nerve 2017, 56, 224–229. [Google Scholar] [CrossRef]
- Sung, K.H.; Chung, C.Y.; Lee, K.M.; Cho, B.C.; Moon, S.J.; Kim, J.; Park, M.S. Difference in body composition according to gross motor function in children with cerebral palsy. Arch. Phys. Med. Rehabil. 2017, 98, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Wyszynska, J.; Podgórska-Bednarz, J.; Drzal-Grabiec, J.; Rachwał, M.; Baran, J.; Czenczek-Lewandowska, E.; Leszczak, J.; Mazur, A. Analysis of relationship between the body mass composition and physical activity with body posture in children. Biomed. Res. Int. 2016, 2016, 1851670. [Google Scholar] [CrossRef]
- Wiech, P.; Cwirlej-Sozanska, A.; Wisniowska-Szurlej, A.; Kilian, J.; Lenart-Domka, E.; Bejer, A.; Domka-Jopek, E.; Sozański, B.; Korczowski, B. The relationship between body composition and muscle tone in children with cerebral palsy: A case-control study. Nutrients 2020, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sugiura, H.; Ito, Y.; Noritake, K.; Ochi, N. Relationship between the skeletal muscle mass index and physical activity of Japanese children: A cross-sectional, observational study. PLoS ONE 2021, 16, e0251025. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Lim, H.J.; Kim, H.W.; Suk, Y.J.; Park, K.-B.; Lee, M.-J. Decrease of muscle mass in young patients with neuromuscular disease: Assessment of sarcopenia. J. Korean Med. Sci. 2023, 38, e187. [Google Scholar] [CrossRef]
- Masaki, M.; Isobe, H.; Uchikawa, Y.; Okamoto, M.; Chiyoda, Y.; Katsuhara, Y.; Mino, K.; Aoyama, K.; Nishi, T.; Ando, Y. Association of gross motor function and activities of daily living with muscle mass of the trunk and lower extremity muscles, range of motion, and spasticity in children and adults with cerebral palsy. Dev. Neurorehabil. 2023, 26, 115–122. [Google Scholar] [CrossRef]
- Vicente-Rodrrguez, G.; Urzanqui, A.; Mesana, M.I.; Ortega, F.B.; Ruiz, J.R.; Ezquerra, J.; Casajús, J.A.; Blay, G.; Blay, V.A.; Gonzalez-Gross, M.; et al. Physical fitness effect on bone mass is mediated by the independent association between lean mass and bone mass through adolescence: A cross-sectional study. J. Bone Miner. Metab. 2008, 26, 288–294. [Google Scholar] [CrossRef]
- Dorsey, K.B.; Thornton, J.C.; Heymsfield, S.B.; Gallagher, D. Greater lean tissue and skeletal muscle mass are associated with higher bone mineral content in children. Nutr. Metab. 2010, 7, 41. [Google Scholar] [CrossRef]
- Wey, H.E.; Binkley, T.L.; Beare, T.M.; Wey, C.L.; Specker, B.L. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J. Clin. Endocrinol. Metab. 2011, 96, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, R.K.; Garg, M.K.; Bhadra, K.; Mahalle, N.; Mithal, A.; Tandon, N. Lean body mass and bone health in urban adolescents from northern India. Indian Pediatr. 2017, 54, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Guisado, E.; Martinez-Rodriguez, A.; Gallardo, L.; Sanchez-Sanchez, J. Bone mass in girls according to their BMI, VO2 max, hours and years of practice. Eur. J. Sport Sci. 2016, 16, 1176–1186. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Vlachopoulos, D.; Ferreira de Moraes, A.C.; Torres-Costoso, A.; Wilkinson, K.; Metcalf, B.; Sánchez-Sánchez, J.; Gallardo, L.; Gracia-Marco, L. Lean mass explains the association between muscular fitness and bone outcomes in 13-year-old boys. Acta Paediatr. 2017, 106, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Hetherington-Rauth, M.; Bea, J.W.; Blew, R.M.; Funk, J.L.; Hingle, M.D.; Lee, V.R.; Roe, D.J.; Wheeler, M.D.; Lohman, T.G.; Going, S.B. Relative contributions of lean and fat mass to bone strength in young Hispanic and non-Hispanic girls. Bone 2018, 113, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Hyde, N.K.; Duckham, R.L.; Wark, J.D.; Brennan-Olsen, S.L.; Hosking, S.M.; Holloway-Kew, K.L.; Pasco, J.A. The association between muscle mass and strength in relation to bone measures in a pediatric population: Sex-specific effects. Calcif. Tissue Int. 2020, 107, 121–125. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, I.; Martin-Garcia, M.; Garcia-Cuartero, B.; González-Vergaz, A.; Carcavilla, A.; Aragonés, Á.; Alegre, L.M.; Ara, I. Body composition as a mediator between cardiorespiratory fitness and bone mass during growth. Med. Sci. Sports Exerc. 2020, 52, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Dencker, M.; Thorsson, O.; Karlsson, M.K.; Lindén, C.; Eiberg, S.; Wollmer, P.; Andersen, L.B. Gender differences and determinants of aerobic fitness in children aged 8–11 years. Eur. J. Appl. Physiol. 2007, 99, 19–26. [Google Scholar] [CrossRef]
- Hopkins, N.; Stratton, G.; Tinken, T.; McWhannell, N.; Ridgers, N.; Graves, L.; George, K.; Cable, N.; Green, D. Relationships between measures of fitness, physical activity, body composition, and vascular function in children. Atherosclerosis 2009, 204, 244–249. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.S. Low muscle mass is associated with metabolic syndrome in Korean adolescents: The Korea National Health and Nutrition Examination Survey 2009–2011. Nutr. Res. 2016, 36, 1423–1428. [Google Scholar] [CrossRef]
