Postural Control and Neuromuscular Activation in 11–13-Year-Old Athletic Boy Swimmers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Measurements
2.3.1. Postural Control
2.3.2. EMG
2.4. Statistical Analysis
3. Results
3.1. CoP Parameters
3.2. EMG Parameter
4. Discussion
Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, J.M.; Rietdyk, S.; Claxton, L.J.; Huber, J.E. Task-dependent postural control throughout the lifespan. Exerc. Sport Sci. Rev. 2013, 41, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.; Melnikov, A.; Skvortsov, D.; Akhmerova, K.; Vavaev, A.; Golov, A.; Draugelite, V.; Nikolaev, R.; Chechelnickaia, S.; Zhuk, D. Postural stability in athletes: The role of sport direction. Gait Posture 2021, 89, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.; Gurfinkel, V.S. Human postural control. Front. Neurosci. 2018, 12, 301583. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, O.; Kelm, J.; Hammes, A.; Schmitt, E.; Fröhlich, M. Neuromuscular performance of balance and posture control in childhood and adolescence. Heliyon 2020, 6, e04541. [Google Scholar] [CrossRef] [PubMed]
- Mallau, S.; Vaugoyeau, M.; Assaiante, C. Postural strategies and sensory integration: No turning point between childhood and adolescence. PLoS ONE 2010, 5, e13078. [Google Scholar] [CrossRef]
- Cuisinier, R.; Olivier, I.; Vaugoyeau, M.; Nougier, V.; Assaiante, C. Reweighting of sensory inputs to control quiet standing in children from 7 to 11 and in adults. PLoS ONE 2011, 6, e19697. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, A.W.; Armitano-Lago, C.N.; Cone, B.L.; Bonnette, S.; Rhea, C.K.; Cummins-Sebree, S.; Riley, M.A. Postural control development from late childhood through young adulthood. Gait Posture 2021, 86, 169–173. [Google Scholar] [CrossRef]
- Orendorz-Frączkowska, K.; Kubacka, M. The development of postural control in 6–17 old years healthy children. Part I Postural control evaluation in modified Clinical Test for The Sensory Interaction on Balance in 6–17 old year children (mctsib). Pol. J. Otolaryngol. 2020, 74, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Dudziak, T.; Czubek, Z.; Grabowski, M.; Urbanski, R. Changes in body composition of children aged of 9 to 12 years with excess body weight under the influenceof eight-week workout. Balt. J. Health Phys. Act. 2018, 10, 10. [Google Scholar] [CrossRef]
- Rogol, A.D.; Clark, P.A.; Roemmich, J.N. Growth and pubertal development in children and adolescents: Effects of diet and physical activity. Am. J. Clin. Nutr. 2000, 72, 521S–528S. [Google Scholar] [CrossRef]
- Mainenti, M.R.M.; de Carvalho Rodrigues, É.; de Oliveira, J.F.; de Sá Ferreira, A.; Dias, C.M.; dos Santos Silva, A.L. Adiposity and postural balance control: Correlations between bioelectrical impedance and stabilometric signals in elderly Brazilian women. Clinics 2011, 66, 1513–1518. [Google Scholar]
- John, C.; Rahlf, A.L.; Hamacher, D.; Zech, A. Influence of biological maturity on static and dynamic postural control among male youth soccer players. Gait Posture 2019, 68, 18–22. [Google Scholar] [CrossRef]
- Stanek, E.; Truszczyńska, A.; Drzał-Grabiec, J.; Tarnowski, A. Postural balance assessment in children aged 7 to 9 years, as related to body weight, height, and physical activity. Biomed. Hum. Kinet. 2015, 7, 135–141. [Google Scholar] [CrossRef]
