A Review of Contemporary and Future Pharmacotherapy for Chronic Heart Failure in Children
Abstract
:1. Introduction
2. Chronic HF with Reduced Ejection Fraction (HFrEF)
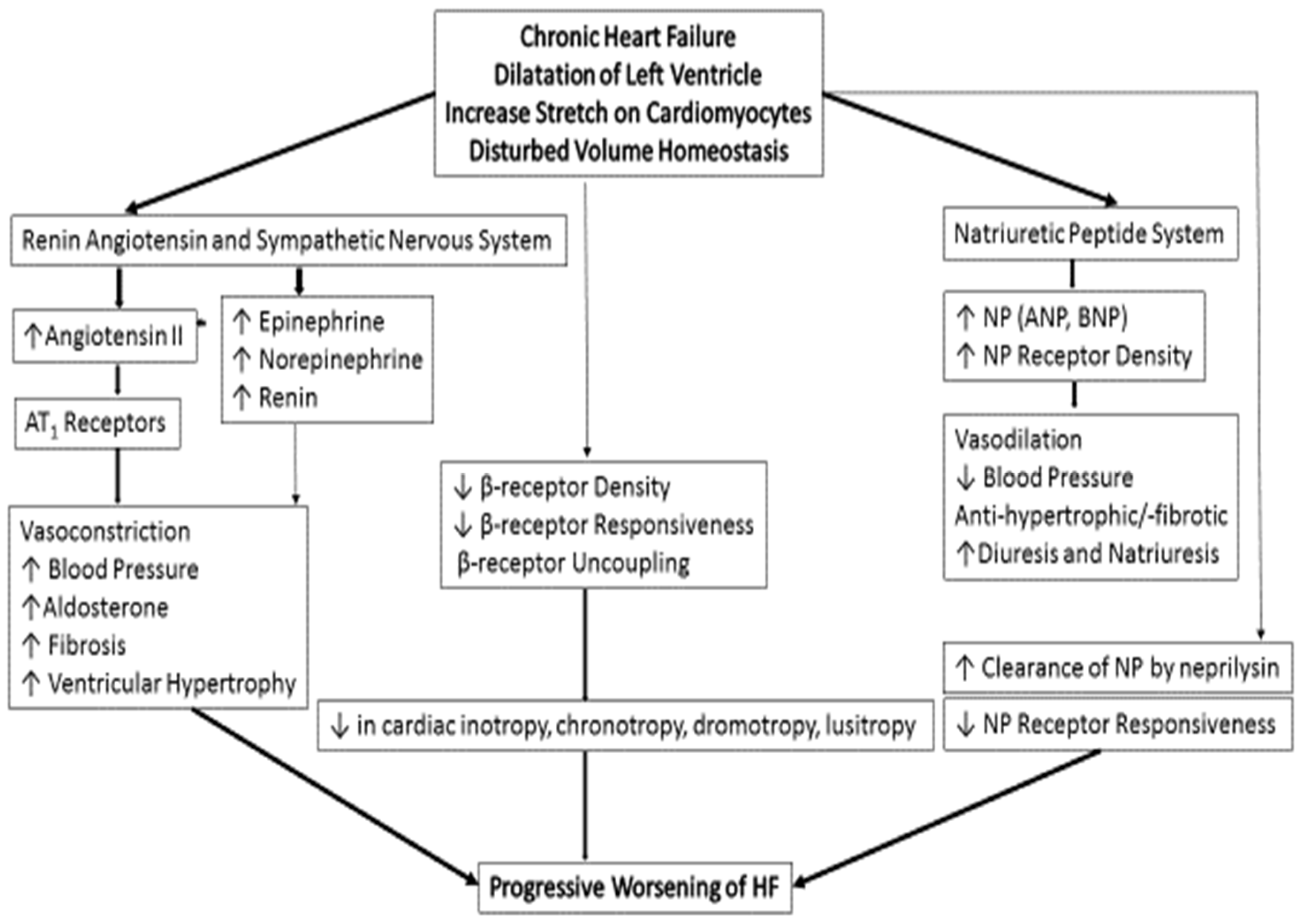
3. Pathogenesis and Pathophysiology of Chronic HFrEF
4. Heart Failure with Preserved Ejection Fraction (HFpEF)
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossano, J.W.; Kim, J.J.; Decker, J.A.; Price, J.F.; Zafar, F.; Graves, D.E.; Morales, D.L.; Heinle, J.S.; Bozkurt, B.; Towbin, J.A.; et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: A population-based study. J. Card. Fail. 2012, 18, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A. Acute Heart Failure Deserves a Log-Scale Boost in Research Support: Call for Multidisciplinary and Universal Actions. JACC Heart Fail. 2018, 6, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.; Dipchand, A.I.; Rosenthal, D.N.; Addonizio, L.; Burch, M.; Chrisant, M.; Dubin, A.; Everitt, M.; Gajarski, R.; Mertens, L.; et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary. J. Heart Lung Transplant. 2014, 33, 888–909. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B. Current State of Pediatric Heart Failure. Children 2018, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.D. The Ross classification for heart failure in children after 25 years: A review and an age-stratified revision. Pediatr. Cardiol. 2012, 33, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Rutkowski, M.; Auslender, M.; Artman, M. The New York University Pediatric Heart Failure Index: A new method of quantifying chronic heart failure severity in children. J. Pediatr. 2001, 138, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, R.; Canter, C.; Halnon, N.; Kochilas, L.; Rossano, J.; Bonnet, D.; Bush, C.; Zhao, Z.; Kantor, P.; Burch, M.; et al. Design for the sacubitril/valsartan (LCZ696) compared with enalapril study of pediatric patients with heart failure due to systemic left ventricle systolic dysfunction (PANORAMA-HF study). Am. Heart J. 2017, 193, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Miller, W.L. Fluid Volume Overload and Congestion in Heart Failure. Circ. Heart Fail. 2016, 9, e002922. [Google Scholar] [CrossRef] [PubMed]