- Burrows, R.; Correa-Burrows, P.; Reyes, M.; Blanco, E.; Albala, C.; Gahagan, S. Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort. Pediatr. Diabetes 2017, 18, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Iniguez, J.A.; Vasquez-Garibay, E.M.; Garcia-Contreras, A.A.; Velarde, E.R.; Sanromán, R.T.; Rocha, J.H.; Rosas, A.R.; León, M.R.; Martínez, E.U. Energy expenditure is associated with age, anthropometric indicators and body composition in children with spastic cerebral palsy. Nutr. Hosp. 2018, 35, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Summer, S.S.; Wong, B.L.; Rutter, M.M.; Horn, P.S.; Tian, C.; Rybalsky, I.; Shellenbarger, K.C.; Kalkwarf, H.J. Age-related changed in appendicular lean mass in males with Duchenne muscular dystrophy: A retrospective review. Muscle Nerve 2021, 63, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, S.G.; Powell, A.W.; Opotowsky, A.R.; Mays, W.W.; Knecht, S.K.; Rivin, G.; Chin, C. Skeletal muscle mass is linked to cardiorespiratory fitness in youth. Med. Sci. Sports Exerc. 2020, 52, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Haapala, E.A.; Gao, Y.; Rantalainen, T.; Finni, T. Associations of age, body size, and maturation with physical activity intensity in different laboratory tasks in children. J. Sports Sci. 2021, 39, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Kwarteng, E.A.; Shank, L.M.; Faulkner, L.M.; Loch, L.K.; Fatima, S.; Gupta, S.; Haynes, H.E.; Ballenger, K.L.; Parker, M.N.; Brady, S.M.; et al. Influence of puberty on relationships between body composition and blood pressure: A cross-sectional study. Pedaitr. Res. 2023, 94, 781–788. [Google Scholar] [CrossRef]
- Schoenau, E. From mechanostat theory to development of the “functional muscle-bone unit”. J. Musculoskelet. Neuronal Interact. 2005, 5, 232–238. [Google Scholar]
| Parameter | Unit | Definition |
|---|---|---|
| Fat-free mass (FFM) | kg or % | Mass of muscle, bone, tissue, and water. No fat mass included [18] |
| Skeletal muscle mass (SMM) | kg | Mass of skeletal muscle using BIA [18] |
| Lean body mass (LBM) or lean soft tissue mass | kg or % | Mass of muscle, tissue, water. No fat or bone mass included [16] |
| Appendicular lean soft tissue mass or appendicular skeletal muscle mass (ASM) | kg | Lean body mass (or lean soft tissue mass) without mass from head and trunk [16] |
| Measurements | Conditions | Outcomes | Relationships | Populations |
|---|---|---|---|---|
| Muscle mass | Cerebral palsy or CP | Outcome | Association | Child |
| Lean mass | Quadriplegia | Gait | Relationship | Adolescent |
| Lean body mass | Paraplegia | Function | Correlation | Pediatric |
| Fat-free mass | Tetraplegia | Recovery | Predictor | |
| Paralysis |
| Author, Year Country Design (Sample Size) | Sample Characteristics and Measurements | Outcome Measures | Conclusions |
|---|---|---|---|
| Functional and Mobility Outcomes | |||
| [31] Palmieri et al., 1996 United States Cross-sectional study (n = 19) | Individuals with DMD (n = 19): Age, median: 11; weight (kg): 34.42 ± 14.7; LBM (%): 65.3 ± 17.7; FBM (%): 31.7 ± 17.9; functional activity score, upper body: 3.7 ± 2.2; functional activity score, lower body: 6.7 ± 3.3 | DXA for total body composition Functional activity scores for muscle function Manual muscle testing for muscle strength | Total LBM was negatively correlated with both upper- and lower-extremity functional activity scores (R = −0.676, p = 0.021; R = −0.679, p = 0.002). LBM was positively correlated with manual muscle testing in 32 muscle groups (R = 0.686, p = 0.007). |
| [32] Campanozzi et al., 2007 Italy Pre–post study (n = 21) | Male = 11; female = 10 Children with CP, improved GMFM after nutritional rehabilitation (n = 9): Age: 6.11 ± 4.45; starting GMFM (%): 9.9 ± 6.7; Final GMFM (%): 13.7 ± 7.1; starting weight (kg): 14 ± 7.6; final weight (kg): 15.7 ± 8.3; starting FFM (kg): 12.8 ± 6.8; final FFM (kg): 13.9 ± 7; starting FBM (kg): 1.3 ± 1.1; final FBM (kg): 1.8 ± 1.4 Children with CP, unchanged GMFM after nutritional rehabilitation (n = 5): Age: 7 ± 3.1; starting GMFM (%): 4.14 ± 1.71; final GMFM (%): 14.3 ± 1.71; starting weight (kg): 15.5 ± 5.4; final weight (kg): 17.1 ± 6.8; starting FFM (kg): 13.7 ± 4.5; final FFM (kg): 14.1 ± 4.5; starting FBM (kg): 1.8 ± 1.6; Final FBM (kg): 2.9 ± 2 | Multiple skinfold anthropometry procedure for FFM and FBM Gross Motor Function Measure for basic, gross motor function | Targeting nutritional status to significantly increase (p < 0.005) FFM significantly (p < 0.05) and positively impacts gross motor function in children with CP. |