- Drzał-Grabiec, J.; Snela, S.; Rykała, J.; Podgórska, J.; Banaś, A. Changes in the body posture of women occurring with age. BMC Geriatr. 2013, 13, 108. [Google Scholar] [CrossRef]
- García-Soidán, J.L.; García-Liñeira, J.; Leirós-Rodríguez, R.; Soto-Rodríguez, A. Physical activity practice and optimal development of postural control in school children: Are they related? J. Clin. Med. 2020, 9, 2919. [Google Scholar] [CrossRef]
- Oliveira Junior, E.d.; Silva, A.F.M.d.; Antunes, F.D.; Jacinto, J.L.; Aguiar, A.F. Analysis of postural balance in children who practice and those who do not practice sports activities. Rev. Bras. Med. Esporte 2021, 27, 588–591. [Google Scholar] [CrossRef]
- Paillard, T. Posture et Équilibration Humaines; De Boeck Superieur: Paris, France, 2016. [Google Scholar]
- Bringoux, L.; Marin, L.; Nougier, V.; Barraud, P.-A.; Raphel, C. Effects of gymnastics expertise on the perception of body orientation in the pitch dimension. J. Vestib. Res. 2000, 10, 251–258. [Google Scholar] [CrossRef]
- Melam, G.R.; Alhusaini, A.A.; Perumal, V.; Buragadda, S.; Kaur, K. Comparison of static and dynamic balance between football and basketball players with chronic ankle instability. Saudi J. Sports Med. 2016, 16, 199–204. [Google Scholar] [CrossRef]
- Jouira, G.; Srihi, S.; Kachouri, H.; Ben Waer, F.; Rebai, H.; Sahli, S. Static postural balance between male athletes with intellectual disabilities and their sedentary peers: A comparative study. J. Appl. Res. Intellect. Disabil. 2021, 34, 1136–1144. [Google Scholar] [CrossRef]
- Hrysomallis, C. Balance ability and athletic performance. Sports Med. 2011, 41, 221–232. [Google Scholar] [CrossRef]
- Franklin, M.; Lomas, J.; Walker, S.; Young, T. An educational review about using cost data for the purpose of cost-effectiveness analysis. Pharmacoeconomics 2019, 37, 631–643. [Google Scholar] [CrossRef]
- Noé, F.; Paillard, T. Is postural control affected by expertise in alpine skiing? Br. J. Sports Med. 2005, 39, 835–837. [Google Scholar] [CrossRef]
- Perrin, P.; Deviterne, D.; Hugel, F.; Perrot, C. Judo, better than dance, develops sensorimotor adaptabilities involved in balance control. Gait Posture 2002, 15, 187–194. [Google Scholar] [CrossRef]
- Paillard, T. Relationship between sport expertise and postural skills. Front. Psychol. 2019, 10, 1428. [Google Scholar] [CrossRef]
- Ting, L.H. Dimensional reduction in sensorimotor systems: A framework for understanding muscle coordination of posture. Prog. Brain Res. 2007, 165, 299–321. [Google Scholar]
- Sousa, A.S.; Silva, A.; Tavares, J.M.R. Biomechanical and neurophysiological mechanisms related to postural control and efficiency of movement: A review. Somatosens. Mot. Res. 2012, 29, 131–143. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Chou, S.-W.; Chen, C.; Wong, A.M.-K.; Chen, C.-K.; Hong, J.-P. Effects of swimming on eye hand coordination and balance in the elderly. J. Nutr. Health Aging 2010, 14, 692–695. [Google Scholar] [CrossRef]
- Sigmundsson, H.; Hopkins, B. Baby swimming: Exploring the effects of early intervention on subsequent motor abilities. Child Care Health Dev. 2010, 36, 428–430. [Google Scholar] [CrossRef]
