Comparison of Metabolic Control in Children and Adolescents Treated with Insulin Pumps
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Recruitment and Study Design
2.2. Ethics Statement
2.3. Collecting Clinical and Continuous Glucose Monitoring Data
2.4. Statistical Analysis
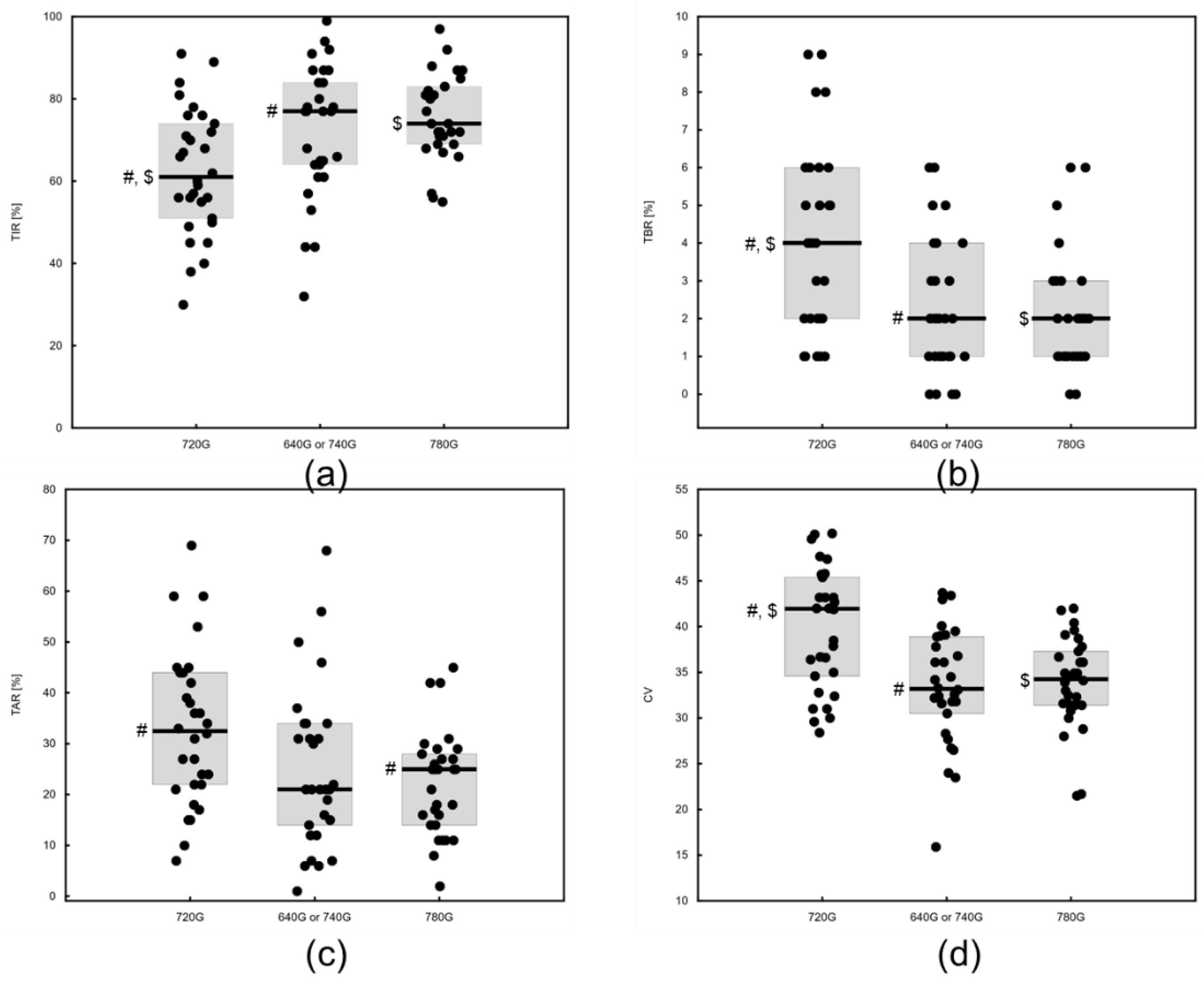
3. Results
3.1. Study Group Characteristics
3.2. Results of Diabetes Control for Different Technologies
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Position of the Polish Diabetes Association. Current Topics in Diabetes 2024. Curr. Top Diabetes 2023, 3, 1–348. [Google Scholar] [CrossRef]
- Stephan, Y.; Sutin, A.R.; Luchetti, M.; Canada, B.; Terracciano, A. Personality and HbA1c: Findings from six samples. Psychoneuroendocrinology 2020, 120, 104782. [Google Scholar] [CrossRef]
- Niemiec, A.; Juruć, A.; Moleda, P.; Safranow, K.; Majkowska, L. Personality traits, metabolic control and the use of insulin pump functions in adults with Type 1 diabetes: An observational single visit study. Diabetes Ther. 2021, 12, 419–430. [Google Scholar] [CrossRef]
- Beck, R.W.; Raghinaru, D.; Calhoun, P.; Bergenstal, R.M. A comparison of continuous glucose monitoring-measured time-in-range 70–180 mg/dL versus time-in-tight-range 70–140 mg/dL. Diabetes Technol. Ther. 2024, 26, 151–155. [Google Scholar] [CrossRef]
- Castaneda, J.; Arrieta, A.; Heuvel, T.V.D.; Cohen, O. The significance of coefficient of variation as a measure of hypoglycaemia risk and glycaemic control in real world users of the automated insulin delivery MiniMed 780G system. Diabetes Obes. Metab. 2023, 25, 2545–2552. [Google Scholar] [CrossRef]
- Beck, R.W.; Bergenstal, R.M.; Cheng, P.; Kollman, C.; Carlson, A.L.; Johnson, M.L.; Aleppo, G. Time in Tight Glucose Range in Type 1 Diabetes. Diabetes Care 2024, 47, 790–797. [Google Scholar]
- Seget, S.; Rusak, E.; Polanska, J.; Jarosz-Chobot, P. Prospective open-label, single-arm, single-center follow-up study of the application of the advanced hybrid closed loop system in well-controlled children and adolescents with type 1 diabetes. Diabetes Technol. Ther. 2022, 24, 824–831. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef]
- Carlson, A.L.; Sherr, J.L.; Shulman, D.I.; Garg, S.K.; Pop-Busui, R.; Bode, B.W.; Lilenquist, D.R.; Brazg, R.L.; Kaiserman, K.B.; Kipnes, M.S.; et al. Safety and glycemic outcomes during the MiniMed™ advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol. Ther. 2022, 24, 178–189. [Google Scholar] [CrossRef]
- Pulkkinen, M.-A.; Varimo, T.J.; Hakonen, E.T.; Harsunen, M.H.; Hyvönen, M.E.; Jane, J.N.; Kiiveri, S.M.; Laakkonen, H.M.; Laakso, S.M.; Wehkalampi, K.; et al. MiniMed 780G™ in 2- to 6-year-old children: Safety and clinical outcomes after the first 12 weeks. Diabetes Technol. Ther. 2023, 25, 100–107. [Google Scholar] [CrossRef]
