Sclerostin and Wnt Signaling in Idiopathic Juvenile Osteoporosis Using High-Resolution Confocal Microscopy for Three-Dimensional Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Biochemistry and Histomorphometry
2.2. Bone Immunohistochemistry (IHC) and Quantification of Osteocytic Sclerostin
2.3. Immunofluorescence Microscopy Analyses
2.4. Statistical Analysis
3. Results
3.1. Biochemical Characteristics and Parameters of Bone Histomorphometry
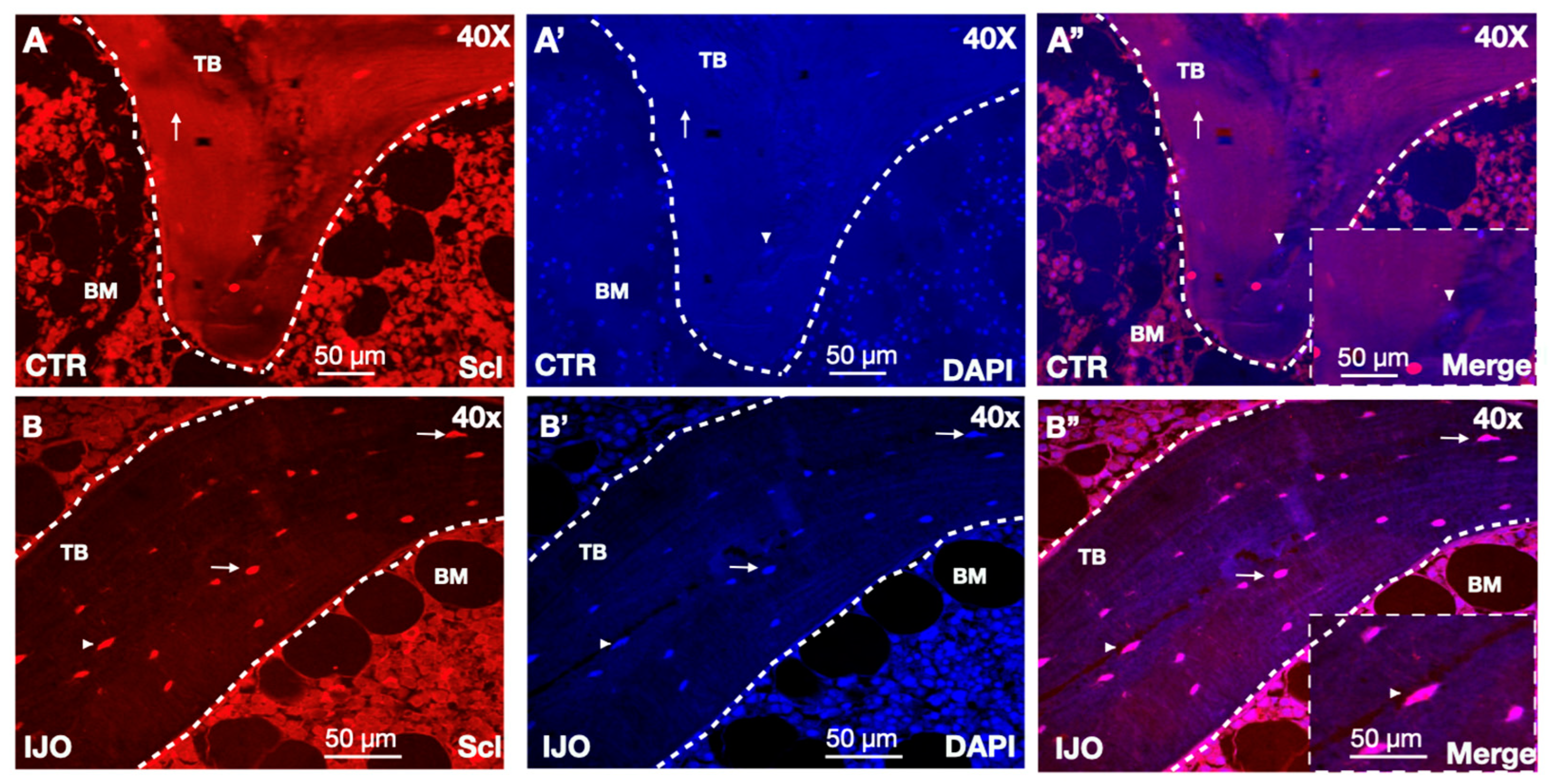
3.2. Sclerostin Bone Staining
3.3. Sclerostin Secretion from Osteocyte Dendrite Vesicles
3.4. Sclerostin Associations with Biochemical Parameters of Bone Histomorphometry
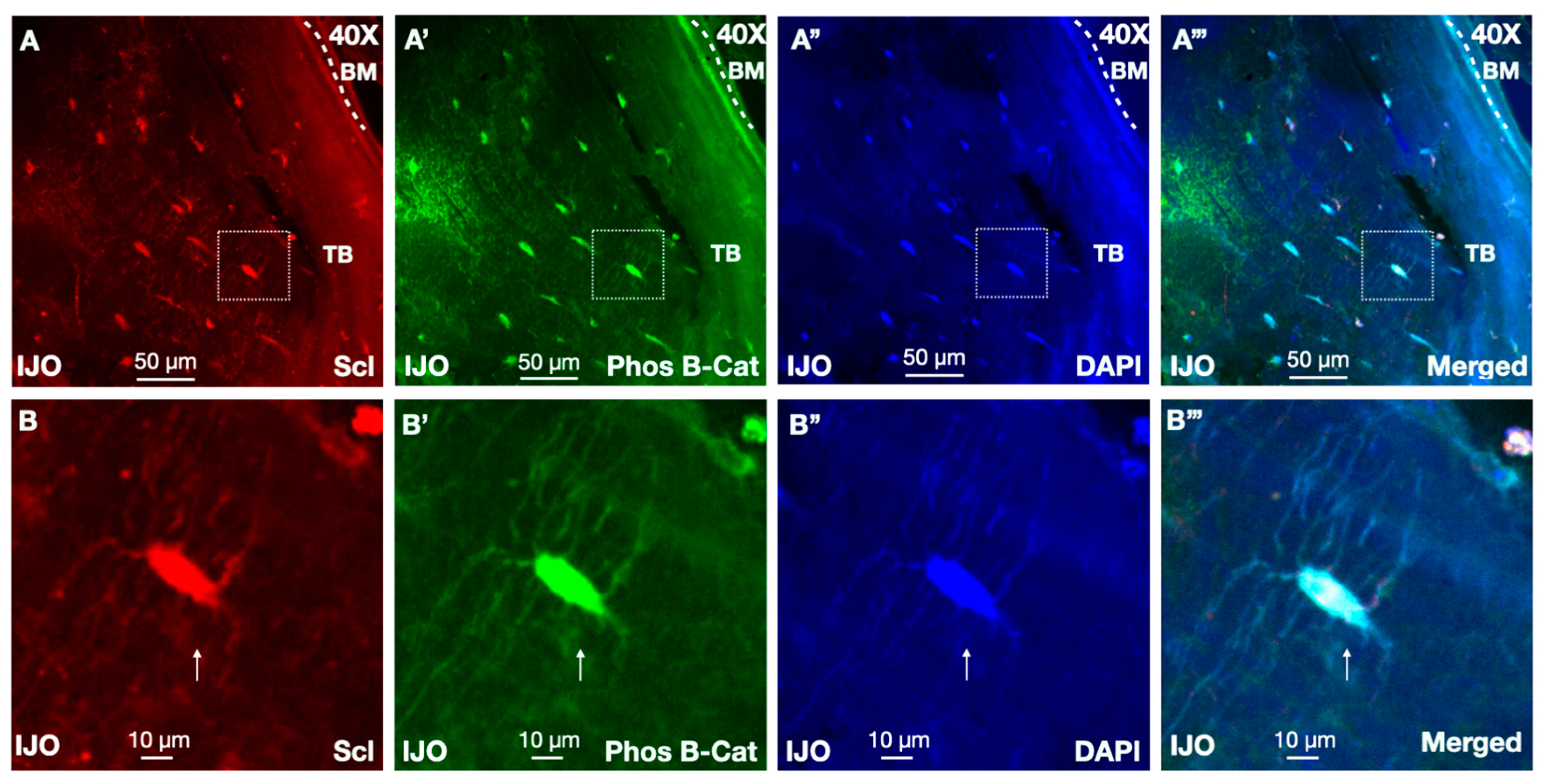
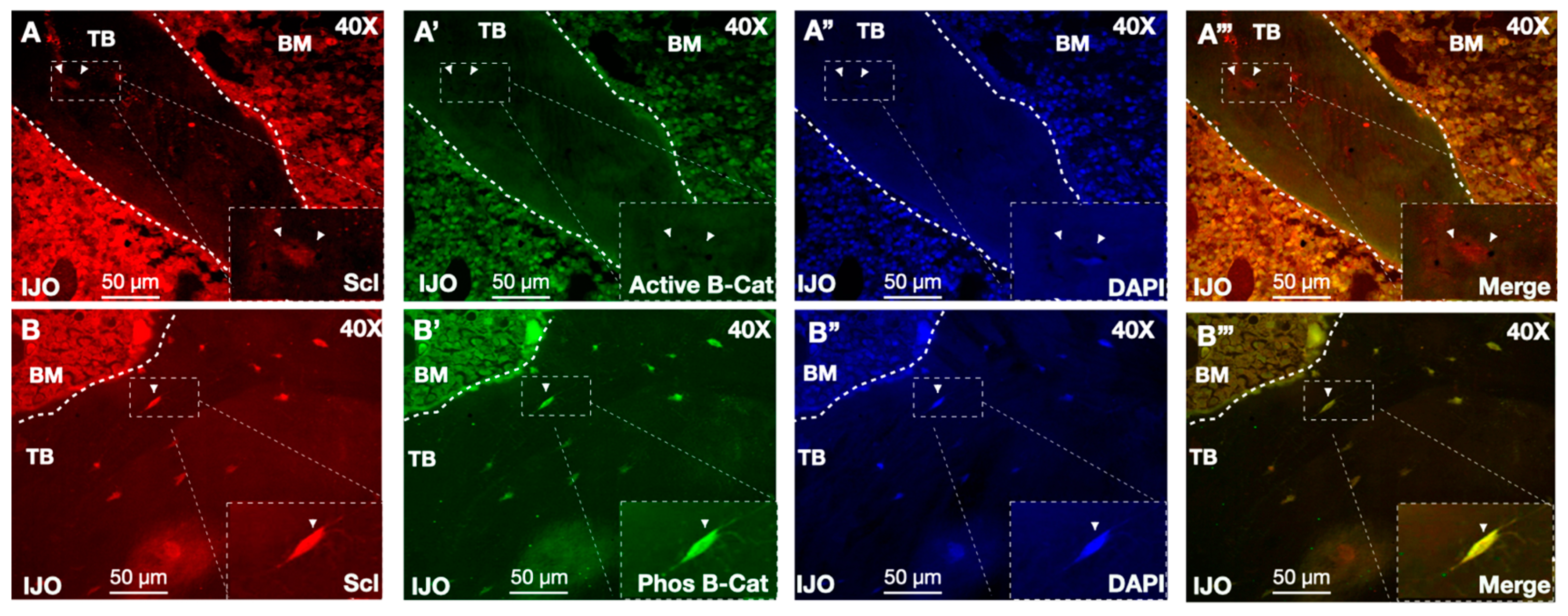
3.5. Sclerostin and Wnt Signaling
4. Discussion
4.1. Sclerostin Secretion from Osteocytes
4.2. Bone Biopsies Provide a Rich Resource to Elucidate Mechanisms of Disease
4.3. Future Applications of Imaging in Osteocyte-Secreted Factors of Native Environments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Increased mortality in patients with a hip fracture-effect of pre-morbid conditions and post-fracture complications. Osteoporos. Int. 2007, 18, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Estell, E.G.; Rosen, C.J. Emerging insights into the comparative effectiveness of anabolic therapies for osteoporosis. Nat. Rev. Endocrinol. 2021, 17, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Min. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Rauch, F.; Glorieux, F.H. Osteogenesis imperfecta. Lancet 2004, 363, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Travers, R.; Norman, M.E.; Taylor, A.; Parfitt, A.M.; Glorieux, F.H. The bone formation defect in idiopathic juvenile osteoporosis is surface-specific. Bone 2002, 31, 85–89. [Google Scholar] [CrossRef]