| [33] Skalsky et al., 2008 United States Cross-sectional study (n = 46) | Individuals with DMD (n = 23): Age: 9.5 ± 2.1; lean tissue mass (kg): 19.76 ± 4.4; BMC (g): 40.44 ± 11.07; arm strength (elbow flexion + extension): 5.8 ± 2.6; thigh strength (knee flexion + extension): 15.0 ± 8.4 Control group (n = 23): Age: 9.3 ± 2.0; lean tissue mass (kg): 24.46 ± 7.9; BMC (g): 48.76 ± 12.33; arm strength (elbow flexion + extension): 26.6 ± 12.6; thigh strength (knee flexion + extension): 76.5 ± 42.5 | DXA for lean tissue mass, fat tissue mass, and BMC Dynamometer for quantitative peak isometric strength in elbow/knee extensors and flexors | Lean tissue mass demonstrated a positive correlation with arm and thigh strength in the control group (R = 0.832, p < 0.001; R = 0.947, p < 0.001). Individuals with DMD lean tissue mass showed a weaker correlation to thigh strength and no correlation to arm strength (R = 0.514, p = 0.012; R = 0.164, p = 0.456). |
| [34] Hazell et al., 2014 Canada Cohort study (n = 81) | Typically developing individuals <3 years (n = 15): Male = 7; female = 8; age: 2.5 ± 0.4; LBM, DXA (kg): 9.2 ± 1.0; FBM, DXA (kg): 4.3 ± 1.1; sit-to-stand, relative peak force (N/kg): 11.1 ± 0.9 in males, 11.7 ± 0.9 in females; sit-to-stand, relative peak power (N/s/kg): 53.4 ± 18.5 in males, 70.4 ± 42.3 in females; jump test, relative peak force (N/kg): 20.0 ± 2.0 in males, 19.7 ± 5.0 in females; jump test, relative peak power (N/s/kg): 4.9 ± 2.5 in males, 7.6 ± 7.4 in females; body sway amplitude (mm): 1.1 ± 1.0 in males, 1.1 ± 0.4 in females; body sway velocity (mm/s): 10.1 ± 7.6 in males, 7.3 ± 3.9 in females Typically developing individuals 3–3.9 years (n = 21): Male = 12; female = 9; age: 3.4 ± 0.3; LBM, DXA (kg): 10.7 ± 1.5; FBM, DXA (kg): 5.1 ± 0.9; sit-to-stand, relative peak force (N/kg): 11.8 ± 1.3 in males, 10.8 ± 1.2 in females; sit-to-stand, relative peak power (N/s/kg): 76.4 ± 32.4 in males, 70.4 ± 18.2 in females; jump test, relative peak force (N/kg): 20.4 ± 2.6 in males, 18.7 ± 2.7 in females; jump test, relative peak power (N/s/kg): 6.1 ± 5.3 in males, 12.0 ± 8.1 in females; body sway amplitude (mm): 1.1 ± 0.5 in males, 0.7 ± 0.3 in females; body sway velocity (mm/s): 7.1 ± 3.9 in males, 4.3 ± 1.9 in females Typically developing individuals 4–4.9 years (n = 28): Male = 13; female = 15; age: 4.5 ± 0.3; LBM, DXA (kg): 12.7 ± 1.6; FBM, DXA (kg): 4.6 ± 1.3; sit-to-stand, relative peak force (N/kg): 13.4 ± 1.4 in males, 13.2 ± 1.0 in females; sit-to-stand, relative peak power (N/s/kg): 94.9 ± 39.9 in males, 94.0 ± 30.0 in females; jump test, relative peak force (N/kg): 20.3 ± 3.7 in males, 20.7 ± 3.9 in females; jump test, relative peak power (N/s/kg): 11.1 ± 6.1 in males, 18.5 ± 21.7 in females; body sway amplitude (mm): 0.8 ± 0.2 in males, 0.6 ± 0.2 in females; body sway velocity (mm/s): 5.5 ± 2.2 in males, 5.0 ± 2.6 in females Typically developing individuals >5 years (n = 17): Male = 10; female = 7; age: 5.3 ± 0.3; LBM, DXA (kg): 14.2 ± 2.1; FBM, DXA (kg): 4.9 ± 1.1; sit-to-stand, relative peak force (N/kg): 13.7 ± 0.6 in males, 13.2 ± 1.0 in females; sit-to-stand, relative peak power (N/s/kg): 94.8 ± 26.8 in males, 81.1 ± 21.7 in females; jump test, relative peak force (N/kg): 19.7 ± 6.3 in males, 19.6 ± 2.8 in females; jump test, relative peak power (N/s/kg): 12.3 ± 34.2 in males, 28.9 ± 9.2 in females; body sway amplitude (mm): 0.6 ± 0.2 in males, 0.7 ± 0.1 in females; body sway velocity (mm/s): 5.5 ± 5.0 in males, 8.1 ± 2.7 in females | DXA for body composition measurements Jumping test and sit-to-stand test for muscle function | LBM demonstrated importance in models to determine sit-to-stand force likely due to its indication of strength. |
| [35] Walker et al., 2014 Australia Cross-sectional study (n = 101) | Individuals with CP (n = 85): male = 58; female = 27 GMFCS scores I and II (n = 52): Age: 2.63 ± 0.79; FFM (kg): 11.0 ± 1.5; body fat (kg): 2.7 ± 1.4 GMFCS score III (n = 13): Age: 2.04 ± 0.47; FFM (kg): 9.0 ± 1.5; body fat (kg): 2.1 ± 0.8 GMFCS scores IV and V (n = 20): Age: 2.94 ± 0.86; FFM (kg): 9.6 ± 1.9; body fat (kg): 3.4 ± 2.0 Typically developing individuals (n = 16): Male = 10; female = 6; age: 3.69 ± 0.48; FFM (kg): 13.0 ± 1.4; body fat (kg): 3.9 ± 0.7 | DXA for FFM and body fat measures Gross Motor Function Classification System for functioning level | Lower FFM was correlated with higher GMFCS classifications. |
| [36] Finbraten et al., 2015 Norway Cross-sectional study (n = 47) | Individuals with CP: 64 invited to participate, 52 agreed, measurements for 47 GMFCS scores I and II: Females = 11; males = 19; age: 13.3 ± 2.75; subscapular skinfold thickness (mm): 10.8 ± 8.2; triceps skinfold thickness (mm): 13.3 ± 6.9; body fat, DXA (%): 25.3 ± 8.5; LBM, DXA (%): 72.8 ± 9.0; bone mass, DXA (%): 3.1 ± 0.6 GMFCS scores III, IV, and V: Females = 7; males = 10; age: 12.83 ± 2.92; subscapular skinfold thickness (mm): 13.4 ± 6.6; triceps skinfold thickness (mm): 13.4 ± 8.0; body fat, DXA (%): 33.5 ± 7.6; LBM, DXA (%): 63.6 ± 7.2; bone mass, DXA (%): 2.9 ± 0.8 | DXA for body composition Gross Motor Function Classification System for functioning level | Lower measurements of LBM were consistent with lower functional levels (i.e., higher GMFCS scores). LBM also had lower correlations to skinfold thickness (R = 0.370 for triceps, 0.382 for subscapular). |
