Abstract
Congenital heart diseases (CHDs) represent a heterogeneous group of congenital defects, with high prevalence worldwide. Non-invasive imaging is essential to guide medical and surgical planning, to follow the patient over time in the evolution of the disease, and to reveal potential complications of the chosen treatment. The application of cardiac magnetic resonance imaging (CMRI) in this population allows for obtaining detailed information on the defects without the necessity of ionizing radiations. This review emphasizes the central role of CMR in the overall assessment of CHDs, considering also the limitations and challenges of this imaging technique. CMR, with the application of two-dimensional (2D) and tri-dimensional (3D) steady-state free precession (SSFP), permits the obtaining of very detailed and accurate images about the cardiac anatomy, global function, and volumes’ chambers, giving essential information in the intervention planning and optimal awareness of the postoperative anatomy. Nevertheless, CMR supplies tissue characterization, identifying the presence of fat, fibrosis, or oedema in the myocardial tissue. Using a contrast agent for angiography sequences or 2D/four-dimensional (4D) flows offers information about the vascular, valvular blood flow, and, in general, the cardiovascular system hemodynamics. Furthermore, 3D SSFP CMR acquisitions allow the identification of coronary artery abnormalities as an alternative to invasive angiography and cardiovascular computed tomography (CCT). However, CMR requires expertise in CHDs, and it can be contraindicated in patients with non-conditional devices. Furthermore, its relatively longer acquisition time and the necessity of breath-holding may limit its use, particularly in children under eight years old, sometimes requiring anesthesia. The purpose of this review is to elucidate the application of CMR during the pediatric age.
1. Introduction
Congenital heart diseases (CHDs) represent the most prevalent group of congenital defects worldwide, exhibiting a prevalence of approximately 0.9% of liveborn children [1,2]. CHDs consist of abnormalities in the development of the heart and great vessels. They are divided in two main categories: cyanotic CHD (CCHD); and acyanotic CHDs. CCHD represents a cardiac emergency in the neonatal period because it is characterized by a right-to-left shunt, which allows deoxygenated blood to mix with the oxygenated blood of the vascular circuit. The acyanotic CHDs, on the other hand, can manifest as either obstruction or shunt lesions. Obstructive lesions may occur in the ventricular inflow tracts, outflow tracts, and in the great vessels, leading to proximal chamber hypertrophy and distal dilatation to the obstruction. Shunt lesions create abnormal communications between the left and right heart chambers, and include conditions such as atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), and atrio-ventricular canal defects [2].
The development of prenatal screening, pediatric diagnostic techniques, and therapeutical innovations have contributed to increased survival among this heterogeneous group of patients, most of whom arrive in adulthood [3,4,5]. Advanced non-invasive imaging, providing anatomical and functional information, guides medical and surgical planning, and permits following the evolution of the disease over time, also revealing potential issues related to the chosen treatment [6,7]. Echocardiography is the most commonly used diagnostic technique for evaluating patients with CHD, with both pediatric and adult populations. However, it has significant limitations, particularly in patients with poor acoustic windows, and its imaging quality and interpretation are highly dependent on the skill and experience of the operator. Nowadays, cardiac magnetic resonance (CMR) is largely used, and overcomes echocardiographic limitation (Table 1), offering detailed information about the cardiac anatomy, function, flow, and tissue properties characteristics, as well as the evaluation of myocardial viability and perfusion without ionizing radiations. Nonetheless, CMR is currently widely available, although it requires high expertise in the CHD context, necessitating that the examination of these patients be performed in highly specialized and dedicated centers [8,9,10,11,12,13].

Table 1.
Advantages and disadvantages of echocardiography and cardiovascular magnetic resonance (CMR). * Unless performed in expert centers where non-MRI conditional devices are performed in adults.