- Aspenes, S.T.; Karlsen, T. Exercise-training intervention studies in competitive swimming. Sports Med. 2012, 42, 527–543. [Google Scholar] [CrossRef]
- Jarchow, T.; Mast, F.W. The effect of water immersion on postural and visual orientation. Aviat. Space Environ. Med. 1999, 70, 879–886. [Google Scholar]
- Marinho-Buzelli, A.R.; Rouhani, H.; Masani, K.; Verrier, M.C.; Popovic, M.R. The influence of the aquatic environment on the control of postural sway. Gait Posture 2017, 51, 70–76. [Google Scholar] [CrossRef]
- Deliagina, T.G.; Zelenin, P.V.; Orlovsky, G.N. Physiological and circuit mechanisms of postural control. Curr. Opin. Neurobiol. 2012, 22, 646–652. [Google Scholar] [CrossRef]
- Pop, N.H.; Ilisei, I. Enhacement of swimming kinematics and performance through proprioception. Stud. Univ. Babeș-Bolyai Educ. Artis Gymnast. 2022, 67, 137–145. [Google Scholar] [CrossRef]
- Varma, K.; Gokhale, P. Assessment of Static and Dynamic Balance in Swimmers. Int. J. Health Sci. Res. 2021, 11, 202–210. [Google Scholar] [CrossRef]
- Tanaka, H.; Costill, D.L.; Thomas, R.; Fink, W.J.; Widrick, J.J. Dry-land resistance training for competitive swimming. Med. Sci. Sports Exerc. 1993, 25, 952. [Google Scholar] [CrossRef]
- Sharp, R.L.; Troup, J.P. Relationship between power and sprint freestyle. Med. Sci. Sports Exerc. 1982, 14, 53–56. [Google Scholar] [CrossRef]
- Wong, A.; Kwak, Y.-S.; Scott, S.D.; Pekas, E.J.; Son, W.-M.; Kim, J.-S.; Park, S.-Y. The effects of swimming training on arterial function, muscular strength, and cardiorespiratory capacity in postmenopausal women with stage 2 hypertension. Menopause 2019, 26, 653–658. [Google Scholar] [CrossRef]
- Lubkowska, W.; Wiażewicz, A.; Eider, J. The correlation between sports results in swimming and general and special muscle strength. J. Educ. Health Sport 2017, 7, 222–236. [Google Scholar]
- Dar, S.A.; Jain, R. Effect of swimming on cardiovascular endurance of secondary school students of District Shopion in J&K UT. Int. J. Phys. Educ. Sports Health 2020, 7, 92–95. [Google Scholar]
- Greco, C.C.; Denadai, B.S. Relationship between critical speed and endurance capacity in young swimmers: Effect of gender and age. Pediatr. Exerc. Sci. 2005, 17, 353–363. [Google Scholar] [CrossRef]
- Marinho-Buzelli, A.R.; Bonnyman, A.M.; Verrier, M.C. The effects of aquatic therapy on mobility of individuals with neurological diseases: A systematic review. Clin. Rehabil. 2015, 29, 741–751. [Google Scholar] [CrossRef]
- Suomi, R.; Koceja, D.M. Postural sway characteristics in women with lower extremity arthritis before and after an aquatic exercise intervention. Arch. Phys. Med. Rehabil. 2000, 81, 780–785. [Google Scholar] [CrossRef]
- Bielec, G.; Peczak-Graczyk, A.; Waade, B. Do swimming exercises induce anthropometric changes in adolescents? Issues Compr. Pediatr. Nurs. 2013, 36, 37–47. [Google Scholar] [CrossRef]
- Conceição, A.; Silva, A.J.; Barbosa, T.; Campaniço, J.; Costa, A.; Louro, H. Neuromuscular and motor patterns in breaststroke technique. Rev. Bras. Cineantropom. Desempenho Hum. 2019, 21, e56408. [Google Scholar]
- McLeod, I.A. Swimming Anatomy; Human Kinetics: Champaign, IL, USA, 2009. [Google Scholar]