| [37] Pucillo et al., 2016 United States Cross-sectional study (n = 64) | Individuals with congenital myotonic dystrophy (n = 37): Male = 18; female = 19; age: 7.4 ± 3.0; 10 m walk (s): 11.3 ± 6.9; 10 m run (s): 6.1 ± 3.1; time to rise from floor (s): 9.4 ± 6.3; 4 stair climb (s): 7.0 ± 7.6; 6 min walk (m): 258.3 ± 176.0; 2 min walk (m): 91 ± 58.9 Control group (n = 27): Male = 12; female = 15; age: 9.7 ± 2.3; 10 m walk (s): 6.8 ± 1.8; 10 m run (s): 3.2 ± 0.5; time to rise from floor (s): 2.0 ± 0.6; 4 stair climb (s): 1.5 ± 0.3; 6 min walk (m): 568.2 ± 73.2; 2 min walk (m): 193 ± 22.7; | DXA for body composition measurements Bruininks–Osteretsky Test of Motor Proficiency for fine motor control measurements | LBM is correlated with right grip strength (r = 0.84, p < 0.001) and right pinch strength (r = 0.76, p < 0.001). Correlations were less significant for the 2 min walk test (r = 0.59), 6 min walk test (r = 0.62), and 10 m walk (r = −0.38). |
| [38] Sung et al., 2016 Korea Retrospective case–control study (n = 146) | Individuals with CP, GMFCS I, II, III (n = 57): Males = 39; females = 18; age: 11.6 ± 4.5; body fat (kg): 7.6 ± 6.9; soft LBM (kg): 29.1 ± 11.8; FFM (kg): 30.6 ± 12.6; SMM (kg): 16.4 ± 7.6; BMC (kg): 1.8 ± 0.8 Individuals with CP, GMFCS IV and V (n = 43): Males = 25; females = 18; age: 11.4 ± 3.9; body fat (kg): 5.9 ± 5.6; soft LBM (kg): 18.4 ± 6.8; FFM (kg): 19.7 ± 7.2; SMM (kg): 9.6 ± 4.3; BMC (kg): 1.1 ± 0.5 Typically developing children (n = 46): Males = 24; females = 22; age: 12.8 ± 4.5; body fat (kg): 11.3 ± 7.2; soft LBM (kg): 31.8 ± 12.5; FFM (kg): 34.4 ± 13.8; SMM (kg): 18.4 ± 8.3; BMC (kg): 2.0 ± 0.8 | BIA for body fat, soft LBM, FFM, SMM, and BMC measurements Gross Motor Function Classification System for functional level | Lower FFM, SMM, BMC, and soft LBM were associated with higher GMFCS scores. |
| [39] Wyszynska et al., 2016 Poland Cross-sectional study (n = 120) | Typically developing primary-school children (n = 120): Males = 59; females = 61; age: 12.09 ± 0.83; FBM (kg): 10.11 ± 5.6; MM (kg): 34.9 ± 7.45; level of physical activity (PAQ score): 2.86 ± 0.89 | BIA for body composition measurements Photogrammetric method to measure posture Physical Activity Questionnaire for Children used for physical activity measurements | Individuals with higher MM showed smaller differences in scapular arrangements. |
| [40] Wiech et al., 2020 PolandCase–control study (n = 236) | Individuals with CP (n = 118): Males = 76, females = 42; age: 11 ± 3.8; GMFCS level I = 32, GMFCS level II = 56, GMFCS level III = 4, GMFCS level IV = 17, GMFCS level V = 9; Ashworth level 0 = 13, level 1 = 54, level 2 = 35, level 3 = 14, level 4 = 2; Control individuals (n = 118): Males = 76; females = 42; age: 11 ± 3.8 | BIA for FBM, FFM, and MM measures Ashworth scale for muscle tone measurements Gross Motor Function Classification System for functional mobility level | FFM and MM are inversely related to GMFCS scores. They are also inversely related to Ashworth scores. |
| [41] Ito et al., 2021 Japan Cross-sectional study (n = 340) | Typically developing children with recommended moderate-to-vigorous activity levels (n = 153): Male = 83; female = 70; age, median: 10.0; SMM index, median (kg/m2): 5.98; gait speed, median (m/s): 1.20; grip strength, median (kg): 14.15; sit-to-stand test, median (5×, s): 5.94; timed up-and-go test, median (s): 7.29; one-leg standing time, median (s): 120.0 Typically developing children with less than the recommended moderate-to-vigorous activity levels (n = 187): Male = 82; female = 105; age, median: 9.0; SMM index, median (kg/m2): 5.59; gait speed, median (m/s): 1.16; grip-strength, median (kg): 12.0; sit-to-stand test, median (5×, s): 6.1; timed up-and-go test, median (s): 7.62; one-leg standing time, median (s): 93.24 | Bioelectrical impedance for SMM measurements Moderate-to-vigorous physical activity questionnaire for activity measurements | There is a strong correlation between SMM and moderate-to-vigorous physical activity. |
| [42] Kim et al., 2023 Cross-sectional study Korea (n = 121) | Children with neurologic disease (n = 79): Male = 40; female = 39; ambulatory = 37, non-ambulatory = 42; age: 11.8 ± 3.7; psoas muscle area (mm2): 890.2 ± 481.5; psoas muscle z-score: −2.6 ± 1.1; psoas muscle index: 1.8 ± 0.9 Children with no neurologic disease (n = 42): Male = 16; female = 26; ambulatory = 42, non-ambulatory = 0; age: 13.1 ± 3.6; psoas muscle area (mm2): 1246.5 ± 754.0; psoas muscle z-score: −2.0 ± 1.2; psoas muscle index: 2.2 ± 1.1 | CT was used to measure bilateral psoas muscle area to measure the degree of sarcopenia Ambulatory function was observed based on ability to walk with or without a walker | Bilateral psoas muscle area z-score (representing the degree of sarcopenia) is associated with non-ambulatory function (β = 0.436, p < 0.001). |