A general standard CMR protocol for evaluating CHDs in pediatric patients includes: real-time localization imaging in three planes without ECG gating, useful for anatomy and extracardiac structures; two-dimensional (2D) balanced steady-state free precession (bSSFP) cine sequence, to report the anatomy, size and function of the ventricles; the 2D phase contrast (PC) flow sequences, to permit the evaluation of vascular and valvular flow, although recently the four-dimensional flow CMR resonance (4DFlow CMR) imaging technique has allowed a comprehensive and detailed analysis of cardiovascular flow in a single free-breathing acquisition, providing both quantitative and qualitative data on flow patterns in the heart and great vessels; whole heart isotropic three-dimensional (3D) SSFP imaging, for vascular evaluation without contrast material administration and visualization of proximal and mid-coronary arteries; and MR angiography (MRA), for vascular evaluation [11,12,13,14,15,16,17,18,19]. Moreover, CMR permits tissue characterization by acquiring T1 and T2 mapping sequences, which uses the proton density of the tissue to identify areas of fibrosis, oedema, and fat [6,20,21]. In addition, late gadolinium enhancement (LGE) sequences identify myocardial inflammation and fibrosis, due to the accumulation and slower wash-out of gadolinium in the myocardial areas affected by these conditions. Early gadolinium enhancements (EGE) can also be acquired and provide information about thromboembolic formations [22,23].
The application of CMR in CHDs demands a high level of expertise, given the intricacies of CHDs’ anatomy and treatment. Moreover, CMR’s relatively longer acquisition time and requirement for breath-holding during scanning may pose challenges, particularly in pediatric patients under eight years old, sometimes necessitating general anesthesia to ensure successful imaging acquisition. Finally, this imaging technique can be contraindicated in patients with non-CMR conditional devices, even if in some centers these patients have been started to be scanned regardless [11,21].
The aim of this review is to clarify the role of CMR in the assessment of CHDs, highlighting its current practice and future perspective and revealing the possible challenges and limitations of this imaging technique.
2. Cardiovascular Magnetic Resonance Applications in the Congenital Heart Diseases Affecting the Pediatric Population
2.1. Cardiovascular Magnetic Resonance in Assessing Atrial Septal Defects, Ventricular Septal Defects, Patent Ductus Arteriosus, and Atrioventricular Septal Defects
Atrial septal defects (ASDs), ventricular septal defects (VSDs), and patent ductus arteriosus (PDA) are among the most common CHDs in adults. These anomalies can vary widely in presentation and impact cardiac function, making accurate and detailed imaging crucial for diagnosis and management. CMR offers distinct advantages over other imaging modalities, clarifying the diagnosis, establishing the defect’s location and size, demonstrating the need and the timing for intervention, and monitoring post-surgical corrections [24].
Different CMR techniques are useful for the characterization of patients with suspected cardiac shunts. First, thanks to the 2D bSFPP images, CMR can quantify left (L) and right ventricular (RV) volumes and functions, which can be challenging with 2D transthoracic echocardiography, especially for the RV, due to its complex anatomy [25]. In addition, CMR via 2D PC flow or 4D Flow images can assess forward stroke volume measurements at the main pulmonary artery (MPA) and proximal ascending aorta (Ao), estimating respectively the pulmonary flow (Qp) and the systemic flow (Qs) with the correspondent pulmonary-to-systemic circulation flow ratio (Qp/Qs). Information on cardiac volumes and functions and Qp/Qs ratio are fundamental for understating the hemodynamic significance of shunts guiding subsequent interventions [26].
2.1.1. Atrial Septal Defects
ASDs represent communication between the atria. Transthoracic (TTE) and transesophageal echocardiography (TEE) remain the initial choice for evaluating ASDs to understand defect anatomy and guide percutaneous closure. However, it may not be sufficient in cases with complex anatomical abnormalities, especially for sinus venosus ASDs with an associated anomalous pulmonary venous return that needs an anatomical description of the pulmonary veins for repair procedure planning. CMR plays a vital role in defining the size, location, and hemodynamic impact of ASDs. Indeed, it can accurately measure the dimensions of the defects and assess the degree of right-sided volume overload, thanks to 2D SSFP sequences, and derive the Qp/Qs from the flow sequences as well as evaluating the presence and extent of associated complications, such as pulmonary hypertension. CMR should be strongly considered when: (1) the calculation of intracardiac shunting has been equivocal by echocardiography or interventional; (2) when RV dilation has been suspected on TTE without obvious detection of the anatomic defect; and (3) when associated anomalous pulmonary venous return is suspected [27,28]. In conclusion, CMR helps in setting an indication for ASDs closure when RV dilatation is detected or confirmed together with consensual increase in the Qp/Qs and the absence of pulmonary hypertension [6].