- Faselis, C.; Arundel, C.; Patel, S.; Lam, P.H.; Gottlieb, S.S.; Zile, M.R.; Deedwania, P.; Filippatos, G.; Sheriff, H.M.; Zeng, Q.; et al. Loop Diuretic Prescription and 30-Day Outcomes in Older Patients with Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, R.; Vagha, J.D.; Meshram, R.J.; Patel, A. A Comprehensive Review on Unveiling the Journey of Digoxin: Past, Present, and Future Perspectives. Cureus 2024, 16, e56755. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Mangeot, C.; Anderson, J.B.; Peterson, L.E.; King, E.C.; Lihn, S.L.; Neish, S.R.; Fleishman, C.; Phelps, C.; Hanke, S.; et al. Digoxin Use Is Associated with Reduced Interstage Mortality in Patients with No History of Arrhythmia after Stage I Palliation for Single Ventricle Heart Disease. J. Am. Heart Assoc. 2016, 5, e002376. [Google Scholar] [CrossRef] [PubMed]
- Mlambo, V.C.; Algaze, C.A.; Mak, K.; Collins, R.T., 2nd. Impact of Abnormal Potassium on Arrhythmia Risk during Pediatric Digoxin Therapy. Pediatr. Cardiol. 2024, 45, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Brunner-La Rocca, H.P.; Vaddadi, G.; Esler, M.D. Recent insight into therapy of congestive heart failure: Focus on ACE inhibition and angiotensin-II antagonism. J. Am. Coll. Cardiol. 1999, 33, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Kantor, P.F.; Abraham, J.R.; Dipchand, A.I.; Benson, L.N.; Redington, A.N. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Scholl, F.; Vandale, B.; Chrisant, M. Sacubitril/Valsartan: Potential treatment for paediatric heart failure. Cardiol. Young 2018, 28, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Solomon, S.D. Neprilysin inhibition for heart failure. N. Engl. J. Med. 2014, 371, 2336–2337. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Shaddy, R.; Burch, M.; Kantor, P.F.; Solar-Yohay, S.; Garito, T.; Zhang, S.; Kocun, M.; Bonnet, D. Baseline Characteristics of Pediatric Patients with Heart Failure Due to Systemic Left Ventricular Systolic Dysfunction in the PANORAMA-HF Trial. Circ. Heart Fail. 2023, 16, 259–269. [Google Scholar] [CrossRef]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef]