- Seget, S.; Tekielak, A.; Rusak, E.; Jarosz-Chobot, P. Commercial hybrid closed-loop systems available for a patient with type 1 diabetes in 2022. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 30–36. [Google Scholar] [CrossRef]
- Forlenza, G.P.; Dai, Z.; Niu, F.; Shin, J.J. Reducing diabetes burden in Medtronic’s automated insulin delivery systems. Diabetes Technol. Ther. 2024, 26, 7–16. [Google Scholar] [CrossRef]
- Mizia, S.; Felińczak, A.; Włodarek, D.; Syrkiewicz-Świtała, M. Evaluation of Eating Habits and Their Impact on Health among Adolescents and Young Adults: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 3996. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, E.; Danne, T.; Ahmad, T.; Ayyavoo, A.; Beran, D.; Ehtisham, S.; Fairchild, J.; Jarosz-Chobot, P.; Ng, S.M.; Paterson, M.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Insulin treatment in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1277–1296. [Google Scholar] [CrossRef]
- Nutall, F. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today. 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Shalit, R.; Minsky, N.; Laron-Hirsh, M.; Cohen, O.; Kurtz, N.; Roy, A.; Grosman, B.; Benedetti, A.; Tirosh, A. Unannounced meal challenges using an advanced hybrid closed-loop system. Diabetes Technol Ther. 2023, 25, 579–588. [Google Scholar] [CrossRef]
- Abraham, M.; Smith, G.; Dart, J.; Davis, E.A.; Jones, T.W. Clinical Outcomes with MiniMedTM 780G Advanced Hybrid Closed-Loop Therapy in 2- to 6-Year-Old Children with Type 1 Diabetes. Diabetes Technol. Ther. 2024. [Google Scholar] [CrossRef]
- Da Silva, J.; Lepore, G.; Battelino, T.; Arrieta, A.; Castañeda, J.; Grossman, B.; Shin, J.; Cohen, O. Real-World Performance of the MiniMed™ 780G System: First Report of Outcomes from 4120 Users. Diabetes Technol. Ther. 2022, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.; Cohen, O.; Vogrin, S.; A Vigersky, R.; Jenkins, A.J. An Assessment of Clinical Continuous Glucose Monitoring Targets for Older and High-Risk People Living with Type 1 Diabetes. Diabetes Technol. Ther. 2023, 25, 108–115. [Google Scholar] [CrossRef]
- Huo, L.; Deng, W.; Shaw, J.; Magliano, D.J.; Zhang, P.; McGuire, H.C.; Kissimova-Skarbek, K.; Whiting, D.; Ji, L. Factors associated with glycemic control in type 1 diabetes patients in China: A cross-sectional study. J. Diabetes Investig. 2020, 11, 1575–1582. [Google Scholar] [CrossRef]
