Nutrient and Hormonal Effects on Long Bone Growth in Healthy and Obese Children: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
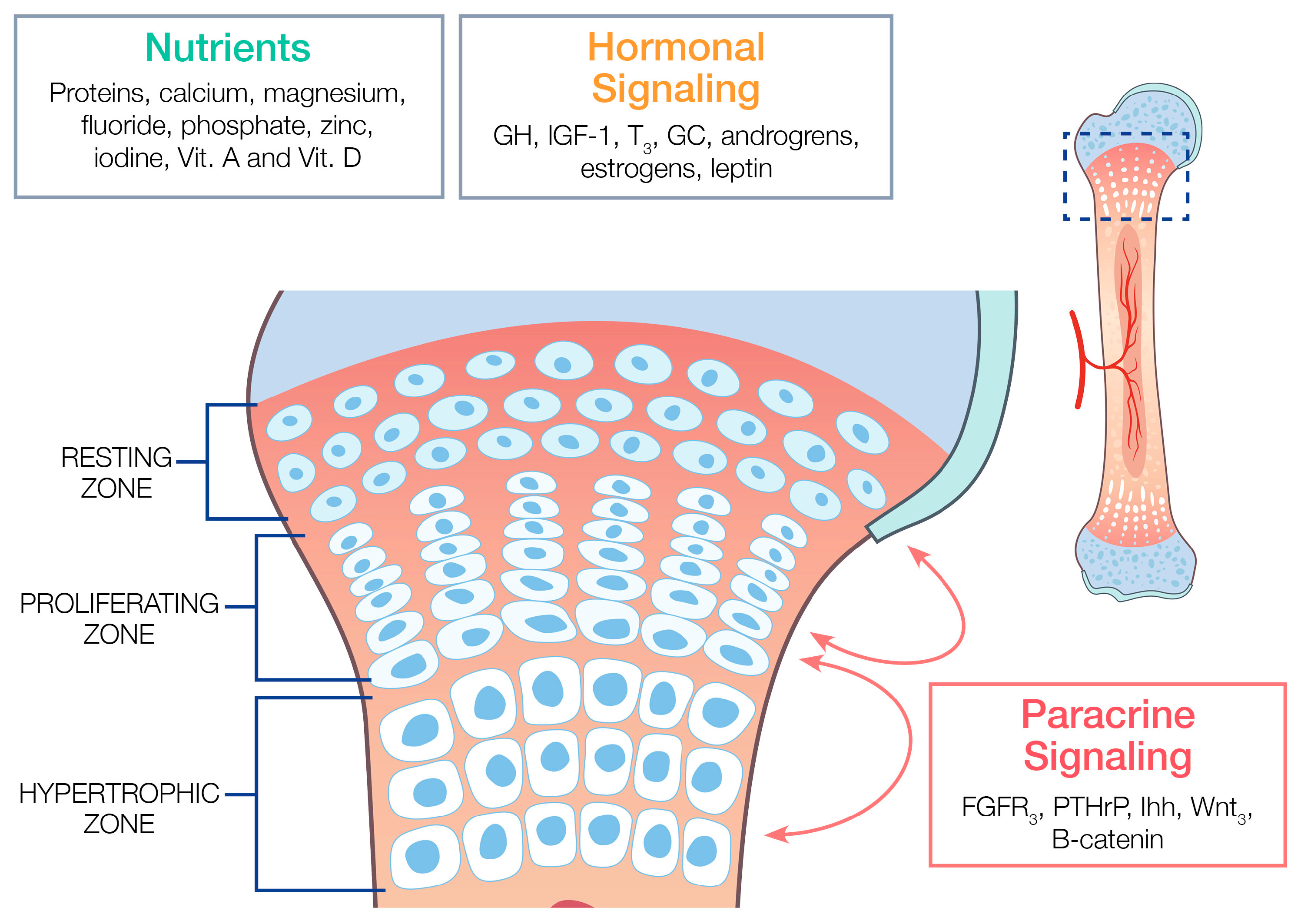
3.1. The Normal Structure and Function of the Epiphyseal Growth Plate
3.2. The Nutritional and Hormonal Regulation of the Epiphyseal Growth Plate under Normal Weight and Obesity Parameters
3.2.1. Major Macronutrients and Micronutrients
Minerals
- Calcium
- Magnesium
- Fluoride
- Phosphate
- Zinc
- Iodine
Vitamins
- Vitamin A
- Vitamin C
- Vitamin D
3.2.2. Major Endocrine Hormones
- Growth Hormone and IGF-1
- Thyroid Hormones
- Glucocorticoids (GCs)
- Androgens and Estrogens
- Leptin
3.2.3. Major Paracrine Factors
- The Ihh-PTHrp Signaling Feedback Loop
- Fibroblast Growth Factors (FGFs)
- Wnt/β-Catenin Pathways
- Vascular Endothelial Growth Factors (VEGFs)
3.3. Clinical Pathology Related to Improper Nutrition and Obesity
3.3.1. Slipped Capital Femoral Epiphysis (SCFE)
3.3.2. Blount’s Disease (Genu Varum, or bowed legs)
3.3.3. Genu Valgum (Knocked Knees)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Price, C.T.; Langford, J.R.; Liporace, F.A. Essential Nutrients for Bone Health and a Review of their Availability in the Average North American Diet. Open Orthop. J. 2012, 6, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Fryar, C.D.; Martin, C.B.; Freedman, D.S.; Carroll, M.D.; Gu, Q.; Hales, C.M. Trends in obesity prevalence by race and hispanic origin—1999–2000 to 2017–2018. JAMA J. Am. Med. Assoc. 2021, 324, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Delmas, P.D. Bone Quality—The Material and Structural Basis of Bone Strength and Fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Abad, V.; Meyers, J.L.; Weise, M.; Gafni, R.I.; Barnes, K.M.; Nilsson, O.L.A.; Bacher, J.D.; Baron, J. The role of the resting zone in growth plate chondrogenesis. Endocrinology 2002, 143, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M.; Davies, P.S.W. Clinical longitudinal standards for height and height velocity for North American children. J. Pediatr. 1985, 107, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Farnum, C.E.; Wilsman, N.J. Morphologic stages of the terminal hypertrophic chondrocyte of growth plate cartilage. Anat. Rec. 1987, 219, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Burdan, F.; Szumiło, J.; Korobowicz, A.; Farooquee, R.; Patel, S.; Patel, A.; Patel, A.; Dave, A.; Szumiło, M.; Solecki, M.; et al. Morphology and physiology of the epiphyseal growth plate. Folia Histochem. Cytobiol. 2009, 47, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; da Sasso, G.R.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Gutin, B.; Stallmann-Jorgensen, I.; Le, A.; Johnson, M.; Dong, Y. Relations of Diet and Physical Activity to Bone Mass and Height in Black and White Adolescents. Pediatr Rep. 2011, 3, e10. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Krebs, N.F.; Baker, R.D. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics 2006, 117, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Lui, J.C. Nutritional Regulation of the Growth Plate. The Biology of the First 1000 Days; CRC Press: Boca Raton, FL, USA, 2017; pp. 237–252. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; De Sanctis, V.; Elalaily, R.; Bedair, S. Advances in pubertal growth and factors influencing it: Can we increase pubertal growth? Indian J. Endocrinol. Metab. 