- Shaddy, R.E.; Boucek, M.M.; Hsu, D.T.; Boucek, R.J.; Canter, C.E.; Mahony, L.; Ross, R.D.; Pahl, E.; Blume, E.D.; Dodd, D.A.; et al. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA 2007, 298, 1171–1179. [Google Scholar] [CrossRef]
- Miyamoto, S.D.; Sucharov, C.C.; Woulfe, K.C. Differential Response to Heart Failure Medications in Children. Prog Pediatr. Cardiol. 2018, 49, 27–30. [Google Scholar] [CrossRef]
- Miyamoto, S.D.; Stauffer, B.L.; Nakano, S.; Sobus, R.; Nunley, K.; Nelson, P.; Sucharov, C.C. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur. Heart J. 2014, 35, 33–41. [Google Scholar] [CrossRef]
- Alabed, S.; Sabouni, A.; Al Dakhoul, S.; Bdaiwi, Y.; Frobel-Mercier, A.-K. Beta-blockers for congestive heart failure in children. Cochrane Database Syst. Rev. 2016, 1, CD007037. [Google Scholar] [CrossRef]
- Pocock, S.J.; Wang, D.; Pfeffer, M.A.; Yusuf, S.; McMurray, J.J.; Swedberg, K.B.; Ostergren, J.; Michelson, E.L.; Pieper, K.S.; Granger, C.B. Predictors of mortality and morbidity in patients with chronic heart failure. Eur. Heart J. 2006, 27, 65–75. [Google Scholar] [CrossRef]
- Fox, K.; Komajda, M.; Ford, I.; Robertson, M.; Bohm, M.; Borer, J.S.; Steg, P.G.; Tavazzi, L.; Tendera, M.; Ferrari, R.; et al. Effect of ivabradine in patients with left-ventricular systolic dysfunction: A pooled analysis of individual patient data from the BEAUTIFUL and SHIFT trials. Eur. Heart J. 2013, 34, 2263–2270. [Google Scholar] [CrossRef]
- Bonnet, D.; Berger, F.; Jokinen, E.; Kantor, P.F.; Daubeney, P.E.F. Ivabradine in Children with Dilated Cardiomyopathy and Symptomatic Chronic Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 1262–1272. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, Y.; Li, D.; Pu, J.; Ding, H.; Zhang, X. Mechanisms of SGLT2 Inhibitors in Heart Failure and Their Clinical Value. J. Cardiovasc. Pharmacol. 2023, 81, 4–14. [Google Scholar] [CrossRef]