- Landau, Z.; Lebenthal, Y.; Mazor-Aronovitch, K.; Brener, A.; Levek, N.; Jacobi-Polishook, T.; Ben Ari, T.; Abiri, S.; Haim, A.; Nir, J.; et al. A comparison of the usage of an open-source automated insulin delivery system and the MiniMed™ 780 G system in children and adolescents with type 1 diabetes in real-world settings: The AWeSoMe study group. Endocrine 2024, 84, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Coutant, R.; Bismuth, E.; Bonnemaison, E.; Dalla-Vale, F.; Morinais, P.; Perrard, M.; Trely, J.; Faure, N.; Bouhours-Nouet, N.; Levaillant, L.; et al. Hybrid closed loop overcomes the impact of missed or suboptimal meal boluses on glucose control in children with Type 1 diabetes compared to sensor-augmented pump therapy. Diabetes Technol. Ther. 2023, 25, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, P.; Hanas, R.; Zaharieva, D.P.; Dovc, K.; Jendle, J. Automated Insulin Delivery Systems in Pediatric Type 1 Diabetes: A Narrative Review. J. Diabetes Sci. Technol. 2024, 24, 19322968241248404. [Google Scholar] [CrossRef]
- Sumnik, Z.; Pavlikova, M.; Neuman, V.; Petruzelkova, L.; Konecna, P.; Venhacova, P.; Skvor, J.; Pomahacova, R.; Neumann, D.; Vosahlo, J.; et al. Glycemic control by treatment modalities: National registry-based population data in children and adolescents with type 1 diabetes. Horm. Res. Paediatr. 2023, 97, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Campbell, J.; Pasha, M.; Day, E.; Hussain, K.; Khalifa, A.; van den Heuvel, T. Simplified meal announcement versus precise carbohydrate counting in adolescents with type 1 diabetes using the MiniMed 780G advanced hybrid closed loop system: A randomized controlled trial comparing glucose control. Diabetes Care. 2023, 46, 544–550. [Google Scholar] [CrossRef]
- Pelkey, M.N.; Boyle, M.; Long, A.; Castro, J.C.; Cook, C.B.; Thompson, B. Hybrid Closed-Loop Insulin Pump Technology Can Be Safely Used in the Inpatient Setting. Endocr. Pract. 2023, 29, 24–28. [Google Scholar] [CrossRef]
- Pollé, O.G.; Delfosse, A.; Martin, M.; Louis, J.; Gies, I.; den Brinker, M.; Seret, N.; Lebrethon, M.-C.; Mouraux, T.; DIATAG Working Group; et al. Glycemic variability patterns strongly correlate with partial remission status in children with newly diagnosed type 1 diabetes. Diabetes Care 2022, 45, 2360–2368. [Google Scholar] [CrossRef]
- Almedia, A.C.; Tavares, F.; Pareira, M.G. Metabolic control and quality of life in type 1 diabetes: Do adherence, family support, and school support matter? Nurs. Health Sci. 2023, 25, 456–465. [Google Scholar] [CrossRef]
- Mohammed Abrahim, A.; Tilahun, T.; Gelana, B. Glycemic Control and Associated Factors Among Children and Adolescents with Type 1 Diabetes Mellitus, Suthwest Ethiopia. Diabetes Metab Syndr Obes. 2023, 4, 2025–2037. [Google Scholar] [CrossRef]
| Group Indices | Medtronic 720G without PLGS | Medtronic 640G or 740G with PLGS | Medtronic 780G | p Value |
|---|---|---|---|---|
| Continuous | Median (25 to 75%) | Median (25 to 75%) | Median (25 to 75%) | |
| Age [years] | 15 (12 to 16) #,$ | 13 (11 to 14) # | 12 (10 to 14) $ | 0.0090 |