2.1.2. Ventricular Septal Defects
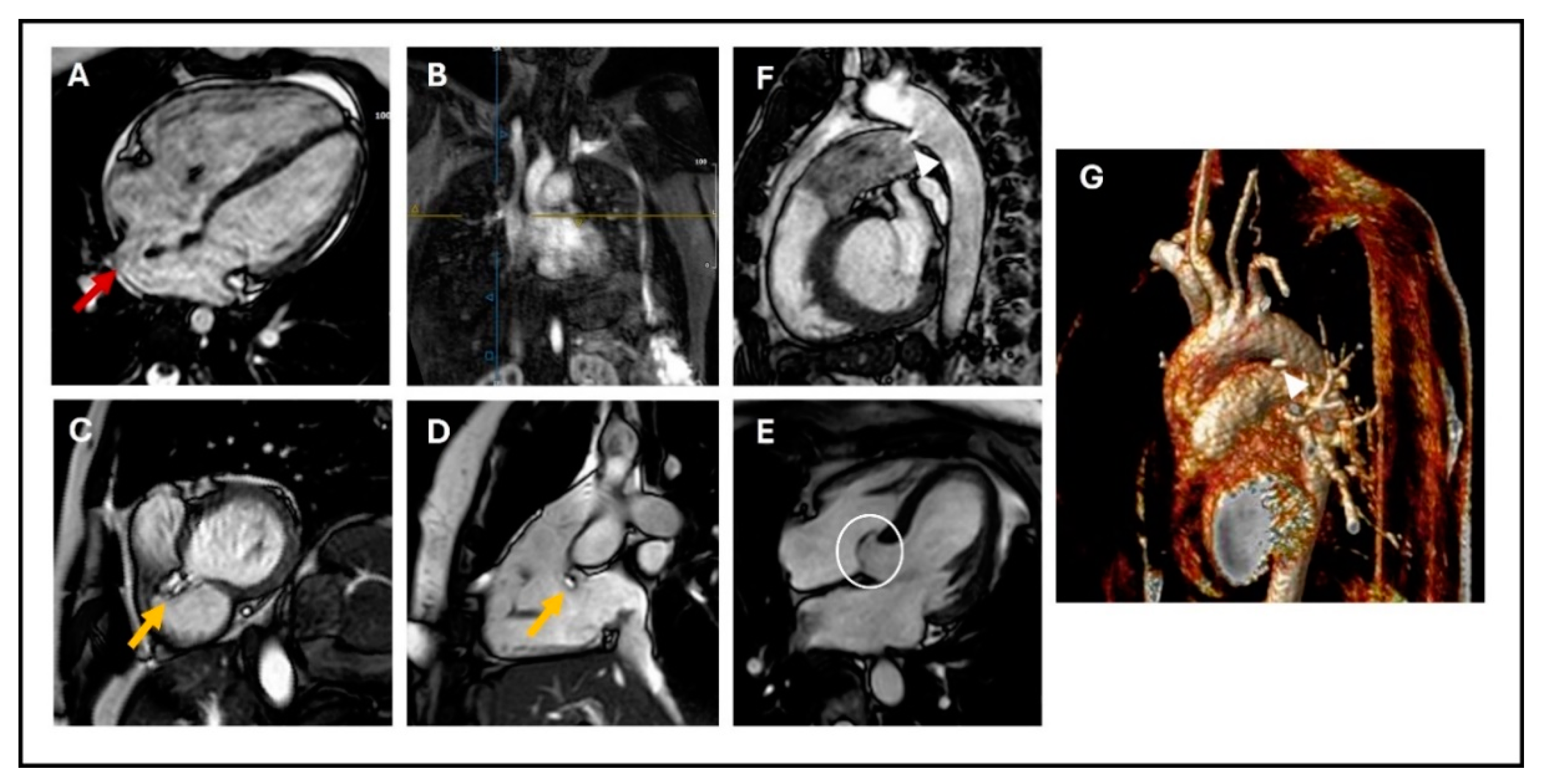
VSDs are the most common CHDs at birth; they can be localized wherever in the septum (membranous, muscular, and outlet defects), but the most common are in the perimembranous area [29] (Figure 1C,D). VSDs tend to close spontaneously during childhood in 40% of the cases. They are defined as restrictive when they are small enough to create a pressure gradient between the ventricles, so that the pulmonary ventricle and pulmonary vasculature are protected from the systemic pressure. As for ASDs, echocardiography is the first-line imaging technique; however, while multiple 2D views of the septum can help evaluate the position of a defect, it can be challenging to visualize the real entirety of the VSD and accurately measure its dimensions [30]. CMR can overcome this limitation, providing precise measurements of defect size and location, thanks to 2D bSSFP and 3D reconstructions sequences, and it can give information about the hemodynamic consequences (LV dilatation, increased Qp/Qs with LV stroke volume greater than the RV stroke volume). Thanks to this information, CMR may be useful for determining the need for interventional closure or surgical repair, indicated by LV dilatation and increased Qp/Qs in the absence of pulmonary hypertension [24,26]. CMR can also visualize healed VSDs, which tend to be associated with the aneurysmal formation of the basal septum and sometimes involve adjacent septal leaflets of the tricuspid valve (Figure 1E) [6,20].