- Billing, A.M.; Kim, Y.C.; Gullaksen, S.; Schrage, B.; Raabe, J.; Hutzfeldt, A.; Demir, F.; Kovalenko, E.; Lassé, M.; Dugourd, A.; et al. Metabolic Communication by SGLT2 Inhibition. Circulation 2024, 149, 860–884. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Jhund, P.S.; Claggett, B.L.; Dewan, P.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Inzucchi, S.E.; et al. Effect of Dapagliflozin in Patients with HFrEF Treated with Sacubitril/Valsartan. JACC Heart Fail. 2020, 8, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Newland, D.M.; Law, Y.M.; Albers, E.L.; Friedland-Little, J.M.; Ahmed, H.; Kemna, M.S.; Hong, B.J. Early Clinical Experience with Dapagliflozin in Children with Heart Failure. Pediatr. Cardiol. 2023, 44, 146–152. [Google Scholar] [CrossRef]
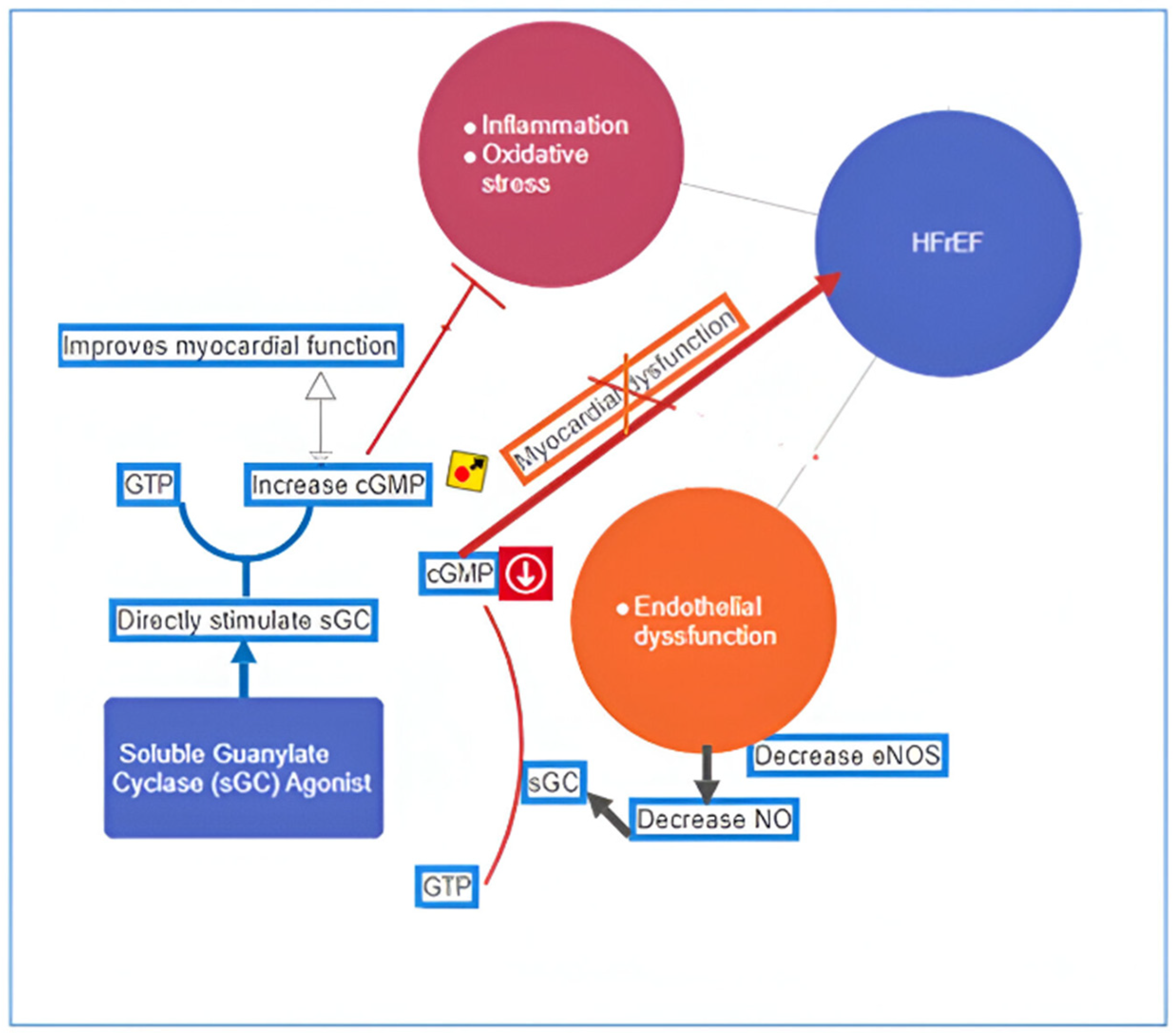
- Armstrong, P.W.; Roessig, L.; Patel, M.J.; Anstrom, K.J.; Butler, J.; Voors, A.A.; Lam, C.S.P.; Ponikowski, P.; Temple, T.; Pieske, B.; et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy and Safety of the Oral Soluble Guanylate Cyclase Stimulator: The VICTORIA Trial. JACC Heart Fail. 2018, 6, 96–104. [Google Scholar] [CrossRef]
- Xia, J.; Hui, N.; Tian, L.; Liang, C.; Zhang, J.; Liu, J.; Wang, J.; Ren, X.; Xie, X.; Wang, K. Development of vericiguat: The first soluble guanylate cyclase (sGC) stimulator launched for heart failure with reduced ejection fraction (HFrEF). Biomed. Pharmacother. 2022, 149, 112894. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Giczewska, A.; Sliwa, K.; Edelmann, F.; Refsgaard, J.; Bocchi, E.; Ezekowitz, J.A.; Hernandez, A.F.; O’Connor, C.M.; Roessig, L.; et al. Clinical Outcomes and Response to Vericiguat According to Index Heart Failure Event: Insights from the VICTORIA Trial. JAMA Cardiol. 2021, 6, 706–712. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Efficacy, Safety and Pharmacokinetics of Vericiguat in Pediatric Participants with Heart Failure Due to Left Ventricular Systolic Dysfunction (MK-1242-036). 2023. Available online: www.clinicaltrials.gov (accessed on 20 June 2024).