- Videler, J.J.; Videler, J.J. Swimming dynamics: Work from muscles. In Fish Swimming; Springer: Dordrecht, The Netherlands, 1993; pp. 139–164. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Sahli, S.; Baccouch, R.; Borji, R.; Sassi, B.; Rebai, H. Postural control and neuromuscular responses in young Kung-Fu practitioners. Sci. Sports 2021, 36, 112–119. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Warburton, D.E.; Janssen, I.; Paterson, D.H.; Latimer, A.E.; Rhodes, R.E.; Kho, M.E.; Hicks, A.; LeBlanc, A.G.; Zehr, L.J.; et al. New Canadian physical activity guidelines. Appl. Physiol. Nutr. Metab. 2011, 36, 36–46. [Google Scholar] [CrossRef]
- Kowalski, K.C.; Crocker, P.R.; Faulkner, R.A. Validation of the physical activity questionnaire for older children. Pediatr. Exerc. Sci. 1997, 9, 174–186. [Google Scholar] [CrossRef]
- Kowalski, K.C.; Crocker, P.R.; Donen, R.M. university of saskatchewan. The physical activity questionnaire for older children (PAQ-C) and adolescents (PAQ-A) manual. Coll. Kinesiol. Univ. Sask. 2004, 87, 1–38. [Google Scholar]
- Benítez-Porres, J.; Alvero-Cruz, J.R.; Sardinha, L.B.; López-Fernández, I.; Carnero, E.A. Cut-off values for classifying active children and adolescents using the Physical Activity Questionnaire: PAQ-C and PAQ-A. Nutr. Hosp. 2016, 33, 1036–1044. [Google Scholar] [CrossRef]
- Qiu, H.; Xiong, S. The influence of foot sizes on human balance. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, San Diego, CA, USA, 30 September–4 October 2013; Volume 57, pp. 920–924. [Google Scholar]
- Rodríguez-Rubio, P.R.; Bagur-Calafat, C.; López-de-Celis, C.; Bueno-Gracía, E.; Cabanas-Valdés, R.; Herrera-Pedroviejo, E.; Girabent-Farrés, M. Validity and reliability of the satel 40 HZ stabilometric force platform for measuring quiet stance and dynamic standing balance in healthy subjects. Int. J. Environ. Res. Public Health 2020, 17, 7733. [Google Scholar] [CrossRef]
- Meshkati, Z.; Namazizadeh, M.; Salavati, M.; Mazaheri, M. Reliability of force-platform measures of postural sway and expertise-related differences. J. Sport Rehabil. 2011, 20, 442–456. [Google Scholar] [CrossRef]
- Baldini, A.; Nota, A.; Assi, V.; Ballanti, F.; Cozza, P. Intersession reliability of a posturo-stabilometric test, using a force platform. J. Electromyogr. Kinesiol. 2013, 23, 1474–1479. [Google Scholar] [CrossRef]
- Jabnoun, S.; Borji, R.; Sahli, S. Postural control of Parkour athletes compared to recreationally active subjects under different sensory manipulations: A pilot study. Eur. J. Sport Sci. 2019, 19, 461–470. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Techniques and methods for testing the postural function in healthy and pathological subjects. BioMed Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef]
- Kollmitzer, J.; Ebenbichler, G.R.; Kopf, A. Reliability of surface electromyographic measurements. Clin. Neurophysiol. 1999, 110, 725–734. [Google Scholar] [CrossRef]