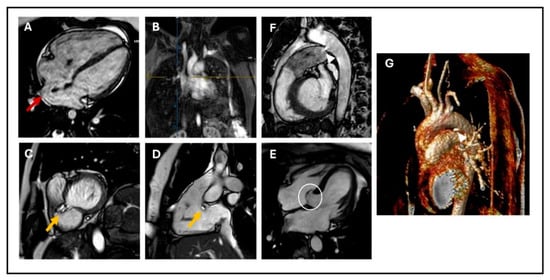
Figure 1.
(A) 4-chamber cine SSFP image showing sinus venosus ASD (red arrow); (B) Angiographic reconstruction showing the right upper pulmonary artery draining into the superior vena cava (blue and yellow cross); (C) Basal short axis cine SSFP image; (D) sagittal RV three-chamber view showing perimembranous VSD (yellow arrow); (E) 4-chamber cine SSFP image showing aneurysmal formation of the basal septum involving adjacent septal leaflet of the tricuspid valve (white circle); (F) Sagittal cine SSFP image; and (G) MRA showing PDA: (arrow-heads). SSFP: steady-state free precession, ASD: atrial septal defect, RV: right ventricle, VSD: ventricular septal defect, PDA: patent ductus arteriosus.
2.1.3. Patent Ductus Arteriosus
Patent ductus arteriosus (PDA) is a fetal vascular structure connecting the proximal descending aorta to the roof of the main pulmonary artery [31]. Although essential in fetal life for the right ventricular ejection into the aorta, PDA typically closes spontaneously after birth. It is frequently observed in pre-term newborns and, depending on its persistence, size, and degree of left-to-right shunting, can cause significant pulmonary overload, leading to increased pulmonary vascular resistance and pulmonary hypertension. Indications for closure include symptomatic left-chamber dilation or dysfunction, with Eisenmenger’s syndrome posing a risk of increased morbidity and mortality.
Transcatheter closure is the established treatment of choice. Cardiac magnetic resonance imaging (CMR) provides a detailed visualization of PDA using techniques such as 2D balanced steady-state free precession (bSSFP) cine imaging, 3D SSFP reconstruction, or angiography sequences (Figure 1F,G). CMR also allows assessment of its hemodynamic consequences, including indirect methods for quantifying the shunt caused by PDA. These methods include calculating the difference between the left ventricular stroke volume and total systemic flow (superior vena cava + descending aorta), which should equal the ductal shunt volume, and using the Qp/Qs ratio, which typically shows less than 1 due to the left-to-right shunting effect [26,32].
2.1.4. Atrio-Ventricular Septal Defects (AVSDs)
AVSDs are characterized by the absence of the muscular atrio-ventricular septum, inlet/outlet disproportion, abnormal lateral rotation of the postero-medial papillary muscle, and abnormal configuration of the atrioventricular valves. These defects can be complete or partial, often accompanied by varying degrees of atrio-ventricular valve abnormalities. Clinical presentation ranges from mild to severe depending on the size of the defect and associated cardiac anomalies.
Diagnosis typically relies on echocardiography, which assesses the anatomy and hemodynamics of the defect. Cardiac magnetic resonance imaging (CMR) complements echocardiography by providing detailed anatomical and functional information in diagnosing and characterizing atrioventricular septal defects (AVSDs). CMR enables precise assessment of the size, location, and extent of AVSDs, as well as the morphology and function of the atrioventricular valves [6,33]. It is also valuable in evaluating associated cardiac abnormalities such as anomalous pulmonary venous drainage and other complex structural anomalies commonly associated with AVSDs.