2014, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; De Sanctis, V.; Elalaily, R. Nutrition and pubertal development. Indian J. Endocrinol. Metab. 2014, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Hassan, A.E.H.I.; Aref, M.K.; Hintz, R.L.; Rosenfeld, R.G.; Rogol, A.D. Serum Insulin-Like Growth Factors I and II Concentrations and Growth Hormone and Insulin Responses to Arginine Infusion in Children with Protein-Energy Malnutrition before and after Nutritional Rehabilitation. Pediatr. Res. 1986, 20, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Nutrition, infection and stunting: The roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev. 2017, 30, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; Yackobovitch-Gavan, M.; Phillip, M. Which dietary components modulate longitudinal growth? Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 211–216. [Google Scholar] [CrossRef]
- Van Vught, A.J.; Heitmann, B.L.; Nieuwenhuizen, A.G.; Veldhorst, M.A.; Andersen, L.B.; Hasselstrom, H.; Brummer, R.-J.M.; Westerterp-Plantenga, M.S. Association between intake of dietary protein and 3-year-change in body growth among normal and overweight 6-year-old boys and girls (CoSCIS). Public Health Nutr. 2010, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Jansen, E.C. Nutritional Determinants of the Timing of Puberty. Annu. Rev. Public Health 2016, 37, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Wallach, S. Effects of magnesium on skeletal metabolism. Magnes Trace Elem. 1990, 9, 1–14. [Google Scholar]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients. 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.J.B.; Murray, S.S.; Grisanti, M.; Duarte, M.E.L.; Urist, M.R. Effect of low dietary calcium on bone metabolism in the SENCAR mouse. J. Orthop. Res. 1997, 15, 585–592. [Google Scholar] [CrossRef]
- Schrager, S. Dietary Calcium Intake and Obesity. J. Am. Board Fam. Med. 2005, 18, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Chen, Z.; Hawthorne, K.M. Magnesium metabolism in 4-year-old to 8-year-old children. J. Bone Min. Res. 2014, 29, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.H.W.; Baylink, D.J. Molecular mechanism of action of fluoride on bone cells. J. Bone Min. Res. 1998, 13, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium Deficiency: Effect on Bone and Mineral Metabolism in the Mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Whitford, G.M. Fluoride metabolism and excretion in children. J. Public Health Dent. 1999, 59, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Penido, M.G.M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Yesİldag, A.; Heybeli, N.; Candır, O.; Oyar, O.; Baykal, B.; Mumcu, E.F.; Gulsoy, U.K. Effects of fluoride on growth plate cartilage in rats: Radiological and histopathological findings. Flouride 2004, 37, 221–230. [Google Scholar]
- Gao, Y.; Gui, F.; Li, D.; Zhang, R.; Sun, Q.; Guo, X. Fluoride regulates the expression of extracellular matrix HSPG and related signaling pathways FGFR3 and Ihh/PTHrP feedback loop during endochondral ossification. Env. Toxicol. Pharmacol. 2020, 73, 103275. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, M.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Li, P.; Zhao, Q.; Dong, L.; Yu, X.; et al. Low-to-moderate fluoride exposure in relation to overweight and obesity among school-age children in China. Ecotoxicol. Environ. Saf. 2019, 183, 109558. [Google Scholar] [CrossRef]