- Ward, L.M.; Glorieux, F.H. CHAPTER 17—The Spectrum of Pediatric Osteoporosis. In Pediatric Bone; Glorieux, F.H., Pettifor, J.M., Jüppner, H., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 401–442. ISBN 978-0-12-286551-0. [Google Scholar]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef]
- Bacchetta, J.; Wesseling-Perry, K.; Gilsanz, V.; Gales, B.; Pereira, R.C.; Salusky, I.B. Idiopathic juvenile osteoporosis: A cross-sectional single-centre experience with bone histomorphometry and quantitative computed tomography. Pediatr. Rheumatol. Online J. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Travers, R.; Norman, M.E.; Taylor, A.; Parfitt, A.M.; Glorieux, F.H. Deficient bone formation in idiopathic juvenile osteoporosis: A histomorphometric study of cancellous iliac bone. J. Bone Min. Res. 2000, 15, 957–963. [Google Scholar] [CrossRef]
- Mäkitie, R.E.; Costantini, A.; Kämpe, A.; Alm, J.J.; Mäkitie, O. New Insights Into Monogenic Causes of Osteoporosis. Front Endocrinol 2019, 10, 70. [Google Scholar] [CrossRef]
- Bachrach, L.K.; Ward, L.M. Clinical review 1: Bisphosphonate use in childhood osteoporosis. J. Clin. Endocrinol. Metab. 2009, 94, 400–409. [Google Scholar] [CrossRef]
- Tsoumpra, M.K.; Muniz, J.R.; Barnett, B.L.; Kwaasi, A.A.; Pilka, E.S.; Kavanagh, K.L.; Evdokimov, A.; Walter, R.L.; Von Delft, F.; Ebetino, F.H.; et al. The inhibition of human farnesyl pyrophosphate synthase by nitrogen-containing bisphosphonates. Elucidating the role of active site threonine 201 and tyrosine 204 residues using enzyme mutants. Bone 2015, 81, 478–486. [Google Scholar] [CrossRef]
- Filleul, O.; Crompot, E.; Saussez, S. Bisphosphonate-induced osteonecrosis of the jaw: A review of 2,400 patient cases. J. Cancer Res. Clin. Oncol. 2010, 136, 1117–1124. [Google Scholar] [CrossRef]
- Drake, M.T.; Khosla, S. Hormonal and Systemic Regulation of Sclerostin. Bone 2017, 96, 8–17. [Google Scholar] [CrossRef]
- Poole, K.E.S.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Löwik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef]