- Silverman, J.D.; Balbinot, G.; Masani, K.; Zariffa, J.; Eng, P. Validity and Reliability of Surface Electromyography Features in Lower Extremity Muscle Contraction in Healthy and Spinal Cord–Injured Participants. Top. Spinal Cord Inj. Rehabil. 2021, 27, 14–27. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Yoon, W.-Y. The acute effect of neuromuscular activation in resistance exercise on human skeletal muscle with the interpolated twitch technique. J. Phys. Ther. Sci. 2015, 27, 2879–2882. [Google Scholar] [CrossRef][Green Version]
- Yoshiko, A.; Watanabe, K.; Akima, H. Relative contribution of neuromuscular activation, muscle size, and muscle quality to maximum strength output of the thigh muscles in young individuals. Physiol. Rep. 2023, 11, e15563. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Seifert, L.; Carmigniani, R. Coordination and stroking parameters in the four swimming techniques: A narrative review. Sports Biomech. 2023, 22, 1617–1633. [Google Scholar] [CrossRef]
- Guignard, B.; Rouard, A.; Chollet, D.; Bonifazi, M.; Dalla Vedova, D.; Hart, J.; Seifert, L. Upper to lower limb coordination dynamics in swimming depending on swimming speed and aquatic environment manipulations. Mot. Control 2019, 23, 418–442. [Google Scholar] [CrossRef]
- Kim, K.H.; Shin, H.K. The effects of water-based exercise on postural control in children with spastic cerebral palsy. Phys. Ther. Rehabil. Sci. 2017, 6, 77–82. [Google Scholar] [CrossRef]
- Carayannopoulos, A.G.; Han, A.; Burdenko, I.N. The benefits of combining water and land-based therapy. J. Exerc. Rehabil. 2020, 16, 20. [Google Scholar] [CrossRef]
- Wilcock, I.M.; Cronin, J.B.; Hing, W.A. Physiological response to water immersion: A method for sport recovery? Sports 2006, 36, 747–765. [Google Scholar] [CrossRef]
- Guo, W.; Soh, K.G.; Zakaria, N.S.; Hidayat Baharuldin, M.T.; Gao, Y. Effect of resistance training methods and intensity on the adolescent swimmer’s performance: A systematic review. Front. Public Health 2022, 10, 840490. [Google Scholar] [CrossRef]
- Ma, Q.; Song, Y.; Zheng, Q.; Meng, X. Effects of postural balance training on stability in swimmers. Rev. Bras. Med. Esporte 2023, 29, e2023_0085. [Google Scholar] [CrossRef]
- Conceição, A.; Fernandes, O.; Baia, M.; Parraca, J.A.; Gonçalves, B.; Batalha, N. Changes in Muscular Activity in Different Stable and Unstable Conditions on Aquatic Platforms. Biology 2022, 11, 1643. [Google Scholar] [CrossRef]
- Robert, G.; Gueguen, N.; Avogadro, P.; Mouchnino, L. Anticipatory balance control is affected by loadless training experiences. Hum. Mov. Sci. 2004, 23, 169–183. [Google Scholar] [CrossRef]
- Baccouch, R.; Ben Waer, F.; Laatar, R.; Borji, R.; Rebai, H.; Sahli, S. Swimming, better than tennis, develops sensorimotor adaptabilities involved in postural balance in 5-6-year-old children. Somatosens. Mot. Res. 2023, 40, 1–7. [Google Scholar] [CrossRef]
- Kavounoudias, A.; Roll, R.; Roll, J.-P. The plantar sole is a ‘dynamometric map’for human balance control. Neuroreport 1998, 9, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T. Plasticity of the postural function to sport and/or motor experience. Neurosci. Biobehav. Rev. 2017, 72, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Jouira, G.; Rebai, H.; Alexe, D.I.; Sahli, S. Postural Balance in Boys With Intellectual Disabilities Who Participate in Soccer Training. Pediatr. Exerc. Sci. 2024, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Onofrei, R.R.; Amaricai, E. Postural balance in relation with vision and physical activity in healthy young adults. Int. J. Environ. Res. Public Health 2022, 19, 5021. [Google Scholar] [CrossRef]