- MacDonald, R.S. The Role of Zinc in Growth and Cell Proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Al Jurayyan, N.A.M.; Mohamed, S.; Al Issa, S.D.A.; Al Jurayyan, A.N.A. Rickets and osteomalacia in Saudi children and adolescents attending endocrine clinic, Riyadh, Saudi Arabia. Sudan J. Paediatr. 2012, 12, 56–63. [Google Scholar] [PubMed]
- Golden, N.H.; Abrams, S.A.; Daniels, S.R.; Abrams, S.A.; Corkins, M.R.; de Ferranti, S.D.; Golden, N.H.; Magge, S.N.; Schwarzenberg, S.J. Optimizing Bone Health in Children and Adolescents. Pediatrics 2014, 134, e1229-43. [Google Scholar] [CrossRef]
- Bosman, A.; Campos-Obando, N.; Medina-Gomez, C.; Voortman, T.; Uitterlinden, A.G.; Zillikens, M.C. Serum Phosphate, BMI, and Body Composition of Middle-Aged and Older Adults: A Cross-Sectional Association Analysis and Bidirectional Mendelian Randomization Study. J. Nutr. 2022, 152, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yan, G.; Guan, M. Zinc Homeostasis in Bone: Zinc Transporters and Bone Diseases. Int. J. Mol. Sci. 2020, 21, 1236. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc deficiency: Its characterization and treatment. Met. Ions Biol. Syst. 2004, 41, 103–137. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Rossi, L.; Migliaccio, S.; Corsi, A.; Marzia, M.; Bianco, P.; Teti, A.; Gambelli, L.; Cianfarani, S.; Paoletti, F.; Branca, F. Reduced Growth and Skeletal Changes in Zinc-Deficient Growing Rats Are Due to Impaired Growth Plate Activity and Inanition. J. Nutr. 2001, 131, 1142–1146. [Google Scholar] [CrossRef]
- Kurtogu, S.; Patiroglu, T.E.; Karakas, S.E. Effect of growth hormone on epiphyseal growth plates in zinc deficiency. Tokai J. Exp. Clin. Med. 1987, 12, 325–329. [Google Scholar] [PubMed]
- Khorsandi, H.; Nikpayam, O.; Yousefi, R.; Parandoosh, M.; Hosseinzadeh, N.; Saidpour, A.; Ghorbani, A. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: A randomized, placebo-controlled, double-blind trial. Diabetol. Metab. Syndr. 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Bao, S.; Bolin, E.R.; Burris, D.L.; Xu, X.; Sun, Q.; Killilea, D.W.; Shen, Q.; Ziouzenkova, O.; Belury, M.A.; et al. Zinc Deficiency Augments Leptin Production and Exacerbates Macrophage Infiltration into Adipose Tissue in Mice Fed a High-Fat Diet1–3. J. Nutr. 2013, 143, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.A.; Naeem, Z.; Alshahat, A.A. Growth Plate Changes Associated with Hypothyroidism amongst the Pre and Postnatal Rats. Int. J. Health Sci. 2013, 7, 31–43. [Google Scholar] [CrossRef]
- Herlihy, J.T.; Stacy, C.; Bertrand, H.A. Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech. Ageing Dev. 1990, 53, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Di Mauro, M.; Paola, G.; Olivieri, A.; Vermiglio, F. Nutritional iodine status and obesity. Thyroid. Res. 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Conaway, H.H.; Henning, P.; Lerner, U.H. Vitamin A Metabolism, Action, and Role in Skeletal Homeostasis. Endocr. Rev. 2013, 34, 766–797. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Uyeda, J.A.; Mericq, V.; Mancilla, E.E.; Yanovski, J.A.; Barnes, K.M.; Zile, M.H.; Baron, J. Retinoic Acid Is a Potent Regulator of Growth Plate Chondrogenesis. Endocrinology 2000, 141, 346–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García, O.P. Effect of vitamin A deficiency on the immune response in obesity. Proc. Nutr. Soc. 2012, 71, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Liakakos, D.; Vlachos, P.; Doulas, N.L.; Litsios, B.; Alexiou, D. Effect of Ascorbic Acid (Vitamin C) on the Epiphyseal Plate of Young Guinea Pigs Receiving Prednisolone. Dev. Pharmacol. Ther. 1981, 2, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Amizuka, N.; Sasaki, T.; Aarts, M.M.; Ozawa, H.; Goltzman, D.; Henderson, J.E.; White, J.H. 1α,25-Dihydroxyvitamin D3 Promotes Vascularization of the Chondro-osseous Junction by Stimulating Expression of Vascular Endothelial Growth Factor and Matrix Metalloproteinase 9. J. Bone Miner. Res. 2002, 17, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.; Leung, K.-C.; Ross, R.J.M.; Iismaa, T.P.; Ho, K.K.Y. Distribution and Abundance of Messenger Ribonucleic Acid for Growth Hormone Receptor Isoforms in Human Tissues1. J. Clin. Endocrinol. Metab. 2000, 85, 2865–2871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dupont, J.; Holzenberger, M. Biology of insulin-like growth factors in development. Birth Defects Res. C Embryo Today 2003, 69, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Mehls, O.; Himmele, R.; Hömme, M.; Kiepe, D.; Klaus, G. The interaction of glucocorticoids with the growth hormone-insulin-like growth factor axis and its effects on growth plate chondrocytes and bone cells. J. Pediatr. Endocrinol. Metab. 2001, 14 (Suppl. 6), 1475–1482. [Google Scholar] [PubMed]
- Siebler, T.; Robson, H.; Shalet, S.M.; Williams, G.R. Glucocorticoids, Thyroid Hormone and Growth Hormone Interactions: Implications for the Growth Plate. Horm. Res. Paediatr. 2001, 56, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Scacchi, M.; Pincelli, A.; Cavagnini, F. Growth hormone in obesity. Int. J. Obes. 1999, 23, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kopp, P. Human Genome and Diseases: Review—The TSH receptor and its role in thyroid disease. Cell. Mol. Life Sci. 2001, 58, 1301–1322. [Google Scholar] [CrossRef] [PubMed]
- Ballock, R.T.; Reddi, A.H. Thyroxine is the serum factor that regulates morphogenesis of columnar cartilage from isolated chondrocytes in chemically defined medium. J. Cell Biol. 1994, 126, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Ballock, R.T.; Zhou, X.; Mink, L.M.; Chen, D.H.C.; Mita, B.C.; Stewart, M.C. Expression of Cyclin-Dependent Kinase Inhibitors in Epiphyseal Chondrocytes Induced to Terminally Differentiate with Thyroid Hormone. Endocrinology 2000, 141, 4552–4557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishikawa, Y.; Genge, B.R.; Wuthier, R.E.; Wu, L.N.Y. Thyroid Hormone Inhibits Growth and Stimulates Terminal Differentiation of Epiphyseal Growth Plate Chondrocytes. J. Bone Miner. Res. 1998, 13, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Moez Ali, B.A.; Mahrous, D.M. Thyroid Function Status in Obese Children. J. Diabetes Metab. 2016, 7, 4. [Google Scholar] [CrossRef]
- Reinehr, T. Thyroid hormones before and after weight loss in obesity. Arch. Dis. Child 2002, 87, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Witbreuk, M.; van Kemenade, F.J.; van der Sluijs, J.A.; Jansma, E.P.; Rotteveel, J.; van Royen, B.J. Slipped capital femoral epiphysis and its association with endocrine, metabolic and chronic diseases: A systematic review of the literature. J. Child Orthop. 2013, 7, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Van der Eerden, B.C.J.; Karperien, M.; Wit, J.M. Systemic and Local Regulation of the Growth Plate. Endocr. Rev. 2003, 24, 782–801. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Sävendahl, L. Promoting Growth in Chronic Inflammatory Disease: Lessons from Studies of the Growth Plate. Horm. Res. Paediatr. 2009, 72, 42–47. [Google Scholar] [CrossRef]