| [43] Masaki et al., 2023 JapanCross-sectional study (n = 32) | Children with CP: Male = 22; female = 10; age: 13 ± 10.8; GMFCS level I = 2; GMFCS level II = 5; GMFCS level III = 2; GMFCS level IV = 8; GMFCS level V = 15; GMFM, lying and rolling (%): 62.9 ± 31.7; GMFM, sitting (%): 47.7 ± 40.4; PEDI, self-care: 26.3 ± 24.1; PEDI, mobility: 16.8 ± 19.5; thoracic erector spinae thickness (cm): 0.81 ± 0.46; lumbar erector spinae thickness (cm): 1.83 ± 0.77; rectus abdominis thickness (cm): 0.73 ± 0.24; obliquus externus abdominus thickness (cm): 0.51 ± 0.21; gluteus maximus thickness (cm): 2.03 ± 0.64; gluteus medius thickness (cm):1.70 ± 0.74; gluteus minimus thickness (cm): 0.77 ± 0.31; rectus femoris thickness (cm): 1.27 ± 0.44; vastus intermedius thickness (cm): 0.9 ± 0.34; vastus lateralis thickness (cm): 1.25 ± 0.45; long head of bicep femoris thickness (cm): 1.41 ± 0.55; tibialis anterior thickness (cm): 1.45 ± 0.42; medial head of gastrocnemius (cm): 0.90 ± 0.27; soleus thickness (cm): 1.22 ± 0.43; spinal alignment: 6.8 ± 4.3; range of motion, lower extremities: 32.0 ± 12.3; hip flexor spasticity: 1.8 ± 0.7; hip adductor spasticity: 3.1 ± 0.7; knee flexor spasticity: 3.4 ± 0.6; plantar flexor spasticity: 3.3 ± 0.9 | B-mode US device for measuring muscle thickness Spinal Alignment and Range of Motion Measure to measure spinal alignment and range of motion of lower extremities Modified Ashworth Scale for spasticity Gross Motor Function Classification System for functional mobility level Gross Motor Function Measure for gross motor function level PEDI for functional ability of daily living | Decreased MM of the thoracic erector spinae is associated with declined gross motor functions lying/rolling and sitting (R2 = 0.74, p = 0.048; R2 = 0.81, p = 0.02). Decreased MM of the rectus abdominus and vastus lateralis is associated with decreased functional abilities of daily living self-care and mobility (R2 = 0.76, p = 0.01; R2 = 0.90, p < 0.01). |
| Bone Outcomes | |||
| [44] Vicente-Rodriguez et al., 2007 Spain Cross-sectional study (n = 278) | Typically developing adolescents, male = 109; female = 169; age, range: 13–18.5 years old; Raw data were included for each age group, split into years 13, 14, 15, 16, and 17–18.5 for each sex | DXA for whole-body BMC, FBM, and LBM | Differences in LBM measurements are related to differences in BMC. This is true across sexes. |
| [45] Dorsey et al., 2010 Cross-sectional study United States (n = 175) | Study 1 subjects, typically developing males (n = 83): Age: 12.0 ± 3.7; FBM, DXA (kg): 11.8 ± 9.3; BMC, DXA (kg): 2.0 ± 0.9; FFM, DXA (kg): 39.3 ± 15.3; non-bone LBM, DXA (kg): 37.3 ± 14.4; SMM, DXA (kg): 18.3 ± 8.4; SMM, MRI (kg) 18.3 ± 8.9 Study 1 subjects, typically developing females (n = 59): Age: 11.6 ± 3.6; FBM, DXA (kg): 15.3 ± 10.1; BMC, DXA (kg): 1.8 ± 0.6; FFM, DXA (kg): 39.3 ± 15.3; non-bone LBM, DXA (kg): 29.9 ± 8.3; SMM, DXA (kg): 14.3 ± 4.8; SMM, MRI (kg) 14.4 ± 5.1 Study 2 subjects, typically developing males (n = 20): Age: 8.8 ± 1.3; FBM, DXA (kg): 8.8 ± 6.3; BMC, DXA (kg): 1.2 ± 0.3; FFM, DXA (kg): 24.9 ± 4.5; non-bone LBM, DXA (kg): 23.7 ± 4.3; SMM, DXA (kg): 10.4 ± 2.7; SMM, MRI (kg) 9.9 ± 2.7 Study 2 subjects, typically developing females (n = 13): Age: 8.2 ± 1.0; FBM, DXA (kg): 8.5 ± 5.8; BMC, DXA (kg): 1.0 ± 0.3; FFM, DXA (kg): 20.8 ± 4.9; non-bone LBM, DXA (kg): 19.8 ± 4.7; SMM, DXA (kg): 8.6 ± 2.4; SMM, MRI (kg) 8.3 ± 2.8 | DXA for BMC, non-bone LBM, skeletal mass and FBM Magnetic resonance imaging for SMM and adipose tissue | SMM measured by MRI can be used as a predictor for BMC (R2 = 0.948, p < 0.001). SMM measured by DXA demonstrated a weaker association (R2 = 0.929, p < 0.001). |
| [46] Wey et al., 2010 United States Cross-sectional, longitudinal study (n = 374) | Typically developing individuals: Males = 234; females = 140; individuals were split into the following age groups: 8–10, 10–12, 12–14, 14–16, and 16+ years old. Data were provided for each group including LBM, FBM, physical activity %, measurements at 4% radius, and measurements 20% radius. These measurements were taken again 18 months after initial tests | DXA for LBM and FBM Peripheral quantitative CT for BMC, BMD, and areaPolar stress strain index for strength | Lean MM was positively associated with cortical area (20R), bone thickness (20R), BMD (4R in males only), BMC (20R and 4R), and strength (20R and female 4R). Lean MM had no association with bone thickness (4R), BMD (20R and female 4R), and strength (4R in males only). |
| [47] Marwaha et al., 2016 India Cross-sectional, secondary data analysis (n = 1403) | Typically developing adolescents: Male = 826; female = 577; age: 13.2 ± 2.7; pubertal stage 1: male = 103; female = 39; total LBM (kg): 22.16 ± 4.21 in males, 17.61 ± 3.34 in females Pubertal stage 2: male = 194; female = 49; total LBM (kg): 27.04 ± 4.72 in males, 20.961 ± 3.02 in females Pubertal stage 3: male = 183; female = 80; total LBM (kg): 34.71 ± 6.49 in males; 25.51 ± 3.85 in females Pubertal stage 4: male = 148; female = 117; total LBM (kg): 39.71 ± 6.49 in males; 28.58 ± 4.12 in females Pubertal stage 1: male = 198; female = 292; total LBM (kg): 44.24 ± 5.59 in males, 30.38 ± 3.65 in females | DXA for LBM and bone | LBM is correlated with BMC. This correlation is strongest in the legs. |