- Fransson, P.-A.; Magnusson, M.; Johansson, R. Analysis of adaptation in anteroposterior dynamics of human postural control. Gait Posture 1998, 7, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Mademli, L.; Mavridi, D.; Bohm, S.; Patikas, D.A.; Santuz, A.; Arampatzis, A. Standing on unstable surface challenges postural control of tracking tasks and modulates neuromuscular adjustments specific to task complexity. Sci. Rep. 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, M.; Prince, F.; Messier, J. Development of postural stability limits: Anteroposterior and mediolateral postural adjustment mechanisms do not follow the same maturation process. Hum. Mov. Sci. 2019, 63, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness control of balance in quiet standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Jouira, G.; Alexe, C.I.; Zinelabidine, K.; Rebai, H.; Mocanu, G.D.; Cojocaru, A.M.; Dragomir, L.; Čaušević, D.; Sahli, S. The Impact of aerobic dance intervention on postural balance in children: A randomized controlled trial. Children 2024, 11, 573. [Google Scholar] [CrossRef]
- Almeida, G.; Carvalho, R.; Talis, V. Postural strategy to keep balance on the seesaw. Gait Posture 2006, 23, 17–21. [Google Scholar] [CrossRef]
- Slobounov, S.; Newell, K. Postural dynamics as a function of skill level and task constraints. Gait Posture 1994, 2, 85–93. [Google Scholar] [CrossRef]
- Streepey, J.W.; Angulo-Kinzler, R.M. The role of task difficulty in the control of dynamic balance in children and adults. Hum. Mov. Sci. 2002, 21, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, H.M.; Hollander, A.P.; Van den Berg, C.; Vorontsov, A. Biomechanics of swimming. Exerc. Sport Sci. 2000, 1, 639–660. [Google Scholar]
- Lumini-Oliveira, J.; Magalhães, J.; Pereira, C.V.; Aleixo, I.; Oliveira, P.J.; Ascensão, A. Endurance training improves gastrocnemius mitochondrial function despite increased susceptibility to permeability transition. Mitochondrion 2009, 9, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Dakin, C.J.; Héroux, M.E.; Luu, B.L.; Inglis, J.T.; Blouin, J.-S.; Mildren, R.L.; Peters, R.M.; Carpenter, M.G.; Uginčius, P.; Yilmaz, G.; et al. Vestibular contribution to balance control in the medial gastrocnemius and soleus. J. Neurophysiol. 2016, 115, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Peterson, M.D.; Ogborn, D.; Contreras, B.; Sonmez, G.T. Effects of low- vs. high-load resistance training on muscle strength and hypertrophy in well-trained men. J. Strength Cond. Res. 2015, 29, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Sacilotto, G.B.; Ball, N.; Mason, B.R. A Biomechanical review of the techniques used to estimate or measure resistive forces in swimming. J. Appl. Biomech. 2014, 30, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Conceição, A.; Frédéric, P.; Louro, H.; Morgado, S.; Seifert, L. Effect of glide on neuromuscular adaptation in breaststroke swimming: A case study of an elite swimmer. Sci. Int. 2018, 2, 233–237. [Google Scholar]