- Mirza, F.S.; Padhi, I.D.; Raisz, L.G.; Lorenzo, J.A. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J. Clin. Endocrinol. Metab. 2010, 95, 1991–1997. [Google Scholar] [CrossRef]
- Mödder, U.I.; Hoey, K.A.; Amin, S.; McCready, L.K.; Achenbach, S.J.; Riggs, B.L.; Melton, L.J.; Khosla, S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J. Bone Min. Res. 2011, 26, 373–379. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- McClung, M.R.; Grauer, A.; Boonen, S.; Bolognese, M.A.; Brown, J.P.; Diez-Perez, A.; Langdahl, B.L.; Reginster, J.-Y.; Zanchetta, J.R.; Wasserman, S.M.; et al. Romosozumab in Postmenopausal Women with Low Bone Mineral Density. N. Engl. J. Med. 2014, 370, 412–420. [Google Scholar] [CrossRef]
- Saito, T.; Mizobuchi, M.; Kato, T.; Suzuki, T.; Fujiwara, Y.; Kanamori, N.; Makuuchi, M.; Honda, H. One-Year Romosozumab Treatment Followed by One-Year Denosumab Treatment for Osteoporosis in Patients on Hemodialysis: An Observational Study. Calcif. Tissue Int. 2023, 112, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Staehling-Hampton, K.; Proll, S.; Paeper, B.W.; Zhao, L.; Charmley, P.; Brown, A.; Gardner, J.C.; Galas, D.; Schatzman, R.C.; Beighton, P.; et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am. J. Med. Genet. 2002, 110, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Brunkow, M.E.; Gardner, J.C.; Van Ness, J.; Paeper, B.W.; Kovacevich, B.R.; Proll, S.; Skonier, J.E.; Zhao, L.; Sabo, P.J.; Fu, Y.; et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 2001, 68, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ominsky, M.S.; Niu, Q.-T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Min. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef]
- Semënov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Koide, M.; Yamashita, T.; Murakami, K.; Uehara, S.; Nakamura, K.; Nakamura, M.; Matsushita, M.; Ara, T.; Yasuda, H.; Penninger, J.M.; et al. Sclerostin expression in trabecular bone is downregulated by osteoclasts. Sci. Rep. 2020, 10, 13751. [Google Scholar] [CrossRef]