| [48] Ubago-Guisado et al., 2016 SpainCross-sectional study (n = 120) | Typically developing females: Age: 11.32 ± 1.6 Prepubertal females, BMI > 16.84 (n = 30): Total BMC (g): 1230.20 ± 263.47; total BMD (g/cm2): 0.86 ± 0.08; total LBM (kg): 27.41 ± 5.11; total FBM (kg): 13.10 ± 4.40; percent body fat (%): 30.90 ± 6.06 Prepubertal females, BMI ≤ 16.84 (n = 30): Total BMC (g): 1043.03 ± 33.86; total BMD (g/cm2): 0.80 ± 0.07; total LBM (kg): 21.20 ± 4.12; total FBM (kg): 7.44 ± 2.35; percent body fat (%): 24.84 ± 5.27 Pubertal females, BMI > 19.84 (n = 30): Total BMC (g): 1911.58 ± 340.64; total BMD (g/cm2): 1.05 ± 0.11; total LBM (kg): 39.70 ± 3.88; total FBM (kg): 19.02 ± 4.32; percent body fat (%): 31.10 ± 4.28 Pubertal females, BMI ≤ 19.84 (n = 30): Total BMC (g): 1423.10 ± 271.08; total BMD (g/cm2): 0.92 ± 0.09; total LBM (kg): 30.52 ± 41.50; total FBM (kg): 10.04 ± 3.12; percent body fat (%): 23.58 ± 5.05 | DXA for FBM, LBM, BMC, and BMD | In pubertal females, LBM is strongly correlated with BMC and BMD (r = 0.918 and r = 0.858; p < 0.001). |
| [49] Ubago-Guisado et al., 2017 Spain Cross-sectional study (n = 121) | Typically developing male participants Swimmers (n = 41): Age: 13.4 ± 1.0; stature (cm): 165.5 ± 9.7; LBM (kg): 41.6 ± 9.1; moderate-to-vigorous physical activity (min/day): 85.9 ± 30.4; vigorous physical activity (min/day): 11.9 ± 7.3; vertical jump (cm): 42.3 ± 6.9; standing long jump (cm): 171.0 ± 28.1; 20 m shuttle run test: 69 ± 20; Areal BMD (g/cm2): 0.918 ± 0.067 Footballers (n = 37): Age: 12.8 ± 0.9; stature (cm): 155.2 ± 9.3; LBM (kg): 35.4 ± 7.2; moderate-to-vigorous physical activity (min/day): 119.8 ± 29.7; vigorous physical activity (min/day): 22.5 ± 9.0; vertical jump (cm): 42.4 ± 6.0; standing long jump (cm): 168.7 ± 24.9; 20 m shuttle run test: 83 ± 18; areal BMD (g/cm2): 0.931 ± 0.071 Cyclists (n = 29): Age: 13.2 ± 1.0; stature (cm): 160.8 ± 9.9; LBM (kg): 37.7 ± 7.5; moderate-to-vigorous physical activity (min/day): 107.2 ± 33.3; vigorous physical activity (min/day): 18.5 ± 12.8; vertical jump (cm): 41.0 ± 6.8; standing long jump (cm): 163.5 ± 25.8; 20 m shuttle run test: 70 ± 21; areal BMD (g/cm2): 0.905 ± 0.086 Nonathletes (n = 14): Age: 12.3 ± 0.5; stature (cm): 154.5 ± 9.9; LBM (kg): 31.7 ± 5.5; moderate-to-vigorous physical activity (min/day): 83.2 ± 26.8; vigorous physical activity (min/day): 8.9 ± 4.0; vertical jump (cm): 39.5 ± 5.8; standing long jump (cm): 137.1 ± 24.5; 20 m shuttle run test: 32 ± 16; areal BMD (g/cm2): 0.828 ± 0.071 | DXA for LBM, areal BMD, and hip structural elements Vertical and standing long jumps used for physical fitness | LBM plays a vital role in connecting fitness and bone outcomes. |
| [50] Heatherington-Rauth et al., 2018 United States Cross-sectional study (n = 326) | Typically developing Hispanic females (n = 241): Age: 10.7 ± 1.1; moderate-to-vigorous physical activity (min/day): 19.5 ± 16.0; LBM (kg): 26.0 ± 7.4; bone strength index (mg/cm4): 56.4 ± 22.1 Typically developing non-Hispanic females (n = 85): Age: 11.0 ± 1.1; moderate-to-vigorous physical activity (min/day): 23.0 ± 25.5; LBM (kg): 26.6 ± 8.6; bone strength index (mg/cm4): 53.1 ± 23.3 | DXA for FBM and LBM Peripheral quantitative CT for bone strength | LBM was not as strong as a predictor in bone strength and bone outcomes in Hispanic females compared to non-Hispanic females. |
| [51] Hyde et al., 2020 Australia Cross-sectional study (n = 172) | Typically developing males (n = 86): Age, median: 10.93; total body (less head) BMC, median (g): 1060.39; total body (less head) BMD, median (g/cm2): 0.814; spine BMC, median (g): 23.6; spine BMD, median (g/cm2): 0.781; max handgrip strength, median (kg): 15.5; max quadriceps strength, median (N): 8.2; max hip abduction strength, median (N): 8.0; max hip flexion strength, median (N): 12.2 Typically developing females (n = 86): Age, median: 10.91; total body (less head) BMC, median (g): 1069.30; total body (less head) BMD, median (g/cm2): 0.816; spine BMC, median (g): 24.8; spine BMD, median (g/cm2): 0.850; max handgrip strength, median (kg): 15.0; max quadriceps strength, median (N): 7.8; max hip abduction strength, median (N): 7.7; max hip flexion strength, median (N): 11.5 | DXA for BMC, BMD, and LBM measures Dynamometer for handgrip strength measures | The association between LBM and bone mass was present in both sexes but was slightly stronger in females. |
| [52] Rodriguez-Gomez et al., 2020 SpainCross-sectional study (n = 169) | Typically developing prepubertal females (n = 98): Age: 10.4 ± 1.2; body fat percentage (%): 33.5 ± 9.4; LBM (kg): 28.3 ± 5.6; whole-body BMD (g/cm2): 0.85 ± 0.07; femoral neck BMD (g/cm2): 0.72 ± 0.10; muscle output power (W/kg): 0.3 ± 0.1; CRF (mL/kg∙min): 59.4 ± 8.7 Typically developing pubertal females (n = 71): Age: 13.1 ± 1.6; body fat percentage (%): 38.0 ± 6.8; LBM (kg): 41.4 ± 10.0; whole-body BMD (g/cm2): 0.96 ± 0.10; femoral neck BMD (g/cm2): 0.87 ± 0.13; muscle output power (W/kg): 0.3 ± 0.1; CRF (mL/kg∙min): 52.2 ± 7.9 | DXA for LBM, FBM, and BMD | LBM is a mediator between bone mass and muscle output power (Sobel test: −3.279, p < 0.001) and between bone mass and CRF (Sobel test: −2.691, p < 0.001). This effect increases with puberty. |