- Chu, L.; Sheng, K.; Liu, P.; Ye, K.; Wang, Y.; Li, C.; Kang, X. Increased Cortisol and Cortisone Levels in Overweight Children. Med. Sci. Monit. Basic Res. 2017, 23, 25–30. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Noppe, G.; Jongejan, M.H.; Kok, C.B.; Mekic, S.; Koper, J.W.; van Rossum, E.F.; van den Akker, E.L. Increased Scalp Hair Cortisol Concentrations in Obese Children. J. Clin. Endocrinol. Metab. 2014, 99, 285–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wongdee, K.; Krishnamra, N.; Charoenphandhu, N. Endochondral bone growth, bone calcium accretion, and bone mineral density: How are they related? J. Physiol. Sci. 2012, 62, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Keenan, B.S.; Richards, G.E.; Ponder, S.W.; Dallas, J.S.; Nagamani, M.; Smith, E.R. Androgen-stimulated pubertal growth: The effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J. Clin. Endocrinol. Metab. 1993, 76, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Oz, O.; Millsaps, R.; Welch, R.; Birch, J.; Zerwekh, J. Expression of aromatase in the human growth plate. J. Mol. Endocrinol. 2001, 27, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Norjavaara, E.; Ankarberg, C.; Albertsson-Wikland, K. Diurnal rhythm of 17 beta-estradiol secretion throughout pubertal development in healthy girls: Evaluation by a sensitive radioimmunoassay. J. Clin. Endocrinol. Metab. 1996, 81, 4095–4102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juul, A. The effects of oestrogens on linear bone growth. Hum. Reprod. Update 2001, 7, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Shalitin, S.; Kiess, W. Putative Effects of Obesity on Linear Growth and Puberty. Horm. Res. Paediatr. 2017, 88, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; Shtaif, B.; Phillip, M. Leptin Stimulates Parathyroid Hormone Related Peptide Expression in the Endochondral Growth Plate. J. Pediatr. Endocrinol. Metab. 2007, 20, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Even-Zohar, N.; Jacob, J.; Amariglio, N.; Rechavi, G.; Potievsky, O.; Phillip, M.; Gat-Yablonski, G. Nutrition-induced catch-up growth increases hypoxia inducible factor 1α RNA levels in the growth plate. Bone 2008, 42, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Halverson, S.J.; Warhoover, T.; Mencio, G.A.; Lovejoy, S.A.; Martus, J.E.; Schoenecker, J.G. Leptin Elevation as a Risk Factor for Slipped Capital Femoral Epiphysis Independent of Obesity Status. J. Bone Jt. Surgery 2017, 99, 865–872. [Google Scholar] [CrossRef]
- Izquierdo-Lahuerta, A. The Parathyroid Hormone-Related Protein/Parathyroid Hormone 1 Receptor Axis in Adipose Tissue. Biomolecules 2021, 11, 1570. [Google Scholar] [CrossRef] [PubMed]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.E.; Hegde, A.; Andrade, A.C.; Nilsson, O.; Baron, J. Fibroblast growth factor expression in the postnatal growth plate. Bone 2007, 40, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.L.; Smith, C.; Partanen, J.; Ornitz, D.M. Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev. Biol. 2006, 296, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wynshaw-Boris, A.; Zhou, F.; Kuo, A.; Leder, P. Fibroblast Growth Factor Receptor 3 Is a Negative Regulator of Bone Growth. Cell 1996, 84, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Ornitz, D.; Werner, S.; Williams, L. Unique Expression Pattern of the FGF Receptor 3 Gene during Mouse Organogenesis. Dev. Biol. 1993, 155, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lavine, K.J.; Hung, I.H.; Ornitz, D.M. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev. Biol. 2007, 302, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. A Ser365→Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum. Mol. Genet. 2001, 10, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, B.G.; Johansson, J.M.; Jennische, E.; Jernås, M.; Itoh, Y.; Peltonen, M.; Olbers, T.; Lönn, L.; Lönroth, H.; Sjöström, L.; et al. Depot-Specific Expression of Fibroblast Growth Factors in Human Adipose Tissue. Obes. Res. 2002, 10, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, X. Wnt/β-catenin signaling: New (and old) players and new insights. Curr. Opin. Cell Biol. 2008, 20, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, Y.; Otani, T.; Kanatani, N.; Koyama, E.; Kitagaki, J.; Komori, T.; Yamada, Y.; Costantini, F.; Wakisaka, S.; Pacifici, M.; et al. Developmental Regulation of Wnt/β-Catenin Signals Is Required for Growth Plate Assembly, Cartilage Integrity, and Endochondral Ossification. J. Biol. Chem. 2005, 280, 19185–19195. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; de Guzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.; Bord, S.; Kelsall, A.W.; Coleman, N.; Compston, J.E. Tie2 ligands angiopoietin-1 and angiopoietin-2 are coexpressed with vascular endothelial cell growth factor in growing human bone. Bone 2001, 28, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Haigh, J.J.; Gerber, H.-P.; Ferrara, N.; Wagner, E.F. Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development 2000, 127, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.E.; Basha, W.; Yousef, R.N.; Awad, M. Serum Vascular Endothelial Growth Factor in Egyptian Obese Women with Insulin Resistance. Open Access Maced. J. Med. Sci. 2019, 7, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Manoff, E.M.; Banffy, M.B.; Winell, J.J. Relationship Between Body Mass Index and Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2005, 25, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.C.; Metcalfe, D.; Lane, S.; Turner, S. Childhood Obesity and Slipped Capital Femoral Epiphysis. Pediatrics 2018, 142, e20181067. [Google Scholar] [CrossRef] [PubMed]
- Kgoedi, M.; Rischbieter, P.; Goller, R. Body mass index and Blount’s disease: A single academic hospital experience. SA Orthop. J. 2019, 18, 15–20. [Google Scholar] [CrossRef]
- Sabharwal, S.; Zhao, C.; McClemens, E. Correlation of Body Mass Index and Radiographic Deformities in Children with Blount Disease. J. Bone Jt. Surg. 2007, 89, 1275–1283. [Google Scholar] [CrossRef]
- Blasier, R.D. Tachdjian’s Pediatric Orthopaedics, 4th Edition. J. Bone Jt. Surg. 2008, 28, 891. [Google Scholar]