- Boltenstål, H.; Qureshi, A.R.; Behets, G.J.; Lindholm, B.; Stenvinkel, P.; D’Haese, P.C.; Haarhaus, M. Association of Serum Sclerostin with Bone Sclerostin in Chronic Kidney Disease is Lost in Glucocorticoid Treated Patients. Calcif. Tissue Int. 2019, 104, 214–223. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Graciolli, F.G.; O’Brien, S.; Tang, W.; dos Reis, L.M.; Ryan, S.; Phillips, L.; Boulanger, J.; Song, W.; Bracken, C.; et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J. Bone Miner. Res. 2012, 27, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Wesseling-Perry, K.; Mäkitie, R.E.; Välimäki, V.-V.; Laine, T.; Laine, C.M.; Välimäki, M.J.; Pereira, R.C.; Mäkitie, O. Osteocyte Protein Expression Is Altered in Low-Turnover Osteoporosis Caused by Mutations in WNT1 and PLS3. J. Clin. Endocrinol. Metab. 2017, 102, 2340–2348. [Google Scholar] [CrossRef]
- Gordon, C.M.; Leonard, M.B.; Zemel, B.S. International Society for Clinical Densitometry 2013 Pediatric Position Development Conference: Executive summary and reflections. J Clin Densitom 2014, 17, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Min. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Pereira, R.C.; Juppner, H.; Azucena-Serrano, C.E.; Yadin, O.; Salusky, I.B.; Wesseling-Perry, K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 2009, 45, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Salusky, I.B.; Roschger, P.; Klaushofer, K.; Yadin, O.; Freymiller, E.G.; Bowen, R.; Delany, A.M.; Fratzl-Zelman, N.; Wesseling-Perry, K. Impaired osteocyte maturation in the pathogenesis of renal osteodystrophy. Kidney Int. 2018, 94, 1002–1012. [Google Scholar] [CrossRef]