| [30] Tajaldeen et al., 2022 Saudi Arabia Cross-sectional study (n = 250) | Control individuals (n = 123): Male = 64; female = 59; age: 19.7 ± 1.41 males, 19.56 ± 1.23 females; fat weight (kg): 16.68 ± 5.89 in males, 33.79 ± 15.36 in females; lean weight (kg): 45.6 ± 11.03 in males, 42.88 ± 13.25 in females Individuals with osteopenia (n = 119): Male = 81; female = 38; age: 19.95 ± 1.31 males, 19.84 ± 1.29 females; fat weight (kg): 15.15 ± 5.42 in males, 29.52 ± 13.01 in females; lean weight (kg): 42.99 ± 8.08 in males, 39.12 ± 13.58 in females Individuals with osteoporosis (n = 8): Male = 5; female = 3; age: 19.4 ± 1.36 males, 18.67 ± 0.94 females; fat weight (kg): 14.82 ± 4.11 in males, 33.53 ± 3.34 in females; lean weight (kg): 37.55 ± 6.22 in males, 50.76 ± 2.85 in females | DXA was used for FBM, LBM, total fat percentages, trunk LBM, BMC, and BMD | LBM was significantly correlated with BMD in females. |
| Health Outcomes | |||
| [53] Dencker et al., 2006 Australia Cross-sectional study (n = 248) | 477 typically developing individuals invited, 248 accepted; males = 140; females = 108 Males: Age: 9.9 ± 0.6; end-diastolic left ventricular inner diameter (mm): 42.1 ± 3.4; total body fat, DXA (kg): 6.4 ± 5.1; LBM, DXA (kg): 26.1 ± 3.4; minutes of vigorous physical activity per day: 46 ± 20; maximal heart rate (BPM): 188 ± 16; VO2 peak (mL/min): 1423 ± 259; VO2 peak (mL/min/LBM): 54.5 ± 7.0 Females: Age: 9.7 ± 0.6; end-diastolic left ventricular inner diameter (mm): 40.7 ± 3.1; total body fat, DXA (kg): 8.1 ± 5.2; LBM, DXA (kg): 24.1 ± 3.5; minutes of vigorous physical activity per day: 35 ± 13; maximal heart rate (BPM): 185 ± 16; VO2 peak (mL/min): 1208 ± 203; VO2 peak (mL/min/LBM): 50.5 ± 6.9 | DXA for LBM and FBM measurements Indirect calorimetry for VO2 peak | LBM was correlated with VO2 peak (r2 = 0.69, p < 0.05), max heart rate (r2 = 0.53, p < 0.001), vigorous activity per day (r2 = 0.61, p = 0.02), and left ventricular inner diastolic diameter (r2 = 0.60 p = 0.009). |
| [54] Hopkins et al., 2008 United Kingdom Cohort study (n = 145) | Typically developing prepubertal individuals (n = 145): Male = 59; female = 86; age: 10.33 ± 0.31; FBM, DXA (%): 27.1 ± 6.9; LBM, DXA (%): 70.1 ± 8.8; SBP (mmHg): 107 ± 10; DBP (mmHg): 64 ± 5; max exercise heart rate (BPM): 202 ± 26; VO2, peak (mL/kg/min): 45 ± 6; flow-mediated dilation (%): 10.1 ± 4.5 | DXA for body composition measurements Actigraphy accelerometer for physical activity | There are significant correlations between LBM percentages and flow-mediated dilation percentages (r = 0.21, p = 0.02) |
| [55] Kim and Park, 2016 KoreaCross-sectional study (n = 1420) | Sex: male = 749; female = 671 Individuals with low MM: Age: 15.5 ± 0.2; ASM (kg): 18.0 ± 0.6; weight (kg): 67.8 ± 1.6; SBP (mmHg): 110.6 ± 1.0; DBP (mmHg): 70.2 ± 0.7; TGs (mg/dL): 162.0 ± 2.0; WC (cm): 79.4 ± 1.2; MetS prevalence: 14.8% (2.7) Individuals without low MM: Age: 15.6 ± 0.9; ASM (kg): 17.8 ± 0.2; weight (kg): 56.2 ± 0.4; SBP (mmHg): 107.9 ± 0.4; DBP (mmHg): 68.7 ± 0.3; TGs (mg/dL): 81.1 ± 1.6; WC (cm): 69.2 ± 0.3; MetS prevalence: 2.4% (0.5) | DXA for SMM | Having low MM was related to higher weight, higher sBP, higher dBP, higher TG levels, higher WC, and higher prevalence of MetS. |
| [56] Burrows et al., 2017 Chile Cross-sectional study (n = 678) | 678 participants, 660 with complete data Male = 52.3%; female = 47.8%; age: 16.8 ± 0.3; obesity prevalence: 16.4%; MetS prevalence: 9.7% Males without low MM (271): Total LBM (%): 78.2 ± 6.0; FBM (%): 18.8 ± 5.9; WC (cm): 77.0 ± 6.5; high-density lipoprotein (mg/dL): 39.4 ± 10.2; SBP (mmHg): 113.7 ± 9.5; DBP (mmHg): 70.2 ± 6.9; TGs (mg/dL): 76.4 ± 38.3 Males with low MM (76): Total LBM (%): 61.2 ± 3.7; FBM (%): 35.5 ± 3.6; WC (cm): 96.3 ± 11.0; high-density lipoprotein (mg/dL): 32.9 ± 8.0; SBP (mmHg): 121.3 ± 11.3; DBP (mmHg): 73.9 ± 7.0; TGs (mg/dL): 128.8 ± 68.9 Females without low MM (214): Total LBM (%): 63.9 ± 5.1; FBM (%): 32.3 ± 5.2; WC (cm): 75.9 ± 8.0; high-density lipoprotein (mg/dL): 43.4 ± 11.1; SBP (mmHg): 106.2 ± 8.2; DBP (mmHg): 66.3 ± 6.4; TGs (mg/dL): 81.9 ± 41.2 Females with low MM (99): Total LBM (%): 51.2 ± 3.4; FBM (%): 44.9 ± 3.4; WC (cm): 92.4 ± 11.0; high-density lipoprotein (mg/dL): 40.3 ± 9.5; SBP (mmHg): 114.3 ± 10.5; DBP (mmHg): 70.3 ± 6.5; TGs (mg/dL): 100.6 ± 57.0 | DXA for total FBM, total lean tissue, and ASM | Low MM is correlated with higher cardiometabolic risk. This was especially true for overweight/obese individuals. |