Furthermore, CMR facilitates accurate measurements of ventricular volumes and function, critical for surgical planning and assessing postoperative outcomes. Its most crucial role lies in post-surgical follow-up, as it is less commonly used before surgery. CMR plays a vital role in monitoring for complications such as residual shunts, atrio-ventricular valve dysfunction, and enlargement and dysfunction of the left and right ventricles, including left ventricular outflow tract obstruction [6,34].
2.2. Cardiovascular Magnetic Resonance in Assessing Conotruncal Congenital Heart Diseases
Conotruncal anomalies (CtA) are a group of CHDs that result from an altered pathway during embryogenesis, with abnormal formation and septation of the outflow tracts of the heart and the great vessels [35]. CtAs account for up to 25–30% of all non-syndromic CHDs and include tetralogy of Fallot (TOF), transposition of the great arteries (TGA), truncus arteriosus (TA), and double outlet right ventricle (DORV) [36]. When not appropriately diagnosed and managed, CtA might lead to significant morbidity and mortality [37]. Therefore, the need to find a proper diagnostic tool to adequately assess cardiac morphology, and at the same time to provide insight into ventricular performance [38].
2.2.1. Dextro-Transposition of the Great Arteries (D-TGA)
Complete transposition of the great arteries (TGA), also referred to as dextro-transposition of the great arteries (D-TGA), is a developmental cardiac defect [39,40] characterized by atrio-ventricular concordance and ventriculo-arterial discordance [41,42]. D-TGA is defined “simple” in the case of no associated congenital anomalies, whereas it is categorized as “complex” in their presence [6]. CMR imaging is rarely performed in the preoperative setting [12,40,43]. Over the years, the surgical treatment