- Albrecht, L.V.; Tejeda-Muñoz, N.; De Robertis, E.M. Cell Biology of Canonical Wnt Signaling. Annu. Rev. Cell Dev. Biol. 2021, 37, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Min. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Elsalam, M.A.; El-Abden, M.Z.; Mahmoud, E.; Zahab, Z.A.; Ahmed, H. Correlation between serum sclerostin level and bone density status in children on regular hemodialysis. Saudi J. Kidney Dis. Transpl. 2019, 30, 1022–1031. [Google Scholar] [CrossRef]
- Stanik, J.; Kratzsch, J.; Landgraf, K.; Vogel, M.; Thiery, J.; Kiess, W.; Körner, A. The Bone Markers Sclerostin, Osteoprotegerin, and Bone-Specific Alkaline Phosphatase Are Related to Insulin Resistance in Children and Adolescents, Independent of Their Association with Growth and Obesity. Horm. Res. Paediatr. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Graciolli, F.G.; Neves, K.R.; Barreto, F.; Barreto, D.V.; Dos Reis, L.M.; Canziani, M.E.; Sabbagh, Y.; Carvalho, A.B.; Jorgetti, V.; Elias, R.M.; et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017, 91, 1436–1446. [Google Scholar] [CrossRef]
- Pereira, R.C.; Delany, A.M.; Khouzam, N.M.; Bowen, R.E.; Freymiller, E.G.; Salusky, I.B.; Wesseling-Perry, K. Primary osteoblast-like cells from patients with end-stage kidney disease reflect gene expression, proliferation, and mineralization characteristics ex vivo. Kidney Int. 2015, 87, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Liu, S.; David, V.; Li, H.; Karydis, A.; Feng, J.Q.; Quarles, L.D. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011, 25, 2551–2562. [Google Scholar] [CrossRef] [PubMed]

| Sex | Age (yrs) | Ca (mg/dL) | PO4 (mg/dL) | ALP (IU/L) | PTH (pg/mL) | 25OH (ng/dL) | Fracture Location | Fracture Number | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 6 | 9.9 | 189.0 | 13.0 | 55.0 | tibia; wrist; femur | >5 | |
| 2 | M | 14 | 9.7 | 4.8 | 188.0 | 36.0 | 36.0 | ankle; wrist; femur; tibia; ulna; ribs | >10 |
| 3 | M | 8 | 9.3 | 5.4 | 234.0 | 20.0 | 54.0 | compression; scoliosis | >3 |
| 4 | M | 14 | 9.8 | 5.6 | 141.0 | 40.0 | 39.0 | compression fractures | >3 |
| 5 | M | 13 | 9.8 | 4.7 | 225.0 | 41.0 | 22.0 | elbow; wrist; forearm; ankle; knee | >5 |