| T1DM duration [years] | 7 (5.2 to 9.1) # | 2.3 (1.2 to 7) # | 3.9 (2.3 to 7.5) | 0.0019 |
| DDI (daily dose of insulin) [units/kg] | 0.8 (0.8 to 1) # | 0.7 (0.5 to 0.9) #,$ | 0.9 (0.7 to 1.1) $ | 0.0031 |
| Weight [kg] z-score percentile | 57.1 (47 to 66.8) 0.4 (−0.1 to 0.9) 64 (47 to 81) | 44.7 (37.5 to 62.3) 0.3 (−0.6 to 0.9) 60.5 (27 to 81) | 47.8 (33.3 to 54) 0.1 (−0.5 to 0.6) 54.5 (31 to 73) | NA 0.3494 NA |
| Height [cm] z-score percentile | 166.7 (157 to 179) 0.4 (−0.6 to 1) 64.5 (29 to 84) | 156.3 (146 to 165.8) 0.1 (−0.7 to 1) 54 (26 to 84) | 153.3 (141.8 to 167) 0.1 (−0.8 to 0.7) 53 (20 to 76) | NA 0.3103 NA |
| BMI [kg/m2] z-score percentile | 20.2 (18.5 to 21.8) 0.3 (−0.3 to 0.7) 61 (37 to 76) | 19.3 (16.6 to 21.5) 0.4 (−0.4 to 0.8) 64.5 (34 to 79) | 18.3 (16.8 to 20.8) 0.1 (−0.3 to 0.5) 56 (38 to 69) | NA 0.7838 NA |
| CSII (continuous subcutaneous insulin infusion) duration [years] | 5.4 (4.2 to 7.6) # | 1.5 (1 to −2) # | 3.5 (1 to 6.7) | 0.0039 |
| Duration of present insulin pump therapy [years] | 0.9 (0.5 to 2.7) | 1.3 (0.7 to 2) | 1.4 (1 to 2.1) | 0.2160 |
| Time of sensor use [%] | 91.5 (88 to 97) #,$ | 85 (64 to 91) # | 96 (95 to 98) $ | <0.0001 |
| Time of SmartGuard use [%] | NA | NA | 97.5 (95 to 99) | NA |
| Group Indices | Medtronic 720G without PLGS | Medtronic 640G or 740G with PLGS | Medtronic 780G | p Value |
|---|---|---|---|---|
| Continuous | Median (25 to 75%) | Median (25 to 75%) | Median (25 to 75%) | |
| TC [mg/dL] | 161.5 (150 to 175) | 170 (157 to 192) | 170.5 (152 to 194) | 0.1537 |
| LDL [mg/dL] | 89 (78 to 104) | 98.5 (90 to 113) | 91 (84 to 111) | 0.1937 |
| HDL [mg/dL] | 57 (50 to 68) | 61.5 (45 to 71) | 62 (49 to 69) | 0.8801 |
| TG [mg/dL] | 61.5 (44 to 72) | 59.5 (47 to 76) | 74.5 (47 to 84) | 0.5567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejk, A.; Myśliwiec, K.; Michalak, A.; Pernak, B.; Fendler, W.; Myśliwiec, M. Comparison of Metabolic Control in Children and Adolescents Treated with Insulin Pumps. Children 2024, 11, 839. https://doi.org/10.3390/children11070839
Lejk A, Myśliwiec K, Michalak A, Pernak B, Fendler W, Myśliwiec M. Comparison of Metabolic Control in Children and Adolescents Treated with Insulin Pumps. Children. 2024; 11(7):839. https://doi.org/10.3390/children11070839
Chicago/Turabian StyleLejk, Agnieszka, Karolina Myśliwiec, Arkadiusz Michalak, Barbara Pernak, Wojciech Fendler, and Małgorzata Myśliwiec. 2024. "Comparison of Metabolic Control in Children and Adolescents Treated with Insulin Pumps" Children 11, no. 7: 839. https://doi.org/10.3390/children11070839
APA StyleLejk, A., Myśliwiec, K., Michalak, A., Pernak, B., Fendler, W., & Myśliwiec, M. (2024). Comparison of Metabolic Control in Children and Adolescents Treated with Insulin Pumps. Children, 11(7), 839. https://doi.org/10.3390/children11070839