- Walker, J.L.; Hosseinzadeh, P.; White, H.; Murr, K.; Milbrandt, T.A.; Talwalkar, V.J.; Iwinski, H.; Muchow, R. Idiopathic Genu Valgum and Its Association with Obesity in Children and Adolescents. J. Pediatr. Orthop. 2019, 39, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, A.Y.; Heyworth, B.E.; Zurakowski, D.; Kocher, M.S. A Reduction in Body Mass Index Lowers Risk for Bilateral Slipped Capital Femoral Epiphysis. Clin. Orthop. Relat. Res. 2013, 471, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Resnik, A.E.; Beavers, D.P.; Mihalko, S.L.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Intentional Weight Loss in Overweight and Obese Patients with Knee Osteoarthritis: Is More Better? Arthritis Care Res. 2018, 70, 1569–1575. [Google Scholar] [CrossRef] [PubMed]

| Current DRIs and RDAs | Ages (Years) | Male | Female |
|---|---|---|---|
| Calories (Kcal/day) | 1–3 | 1000 | 1000 |
| 4–8 | 1200 | 1400–1600 | |
| 9–13 | 1600 | 1800 | |
| 14–18 | 1800 | 2200–3200 | |
| Proteins (grams/day) | 1–3 | 13 | 13 |
| 4–8 | 19 | 19 | |
| 9–13 | 34 | 34 | |
| 14–18 | 46 | 52 |
| Current RDAs | Ages | Requirements |
|---|---|---|
| Calcium mg/day | 0–6 months | 200 |
| 7–12 months | 260 | |
| 1–3 years | 700 | |
| 4–8 years | 1000 | |
| 9–18 years | 1300 | |
| Magnesium mg/day | 0–6 months | 30 |
| 7–12 months | 75 | |
| 1–3 years | 80 | |
| 4–8 years | 130 | |
| 9–13 years | 240 | |
| 14–18 years | Male: 410 Female: 360 | |
| Fluoride mg/day | 0–6 months | 0.01 |
| 7–12 months | 0.5 | |
| 1–3 years | 0.7 | |
| 4–8 years | 1 | |
| 9–13 years | 2 | |
| 14–18 years | 3 | |
| Phosphate mg/day | 0–6 months | 100 |
| 7–12 months | 275 | |
| 1–3 years | 460 | |
| 4–8 years | 500 | |
| 9–18 years | 1250 | |
| Zinc mg/day | 0–6 months | 2 |
| 7–12 months | 3 | |
| 1–3 years | 3 | |
| 4–8 years | 5 | |
| 9–13 years | 8 | |
| 14–18 years | Male: 11 Female: 9 | |
| Iodine mcg/day | 0–6 months | 110 |
| 7–12 months | 130 | |
| 1–8 years | 90 | |
| 9–13 years | 120 | |
| 14–18 years | 150 |
| Current RAEs and RDAs | Ages | Requirement |
|---|---|---|
| Vitamin A mcg/day (RAE) | 0–6 months | 400 |
| 7–12 months | 500 | |
| 1–3 years | 300 | |
| 4–8 years | 400 | |
| 9–13 years | 600 | |
| 14–18 years | 900 | |
| Vitamin C mg/day | 0–6 months | 40 |
| 7–12 months | 50 | |
| 1–3 years | 15 | |
| 4–8 years | 25 | |
| 9–13 years | 45 | |
| 14–18 years | M: 75 F: 65 | |
| Vitamin D IU/day | 0–12 months | 400 |
| 1–18 years | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, S.; Naseer, S.; Zamzam, M.; Mohilldean, H.; Van Wagoner, C.; Hasan, A.; Saleh, E.S.; Uhley, V.; Kamel-ElSayed, S. Nutrient and Hormonal Effects on Long Bone Growth in Healthy and Obese Children: A Literature Review. Children 2024, 11, 817. https://doi.org/10.3390/children11070817
Hasan S, Naseer S, Zamzam M, Mohilldean H, Van Wagoner C, Hasan A, Saleh ES, Uhley V, Kamel-ElSayed S. Nutrient and Hormonal Effects on Long Bone Growth in Healthy and Obese Children: A Literature Review. Children. 2024; 11(7):817. https://doi.org/10.3390/children11070817
Chicago/Turabian StyleHasan, Sazid, Shahrukh Naseer, Mazen Zamzam, Hashem Mohilldean, Colin Van Wagoner, Ahmad Hasan, Ehab S. Saleh, Virginia Uhley, and Suzan Kamel-ElSayed. 2024. "Nutrient and Hormonal Effects on Long Bone Growth in Healthy and Obese Children: A Literature Review" Children 11, no. 7: 817. https://doi.org/10.3390/children11070817
APA StyleHasan, S., Naseer, S., Zamzam, M., Mohilldean, H., Van Wagoner, C., Hasan, A., Saleh, E. S., Uhley, V., & Kamel-ElSayed, S. (2024). Nutrient and Hormonal Effects on Long Bone Growth in Healthy and Obese Children: A Literature Review. Children, 11(7), 817. https://doi.org/10.3390/children11070817