| 6 | F | 10 | 9.7 | 4.7 | 232.0 | NA | NA | NA | |
| 7 | F | 11 | 9.8 | 5.1 | 365.0 | 45.0 | 42.0 | wrist; metacarpal; arm; scapula | >4 |
| 8 | M | 8 | 9.5 | 4.7 | 337.0 | 38.0 | 23.0 | hip; vertebral; tibia | >3 |
| 9 | M | 13 | 10.2 | 4.8 | 385.0 | 33.0 | 30.0 | fingers; arm | >3 |
| 10 | M | 15 | 10.2 | 4.6 | 280.0 | 25.0 | 40.0 | vertebral; finger | >2 |
| 11 | F | 3 | 10.2 | 5.6 | 257.0 | 41.0 | 38.0 | long bone; tibia; elbow | >3 |
| 12 | M | 13 | 10.0 | 4.1 | 228.0 | 27.0 | 33.0 | wrist; thumb; vertebrae; compression | >6 |
| 13 | M | 18 | 9.9 | 4.1 | 96.0 | 22.0 | 46.0 | NA |
| BV/TV (%) | OV/BV (%) | OS/BS (%) | Ob.S/BS (%) | ES/BS (%) | Oc.S/BS (%) | Tb.Th (μm) | O.Th (μm) | Tb.Sp (μm) | Tb.N (mm) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13.6 | 0.4 | 4.9 | 1.1 | 5.2 | 0.2 | 96.0 | 3.4 | 609.6 | 1.4 |
| 2 | 18.1 | 1.1 | 7.7 | 2.2 | 13.3 | 0.9 | 104.9 | 7.2 | 473.9 | 1.7 |
| 3 | 23.6 | 1.6 | 13.2 | 0.6 | 0.8 | 0.3 | 132.8 | 8.0 | 429.5 | 1.8 |
| 4 | 31.2 | 2.2 | 10.6 | 1.3 | 13.8 | 1.9 | 77.6 | 8.2 | 171.6 | 4.0 |
| 5 | 20.5 | 0.9 | 8.4 | 0.9 | 0.0 | 0.0 | 131.2 | 7.2 | 508.2 | 1.6 |
| 6 | 26.8 | 2.1 | 19.3 | 5.3 | 3.6 | 1.0 | 144.7 | 7.8 | 395.3 | 1.9 |
| 7 | 12.6 | 2.0 | 12.0 | 3.9 | 0.6 | 0.0 | 104.3 | 8.6 | 721.6 | 1.2 |
| 8 | 16.9 | 1.6 | 18.5 | 0.5 | 1.6 | 0.1 | 89.8 | 3.9 | 442.4 | 1.9 |
| 9 | 23.5 | 4.2 | 31.6 | 2.6 | 3.8 | 1.2 | 122.9 | 8.1 | 399.9 | 1.9 |
| 10 | 26.7 | 4.1 | 31.0 | 8.6 | 7.0 | 1.8 | 141.4 | 9.3 | 388.2 | 1.9 |
| 11 | 16.3 | 1.3 | 10.4 | 2.5 | 0.4 | 0.0 | 81.1 | 5.0 | 418.2 | 2.0 |
| 12 | 31.0 | 1.2 | 9.6 | 3.9 | 1.6 | 0.5 | 129.2 | 8.1 | 288.0 | 2.4 |
| 13 | 28.0 | 2.9 | 14.3 | 1.3 | 2.1 | 1.0 | 117.3 | 12.0 | 302.2 | 2.4 |
| C | 8.9–34.3 | 0.2–5.8 | 4.3–37.0 | - | 0.5–4.3 | - | 91–177 | 2–13.2 | 351–737 | 1.1–2.2 |
| MS/BS (%) | MS/OS (%) | MAR (μm/d) | Aj.AR (μm/d) | BFR/BS (μ3m/μ2m/y) | Mlt (Days) | Omt (Days) | |
|---|---|---|---|---|---|---|---|
| 1 | 3.7 | 75.3 | 0.8 | 0.6 | 10.1 | 6.1 | 4.6 |