- Psotka, M.A.; Gottlieb, S.S.; Francis, G.S.; Allen, L.A.; Teerlink, J.R.; Adams, K.F.; Rosano, G.M.C.; Lancellotti, P. Cardiac Calcitropes, Myotropes, and Mitotropes. J. Am. Coll. Cardiol. 2019, 73, 2345–2353. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Diaz, R.; Felker, G.M.; McMurray, J.J.V.; Metra, M.; Solomon, S.D.; Adams, K.F.; Anand, I.; Arias-Mendoza, A.; Biering-Sørensen, T.; et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F.; et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Voors, A.A.; et al. Effects of Serelaxin in Patients with Acute Heart Failure. N. Engl. J. Med. 2019, 381, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Metra, M.; Blair, J.E.; Vogel, M.; Harinstein, M.E.; Filippatos, G.S.; Sabbah, H.N.; Porchet, H.; Valentini, G.; Gheorghiade, M. Istaroxime, a first in class new chemical entity exhibiting SERCA-2 activation and Na-K-ATPase inhibition: A new promising treatment for acute heart failure syndromes? Heart Fail. Rev. 2009, 14, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L. SERCA2a stimulation by istaroxime: A novel mechanism of action with translational implications. Br. J. Pharmacol. 2013, 170, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Arici, M.; Lodrini, A.M.; Ferrandi, M.; Barassi, P.; Hsu, S.C.; Chang, G.J.; Boz, E.; Sala, E.; Vagni, S.; et al. SERCA2a stimulation by istaroxime improves intracellular Ca2+ handling and diastolic dysfunction in a model of diabetic cardiomyopathy. Cardiovasc. Res. 2022, 118, 1020–1032. [Google Scholar] [CrossRef]
- Shah, S.J.; Blair, J.E.A.; Filippatos, G.S.; Macarie, C.; Ruzyllo, W.; Korewicki, J.; Bubenek-Turconi, S.I.; Ceracchi, M.; Bianchetti, M.; Carminati, P.; et al. Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: Results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: A Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial. Am. Heart J. 2009, 157, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, V.; Seferovic, P.M.; Simeunovic, D.; Ristic, A.D.; Miric, M.; Moiseyev, V.S.; Kobalava, Z.; Nitsche, K.; Forssmann, W.G.; Luss, H.; et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur. Heart J. 2006, 27, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Ponikowski, P.; Mitrovic, V.; Peacock, W.F.; Filippatos, G. Ularitide for the treatment of acute decompensated heart failure: From preclinical to clinical studies. Eur. Heart J. 2015, 36, 715–723. [Google Scholar] [CrossRef]
- Packer, M.; Holcomb, R.; Abraham, W.T.; Anker, S.; Dickstein, K.; Filippatos, G.; Krum, H.; Maggioni, A.P.; McMurray, J.J.V.; Mebazaa, A.; et al. Rationale for and design of the TRUE-AHF trial: The effects of ularitide on the short-term clinical course and long-term mortality of patients with acute heart failure. Eur. J. Heart Fail. 2017, 19, 673–681. [Google Scholar] [CrossRef]
- Chatfield, K.C.; Sparagna, G.C.; Chau, S.; Phillips, E.K.; Ambardekar, A.V.; Aftab, M.; Mitchell, M.B.; Sucharov, C.C.; Miyamoto, S.D.; Stauffer, B.L. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl. Sci. 2019, 4, 147–157. [Google Scholar] [CrossRef]
- Dai, W.; Shi, J.; Gupta, R.C.; Sabbah, H.N.; Hale, S.L.; Kloner, R.A. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J. Cardiovasc. Pharmacol. 2014, 64, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Hachem, S.; Zhang, K. Chronic Therapy with Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs with Advanced Heart Failure. Circ. Heart Fail. 2016, 9, e002206. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Khan, M.S.; Anker, S.D.; Fonarow, G.C.; Kim, R.J.; Nodari, S.; O’Connor, C.M.; Pieske, B.; Pieske-Kraigher, E.; Sabbah, H.N.; et al. Effects of Elamipretide on Left Ventricular Function in Patients with Heart Failure with Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. J. Card. Fail. 2020, 26, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10, e004389. [Google Scholar] [CrossRef] [PubMed]