- Johnson, T.K.; Woollacott, M.H. Neuromuscular responses to platform perturbations in power-versus endurance-trained athletes. Percept. Mot. Ski. 2011, 112, 3–20. [Google Scholar] [CrossRef]
- Lee, W.-S.; Cheung, W.-H.; Qin, L.; Tang, N.; Leung, K.-S. Age-associated decrease of type IIA/B human skeletal muscle fibers. Clin. Orthop. Relat. Res. 2006, 450, 231–237. [Google Scholar] [CrossRef]
- Kons, R.L.; Sakugawa, R.L.; Rossato, M.; Diefenthaeler, F.; Detanico, D. Neuromuscular and postural control in visually and nonvisually impaired judo athletes: Case study. J. Exerc. Rehabil. 2019, 15, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wein, F.; Peultier-Celli, L.; van Rooij, F.; Saffarini, M.; Perrin, P. No significant improvement in neuromuscular proprioception and increased reliance on visual compensation 6 months after ACL reconstruction. J. Exp. Orthop. 2021, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, X.; Fan, M.; Deng, L.; Jian, C.; Wei, M.; Luo, J. The effect of visual stimuli on stability and complexity of postural control. Front. Neurol. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Blenkinsop, G.M.; Pain, M.T.G.; Hiley, M.J. Balance control strategies during perturbed and unperturbed balance in standing and handstand. R. Soc. Open Sci. 2017, 4, 161018. [Google Scholar] [CrossRef]
- Trajković, N.; Kozinc, Ž.; Smajla, D.; Šarabon, N. Relationship between ankle strength and range of motion and postural stability during single-leg quiet stance in trained athletes. Sci. Rep. 2021, 11, 11749. [Google Scholar] [CrossRef]
- Afschrift, M.; De Groote, F.; Verschueren, S.; Jonkers, I. Increased sensory noise and not muscle weakness explains changes in non-stepping postural responses following stance perturbations in healthy elderly. Gait Posture 2018, 59, 122–127. [Google Scholar] [CrossRef]
| A-S (N = 10) | N-A-S (N = 10) | Degrees of Freedom | Independent-t Test | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Coefficient of Variation | Mean (SD) | Coefficient of Variation | ||||
| Age (years) | 12.10 ± 0.31 | 2.56 | 11.9 ± 0.33 | 2.77 | 18 | NS | 0.3 |
| Height (cm) | 147 ± 2.9 | 1.97 | 146 ± 3.2 | 2.19 | 18 | NS | 0.4 |
| Mass (kg) | 38.01 ± 1.7 | 4.47 | 37.00 ± 2.5 | 6.75 | 18 | NS | 0.3 |
| Foot size (cm) | 38.02 ± 2.2 | 3.15 | 38.00 ± 2.00 | 5.26 | 18 | NS | 0.1 |
| F | Degrees of Freedom | p | ηp² | |
|---|---|---|---|---|
| CoParea | ||||
| Group | 22.27 | 1.18 | <0.001 | 0.55 |
| Vision | 452.12 | 1.18 | <0.001 | 0.96 |
| Vision × Group | 1.045 | 1.18 | =0.32 | - |
| Surface | 392.87 | 2.17 | <0.001 | 0.97 |
| Surface × Group | 14.35 | 2.17 | <0.001 | 0.62 |
| Surface × Vision | 166.42 | 2.17 | <0.001 | 0.95 |
| Surface × Group × Vision | 0.63 | 2.17 | 0.544 | - |
| CoPVm | ||||
| Group | 75.61 | 1.18 | <0.001 | 0.80 |
| Vision | 193.48 | 1.18 | <0.001 | 0.91 |
| Vision × Group | 11.56 | 1.18 | =0.003 | 0.39 |
| Surface | 259.63 | 2.17 | <0.001 | 0.96 |
| Surface × Group | 11.69 | 2.17 | <0.001 | 0.57 |
| Surface × Vision | 123.23 | 2.17 | <0.001 | 0.93 |
| Surface × Group × Vision | 4.98 | 2.17 | =0.020 | 0.37 |
| RMS | ||||
| Group | 28.97 | 1.18 | =0.001 | 0.61 |
| Vision | 46.87 | 1.18 | <0.001 | 0.72 |
| Vision × Group | 0.86 | 1.18 | =0.35 | - |