for D-TGA has evolved from the atrial switch procedure (AtSO) to the arterial switch operation (ASO). Complex D-TGA is often repaired using the Rastelli procedure or its variants [6]. In post-surgical management, CMR is addressed to depict the most common complications and potential residual findings after these procedures and it is usually repeated every 2–4 years [12,38].
Atrial Switch Operation and the Role of CMR Imaging
Complications after AtSO include baffle stenosis or leaks, systemic tricuspid valve (TV) regurgitation, and systemic right ventricle (sRV) dysfunction, with potential pulmonary hypertension often identified during routine imaging [44,45,46]. CMR is the gold standard for assessing sRV issues, offering detailed insights into heart morphology, function, and ejection fraction [12,40,47,48,49,50,51] thanks to the cine sequences, and it is especially recommended for evaluating systemic TV and baffles (Figure 2 and Figure 3) that are well studied from the 2D bSSFP, 3D whole heart, and angiographies [12,40]. Tricuspid regurgitation (TR) often stems from annulus dilatation, valve prolapse, or medial cuspid tethering, with occasional surgical damage to the valve leaflets [52,53,54]. CMR is also essential for detecting and assessing the severity of leaks and stenosis in the interatrial baffle [12,40,45] obtained through flow sequences. Myocardial performance, particularly fibrosis detection, is crucial, as it correlates with adverse outcomes—up to 60% of sRV patients exhibit LGE [45,55,56].
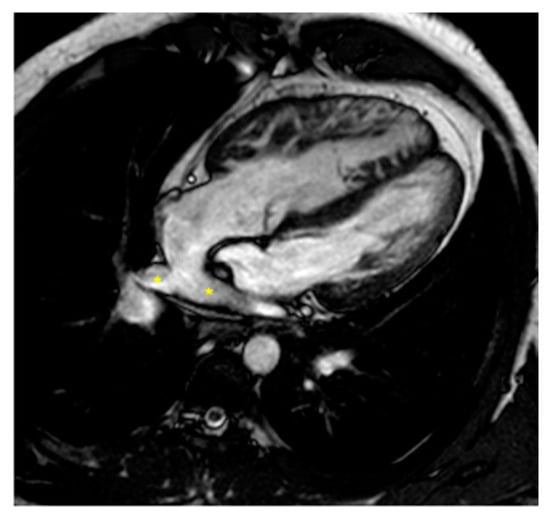
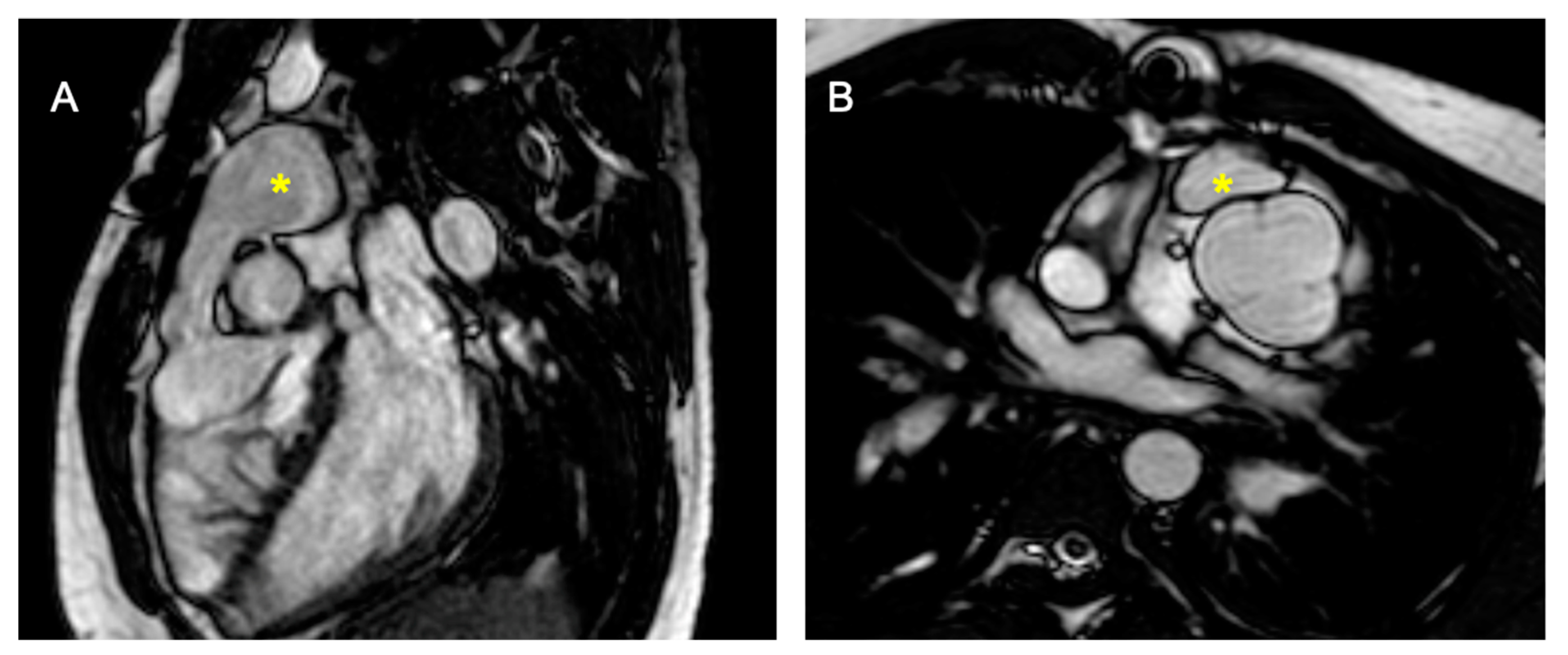
Figure 2.
Cine bSSFP image of D-TGA post-atrial switch operation using the Senning technique. The image shows the pulmonary veins (*) being redirected through the baffle into the right atrium and then to the subaortic positioned sRV.