- Piko, N.; Bevc, S.; Hojs, R.; Ekart, R. Finerenone: From the Mechanism of Action to Clinical Use in Kidney Disease. Pharmaceuticals 2024, 17, 418. [Google Scholar] [CrossRef] [PubMed]
- Robert, L. Heart Failure Drugs in the Pipeline: Six Novel Therapeutics to Note; Patient Care: Cranbury, NJ, USA, 2021. [Google Scholar]
- Nguyen, N.; Page, G.; Abi-Gerges, N.; Miller, P.E.; Adams, J.W. Selective beta-3 adrenergic receptor blockade increases contractility of human ventricular trabeculae from HFrEF donors. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946.1229. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Das, B.; Deshpande, S.; Akam-Venkata, J.; Shakti, D.; Moskowitz, W.; Lipshultz, S.E. Heart Failure with Preserved Ejection Fraction in Children. Pediatr. Cardiol. 2023, 44, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B. Therapeutic Approaches in Heart Failure with Preserved Ejection Fraction (HFpEF) in Children: Present and Future. Paediatr. Drugs 2022, 24, 235–246. [Google Scholar] [CrossRef]
- Arnold, S.V.; Silverman, D.N.; Gosch, K.; Nassif, M.E.; Infeld, M.; Litwin, S.; Meyer, M.; Fendler, T.J. Beta-Blocker Use and Heart Failure Outcomes in Mildly Reduced and Preserved Ejection Fraction. JACC Heart Fail. 2023, 8 Pt 1, 893–900. [Google Scholar] [CrossRef]
- Fu, E.L.; Uijl, A.; Dekker, F.W.; Lund, L.H.; Savarese, G.; Carrero, J.J. Association Between β-Blocker Use and Mortality/Morbidity in Patients with Heart Failure with Reduced, Midrange, and Preserved Ejection Fraction and Advanced Chronic Kidney Disease. Circ. Heart Fail. 2020, 13, e007180. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Manoharan, K.; Davies, C.; Lumbers, R.T. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2021, 5, CD012721. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.; LeWinter, M.M.; Rouleau, J.L.; Bull, D.A.; Mann, D.L.; et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure with Preserved Ejection Fraction. A Randomized Clinical Trial. JAMA 2013, 309, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, K.; Xiao, C.; He, Z.; Liu, S.; Wu, X.; Shi, S.; Guo, Y. Phosphodiesterase inhibitor for heart failure with preserved ejection fraction: A systematic review and meta-analysis. Saudi Pharm. J. 2022, 30, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Hoendermis, E.S.; Liu, L.C.Y.; Hummel, Y.M.; van der Meer, P.; de Boer, R.A.; Berger, R.M.; van Veldhuisen, D.J.; Voors, A.A. Effects of sildenafil on invasive hemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur. Heart J. 2015, 36, 2565. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]



| Drug | Doses | FDA Approval |
|---|---|---|
| Ivabradine | 0.02–0.05 mg/kg twice a day in <40 kg 25 mg twice a day in >40 kg | 2019; off label use in children if tachycardia persists despite use of β-blockers |
| Sacubitril/Valsartan | Starting dose 1.6 mg/kg of the combined amount of both Valsartan and Sacubitril in <40 kg; increase every 2 weeks upward from 2.3 mg/kg up to a max dose of 3.1 mg/kg based on tolerance Valsartan 51 mg and Sacubitril 49 mg twice a day and titrate upward as tolerated in >40 kg | 2019; in patients with symptomatic HF with LV dysfunction, over one-year-old |
| Dapagliflozin | 0.1–0.2 mg/kg once daily, (Max 10 mg) | No approval: dose is determined empirically |
| Omecamtiv Mecarbil | No data | No data |
| Vericiguat | No data | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.B. A Review of Contemporary and Future Pharmacotherapy for Chronic Heart Failure in Children. Children 2024, 11, 859. https://doi.org/10.3390/children11070859
Das BB. A Review of Contemporary and Future Pharmacotherapy for Chronic Heart Failure in Children. Children. 2024; 11(7):859. https://doi.org/10.3390/children11070859
Chicago/Turabian StyleDas, Bibhuti B. 2024. "A Review of Contemporary and Future Pharmacotherapy for Chronic Heart Failure in Children" Children 11, no. 7: 859. https://doi.org/10.3390/children11070859