| Direction | 7.93 | 1.18 | =0.011 | 0.30 |
| Direction × Group | 9.34 | 1.18 | =0.007 | 0.34 |
| Direction × Vision | 0.63 | 1.18 | =0.43 | - |
| Direction × Group × Vision | 0.37 | 1.18 | =0.54 | - |
| A-S | N-A-S | p-Value | 95% CI | d | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | ||||
| CoParea | |||||||
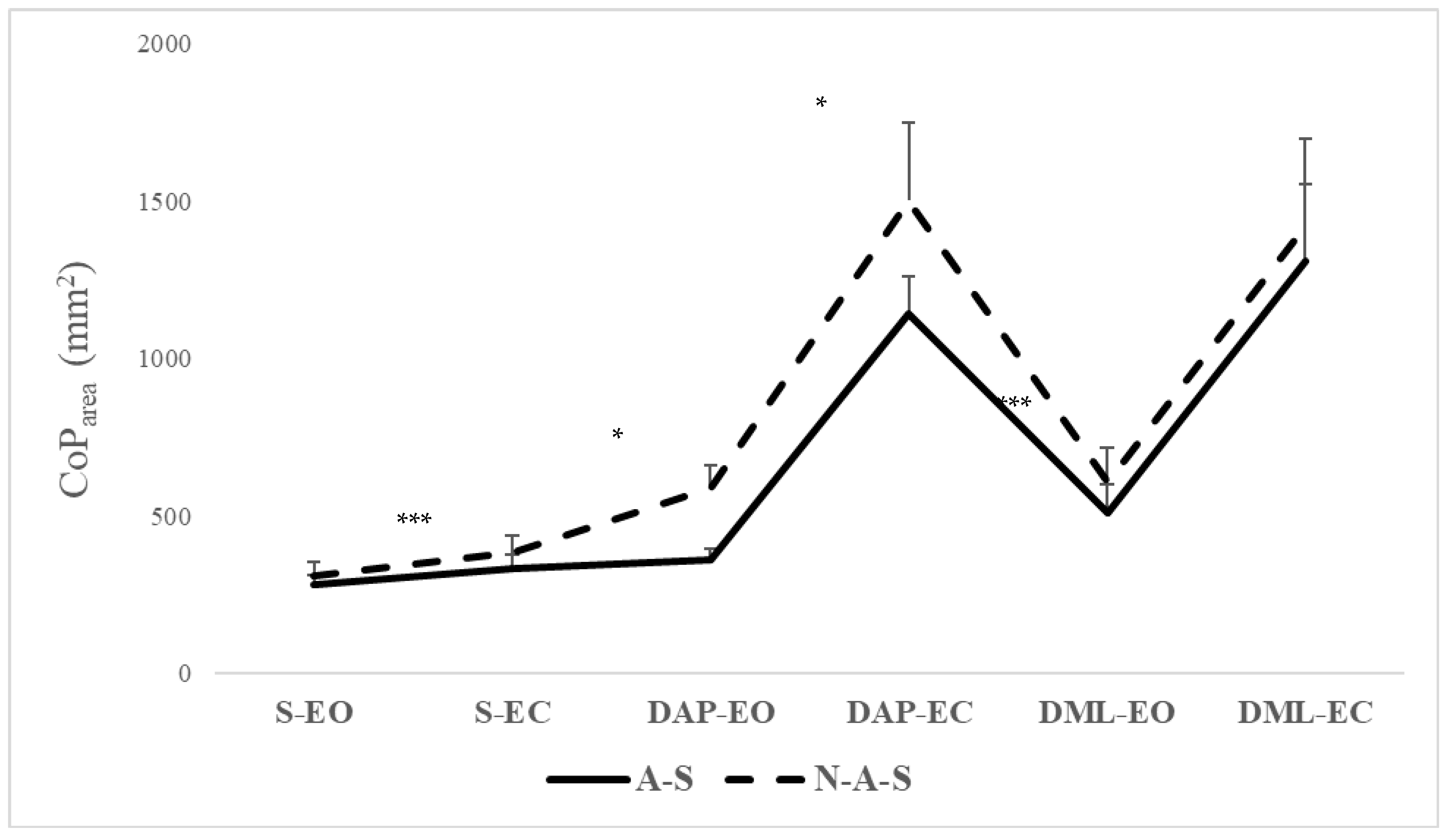
| S-EO | 281.55 (31.30) | 259.15 to 303.94 | 307.5 (44.24) | 275.86 to 339.15 | =0.147 | −10.04 to 61.96 | - |
| S-EC | 330.59 (44.80) | 298.53 to 362.64 | 382.37 (53.39) | 344.17 to 420.57 | <0.001 | 178.15 to 282.690 | −1.05 |
| DAP-EO | 359.73 (33.94) | 335.44 to 384.01 | 590.15 (70.97) | 539.37 to 640.92 | =0.038 | 6.22 to 194.99 | −4.14 |
| DAP-EC | 1142.80 (116.95) | 1059.15 to 1226.48 | 1500.68 (248.79) | 1322.72 to 1678.63 | =0.034 | 5.47 to 98.09 | −1.84 |
| DML-EO | 507.15 (93.28) | 440.41 to 573.88 | 607.79 (107.13) | 531.11 to 684.40 | <0.001 | 175.23 to 540.49 | −1.00 |
| DML-EC | 1309.99 (240.90) | 1137.65 to 1482.32 | 1422.45 (275.19) | 1225.58 to 1619.13 | =0.340 | −130.53 to 355.45 | - |
| CoPVm | |||||||
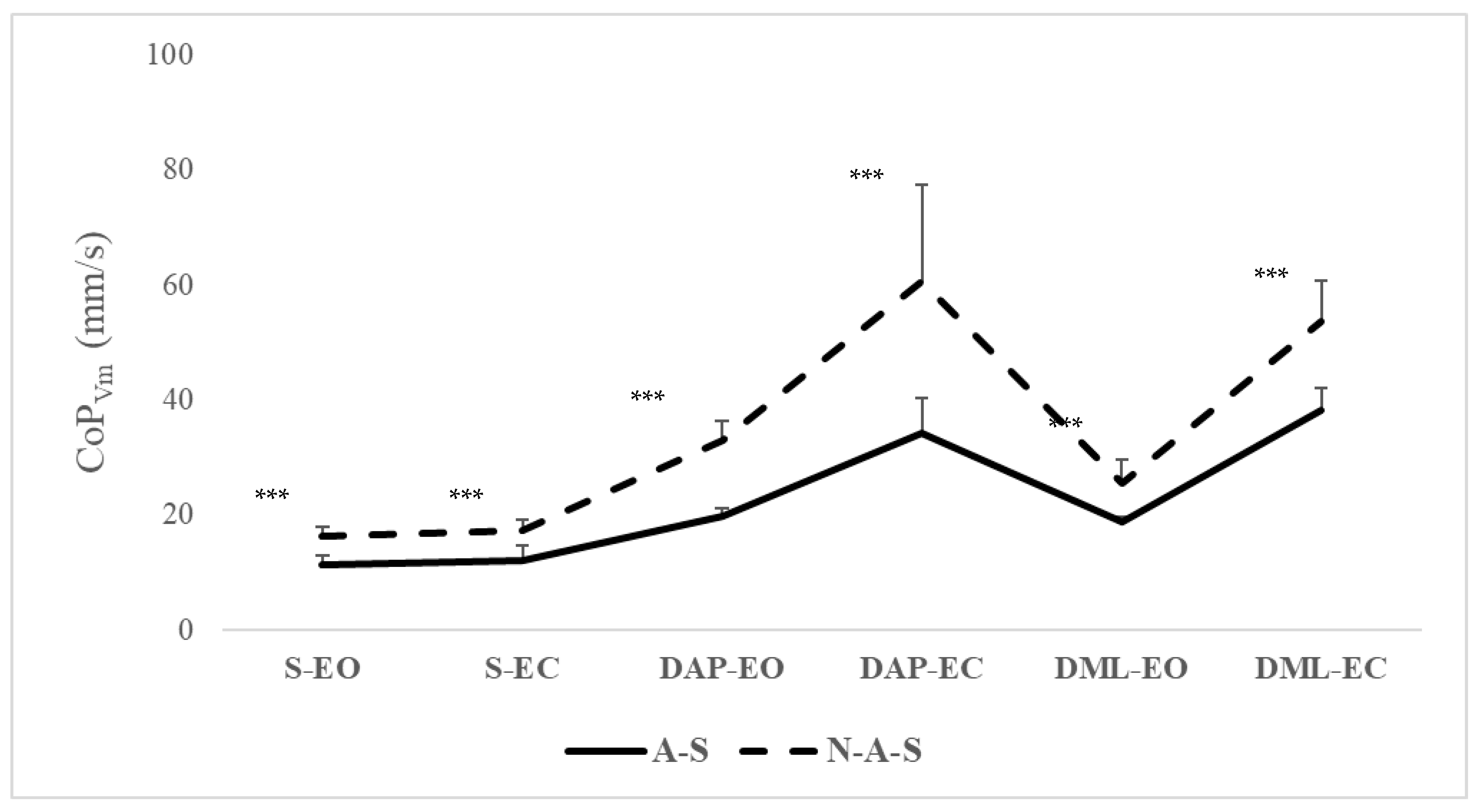
| S-EO | 11.48 (1.46) | 10.43 to 12.53 | 16.22 (1.66) | 15.02 to 17.44 | <0.001 | 3.26 to 6.20 | −3.03 |
| S-EC | 12.08 (2.68) | 10.16 to 14.00 | 17.38 (1.82) | 16.07 to 18.68 | <0.001 | 11.06 to 15.70 | −2.31 |
| DAP-EO | 19.71 (1.52) | 18.68 to 20.80 | 33.10 (3.14) | 30.84 to 35.35 | <0.001 | 3.64 to 9.53 | −5.42 |
| DAP-EC | 34.21 (6.01) | 29.90 to 38.51 | 60.54 (16.85) | 48.48 to 72.59 | <0.001 | 3.13 to 7.45 | −2.02 |
| DML-EO | 18.82 (0.93) | 18.16 to 19.49 | 25.42 (4.33) | 22.32 to 28.51 | <0.001 | 14.43 to 38.21 | −2.10 |
| DML-EC | 38.19 (3.80) | 35.47 to 40.91 | 53.59 (7.09) | 48.51 to 58.66 | <0.001 | 10.04 to 20.74 | −2.70 |
| RMS | |||||||
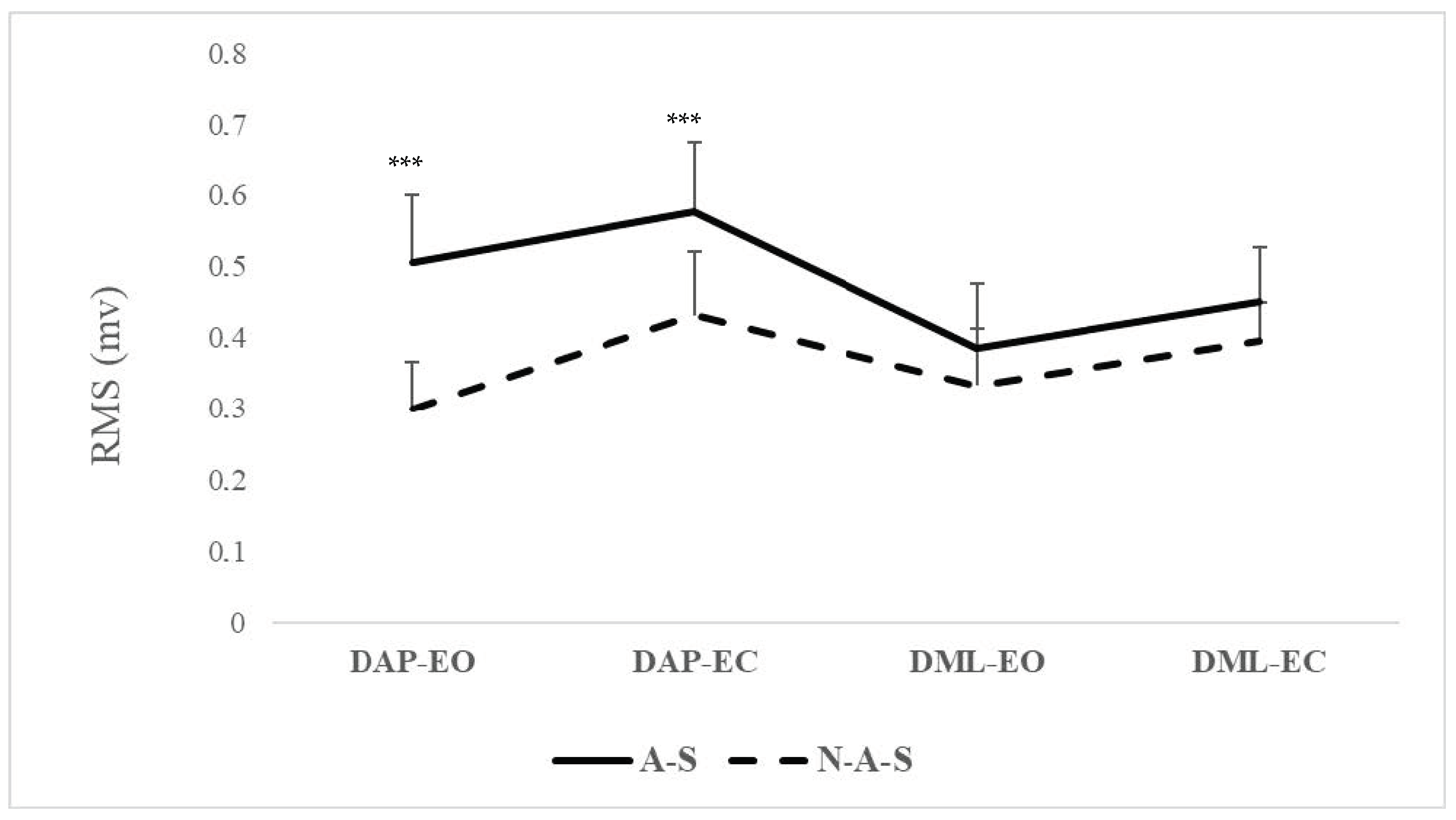
| DAP-EO | 0.50 (0.09) | 0.43 to 0.57 | 0.30 (0.06) | 0.25 to 0.34 | <0.001 | −0.28 to −1.27 | 2.61 |
| DAP-EC | 0.57 (0.09) | 0.50 to 0.64 | 0.41 (0.10) | 0.34 to 0.49 | <0.001 | −0.25 to −0.67 | 1.68 |
| DML-EO | 0.38 (0.09) | 0.32 to 0.45 | 0.33 (0.08) | 0.27 to 0.39 | =0.182 | −0.13 to 0.27 | - |
| DML-EC | 0.45 (0.07) | 0.39 to 0.50 | 0.39 (0.05) | 0.35 to 0.50 | =0.073 | −0.11 to −0.006 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baccouch, R.; Jouira, G.; Alexe, C.I.; Tohănean, D.I.; Alexe, D.I. Postural Control and Neuromuscular Activation in 11–13-Year-Old Athletic Boy Swimmers. Children 2024, 11, 863. https://doi.org/10.3390/children11070863
Baccouch R, Jouira G, Alexe CI, Tohănean DI, Alexe DI. Postural Control and Neuromuscular Activation in 11–13-Year-Old Athletic Boy Swimmers. Children. 2024; 11(7):863. https://doi.org/10.3390/children11070863
Chicago/Turabian StyleBaccouch, Rym, Ghada Jouira, Cristina Ioana Alexe, Dragoș Ioan Tohănean, and Dan Iulian Alexe. 2024. "Postural Control and Neuromuscular Activation in 11–13-Year-Old Athletic Boy Swimmers" Children 11, no. 7: 863. https://doi.org/10.3390/children11070863
APA StyleBaccouch, R., Jouira, G., Alexe, C. I., Tohănean, D. I., & Alexe, D. I. (2024). Postural Control and Neuromuscular Activation in 11–13-Year-Old Athletic Boy Swimmers. Children, 11(7), 863. https://doi.org/10.3390/children11070863