| [57] Garcia Iniguez et al., 2018 MexicoCross-sectional study (n = 79) | Children with CP: Age: 8y5mo ± 4y5mo; FFM, BIA (kg): 12.3 ± 5.5; FBM, BIA (kg): 4.9 ± 3.2; FFM, anthropometry (kg): 15.2 ± 6.0; FBM, anthropometry (kg): 2.1 ± 6.9; GMFCS levels I and II = 5.1%; GMFCS level III = 2.5%; GMFCS level IV = 17.7%; GMFCS level V = 69.9% Males (n = 41): REE (kcal/d): 911 ± 186; REE (kcal/kg/d): 53.2 ± 9.4; TEE (kcal/d): 1274 ± 261; TEE (kcal/cm/d): 11.3 ± 1.0 Females (n = 38): REE (kcal/d): 867 ± 177; REE (kcal/kg/d): 57.3 ± 13.7; TEE (kcal/d): 1185 ± 229; TEE (kcal/cm/d): 11 ± 1.3 | BIA for FBM and FFM BIA QuadScan equations for estimations of REE and TEE | FFM measures by BIA and anthropometry are directly correlated to REE (kcal/d) and both TEE measurements (kcal/d; kcal/cm/d). FFM measures by BIA and anthropometry are negatively correlated with REE (kcal/kg/d). |
| [58] Summer et al., 2020 United States Retrospective cohort study (n = 1192) | Individuals with DMD (n = 499, 2331 DXA scans) Age, median: 11.0; whole-body LBM (g), median: 20.6Functional mobility status (FMS) level 1: n = 649 scans; FMS level 2: n = 856 scans; FMS level 3: n = 188 scans; FMS level 4: n = 71 scans; FMS level 5: n = 145 scans; FMS level 6: n = 389; FMS level 7: n = 21 scans; FMS level 8: n = 2 scans Control group (n = 693, 3670 DXA scans) Age, median: 13.9; whole-body LBM (g), median: 39.3 | DXA for appendicular LBM National Health and Nutrition Examination Survey | Appendicular LBM and the ALM index measurements from DXA scanning can be used as biomarkers for muscle function to monitor disease progressions. |
| [59] Wittekind et al., 2020 United States Retrospective, cross-sectional study (n = 165) | Typically developing children: Age: 14.4 ± 2.5; male = 79; female = 86; total LBM (kg): 46.6 ± 12.0; LBM of right upper extremity (kg): 2.4 ± 0.9; LBM of left upper extremity (kg): 2.3 ± 0.8; LBM of trunk (kg): 20.5 ± 5.3; LBM of right lower extremity (kg): 7.2 ± 2.1; LBM of left lower extremity (kg): 7.1 ± 2.1; SMM (kg): 25.8 ± 7.3; percent body fat (%): 22.4 ± 9.6; resting exchange ratio, median: 1.24; peak absolute VO2, median (mL/min): 1914 | Bioelectrical impedance for body compositional measurements Cardiopulmonary exercise testing to measure peak oxygen consumption | SMM was a stronger correlate of VO2 and CRF than total body mass. |
| [60] Haapala et al., 2021 FinlandCross-sectional study (n = 35) | Typically developing males and females: Male = 14; female = 21; age: 9.6 ± 3.0; stature (cm): 137.6 ± 9.2; SMM (kg): 14.0 ± 2.9; WC (cm): 61.1 ± 6.4; Hip circumference (cm): 74.1 ± 6.1; SMM (kg): 16.2 ± 2.4 post-pubertal adolescents, 13.1 ± 2.7 prepubertal adolescents | BIA for SMM, FBM, and FFM measures Textile electromyography for muscle activity measures | SMM showed an inverse association with VO2 reserves. |
| [61] Kwarteng et al., 2023 United States Cross-sectional study (n = 1405) | Typically developing prepubertal males (n = 137): Age: 9.7 ± 2.3; FBM (kg): 20.5 ± 13.5; FFM (kg): 27.2 ± 15.8; sBP (mmHg): 108.8 ± 12.0; dBP (mmHg): 61.9 ± 7.1; standardized sBP: 0.5 ± 1.1; standardized dBP: 0.1 ± 0.6 Typically developing prepubertal females (n = 105): Age: 9.3 ± 2.1; FBM (kg): 19.3 ± 9.7; FFM (kg): 27.1 ± 11.4; sBP (mmHg): 110.4 ± 12.3; dBP (mmHg): 63.2 ± 7.4; standardized sBP: 0.8 ± 1.2; standardized dBP: 0.2 ± 0.7 Typically developing early/mid-pubertal males (n = 192): Age: 12.7 ± 2.1; FBM (kg): 25.6 ± 18.5; FFM (kg): 44.6 ± 15.0; sBP (mmHg): 116.1 ± 12.6; dBP (mmHg): 65.0 ± 8.1; standardized sBP: 0.6 ± 1.1; standardized dBP: 0.1 ± 0.7 Typically developing early/mid-pubertal females (n = 212): Age: 11.6 ± 2.2; FBM (kg): 26.9 ± 13.2; FFM (kg): 37.4 ± 12.9; sBP (mmHg): 113.2 ± 12.5; dBP (mmHg): 64.3 ± 7.7; standardized sBP: 0.7 ± 1.2; standardized dBP: 0.1 ± 0.7 Typically developing late-pubertal males (n = 157): Age: 15.6 ± 1.4; FBM (kg): 18.3 ± 16.0; FFM (kg): 58.2 ± 11.4; sBP (mmHg): 121.7 ± 11.8; dBP (mmHg): 65.8 ± 8.0; standardized sBP: 0.5 ± 1.1; standardized dBP: −0.04 ± 0.7 Typically developing late-pubertal females (n = 602): Age: 15.0 ± 1.6; FBM (kg): 30.3 ± 15.2; FFM (kg): 48.6 ± 9.8; sBP (mmHg): 115.8 ± 10.8; dBP (mmHg): 65.2 ± 7.5; standardized sBP: 0.5 ± 1.0; standardized dBP: −0.04 ± 0.7 | DXA for FBM and FFM Automated sphygmomanometer for BP | FFM is positively associated with standardized BP values. This relationship is mediated by pubertal stage for standardized systolic pressures. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, I.R.; Sadowsky, C.L. Muscle Mass as a Biomarker for Health Status and Function in Pediatric Individuals with Neuromuscular Disabilities: A Systematic Review. Children 2024, 11, 815. https://doi.org/10.3390/children11070815
Ferrara IR, Sadowsky CL. Muscle Mass as a Biomarker for Health Status and Function in Pediatric Individuals with Neuromuscular Disabilities: A Systematic Review. Children. 2024; 11(7):815. https://doi.org/10.3390/children11070815
Chicago/Turabian StyleFerrara, Isabella R., and Cristina L. Sadowsky. 2024. "Muscle Mass as a Biomarker for Health Status and Function in Pediatric Individuals with Neuromuscular Disabilities: A Systematic Review" Children 11, no. 7: 815. https://doi.org/10.3390/children11070815
APA StyleFerrara, I. R., & Sadowsky, C. L. (2024). Muscle Mass as a Biomarker for Health Status and Function in Pediatric Individuals with Neuromuscular Disabilities: A Systematic Review. Children, 11(7), 815. https://doi.org/10.3390/children11070815