| 2 | 3.0 | 39.3 | 0.8 | 0.3 | 8.9 | 22.7 | 8.9 |
| 3 | 5.2 | 39.4 | 0.9 | 0.3 | 16.5 | 23.3 | 9.2 |
| 4 | 4.6 | 43.3 | 0.9 | 0.4 | 15.5 | 20.5 | 8.9 |
| 5 | 5.4 | 64.2 | 1.0 | 0.6 | 19.6 | 11.3 | 7.2 |
| 6 | 5.2 | 26.7 | 0.5 | 0.1 | 9.4 | 58.3 | 15.6 |
| 7 | 3.4 | 28.3 | 0.7 | 0.2 | 8.3 | 45.4 | 12.8 |
| 8 | 7.5 | 40.4 | 0.7 | 0.3 | 19.5 | 13.6 | 5.5 |
| 9 | 18.3 | 58.1 | 0.8 | 0.4 | 50.3 | 18.5 | 10.7 |
| 10 | 17.8 | 57.6 | 1.0 | 0.6 | 67.6 | 15.6 | 9.0 |
| 11 | 2.0 | 19.2 | 0.7 | 0.1 | 5.4 | 35.2 | 6.8 |
| 12 | 3.4 | 35.2 | 0.9 | 0.3 | 10.5 | 27.2 | 9.6 |
| 13 | 5.0 | 34.6 | 0.6 | 0.2 | 10.1 | 62.3 | 21.6 |
| C | 2.2–19.0 | 7.3–66.2 | 1.1–1.5 | 0.14 ± 1.20 | 10–73.4 | 2.3–63.8 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.C.; Noche, K.J.; Gales, B.; Chen, Z.; Salusky, I.B.; Albrecht, L.V. Sclerostin and Wnt Signaling in Idiopathic Juvenile Osteoporosis Using High-Resolution Confocal Microscopy for Three-Dimensional Analyses. Children 2024, 11, 820. https://doi.org/10.3390/children11070820
Pereira RC, Noche KJ, Gales B, Chen Z, Salusky IB, Albrecht LV. Sclerostin and Wnt Signaling in Idiopathic Juvenile Osteoporosis Using High-Resolution Confocal Microscopy for Three-Dimensional Analyses. Children. 2024; 11(7):820. https://doi.org/10.3390/children11070820
Chicago/Turabian StylePereira, Renata C., Kathleen J. Noche, Barbara Gales, Zhangying Chen, Isidro B. Salusky, and Lauren V. Albrecht. 2024. "Sclerostin and Wnt Signaling in Idiopathic Juvenile Osteoporosis Using High-Resolution Confocal Microscopy for Three-Dimensional Analyses" Children 11, no. 7: 820. https://doi.org/10.3390/children11070820
APA StylePereira, R. C., Noche, K. J., Gales, B., Chen, Z., Salusky, I. B., & Albrecht, L. V. (2024). Sclerostin and Wnt Signaling in Idiopathic Juvenile Osteoporosis Using High-Resolution Confocal Microscopy for Three-Dimensional Analyses. Children, 11(7), 820. https://doi.org/10.3390/children11070820