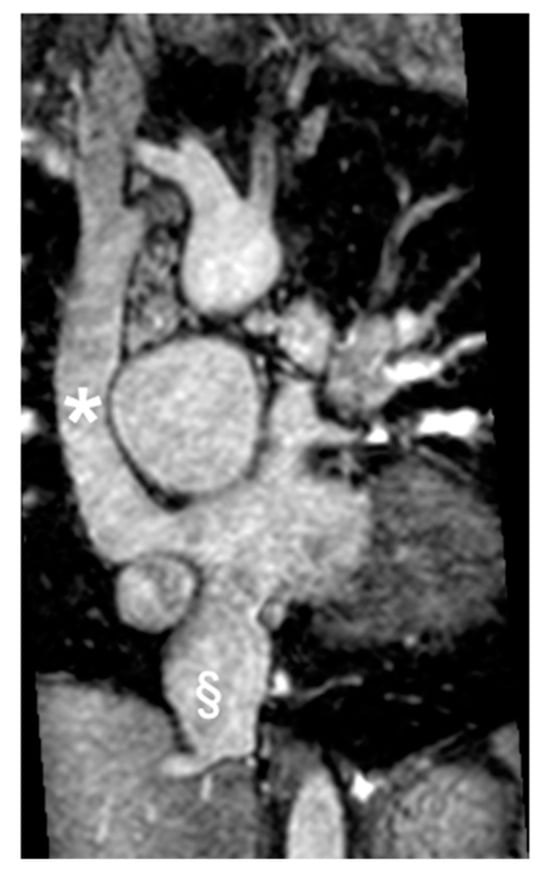
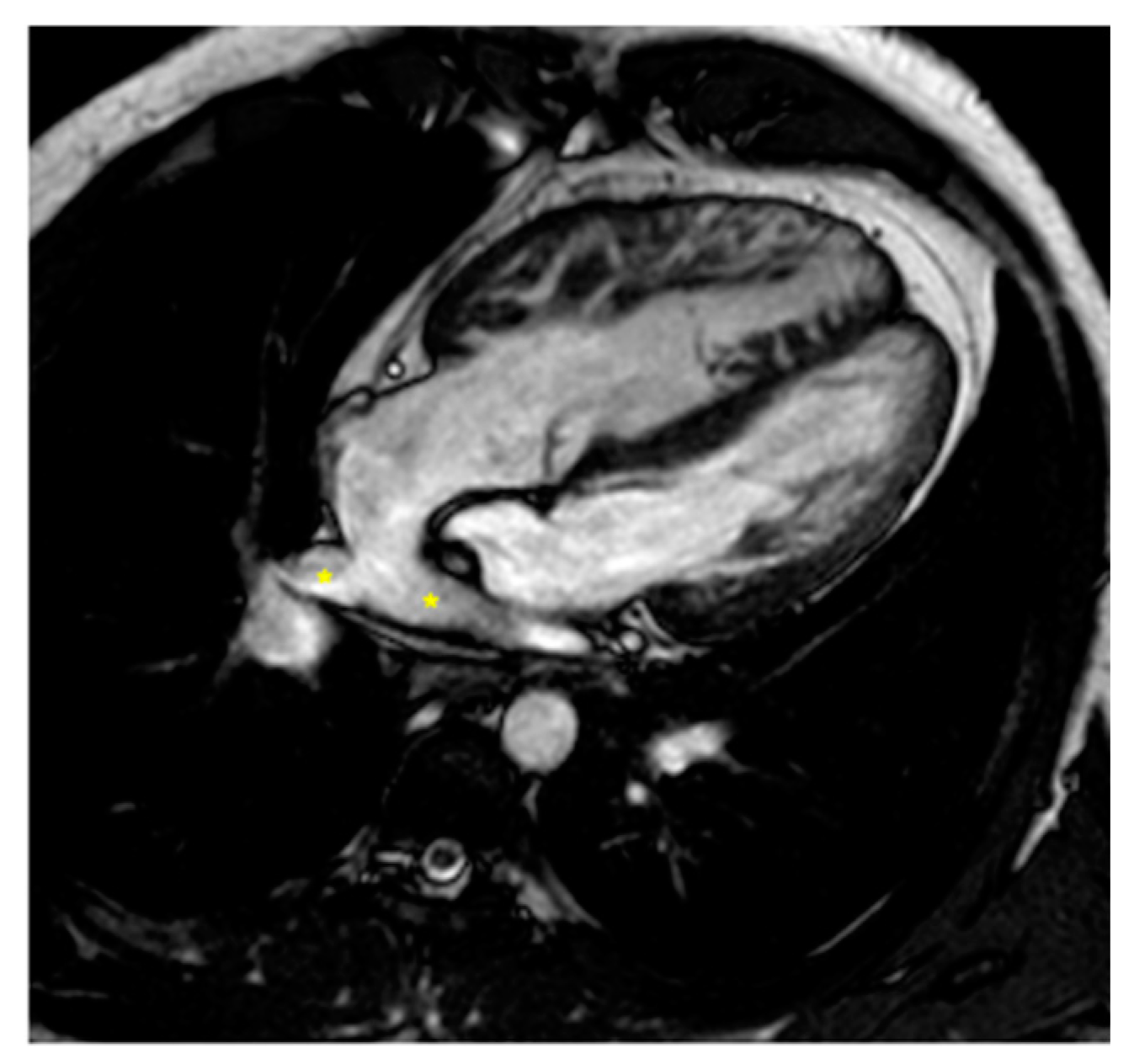
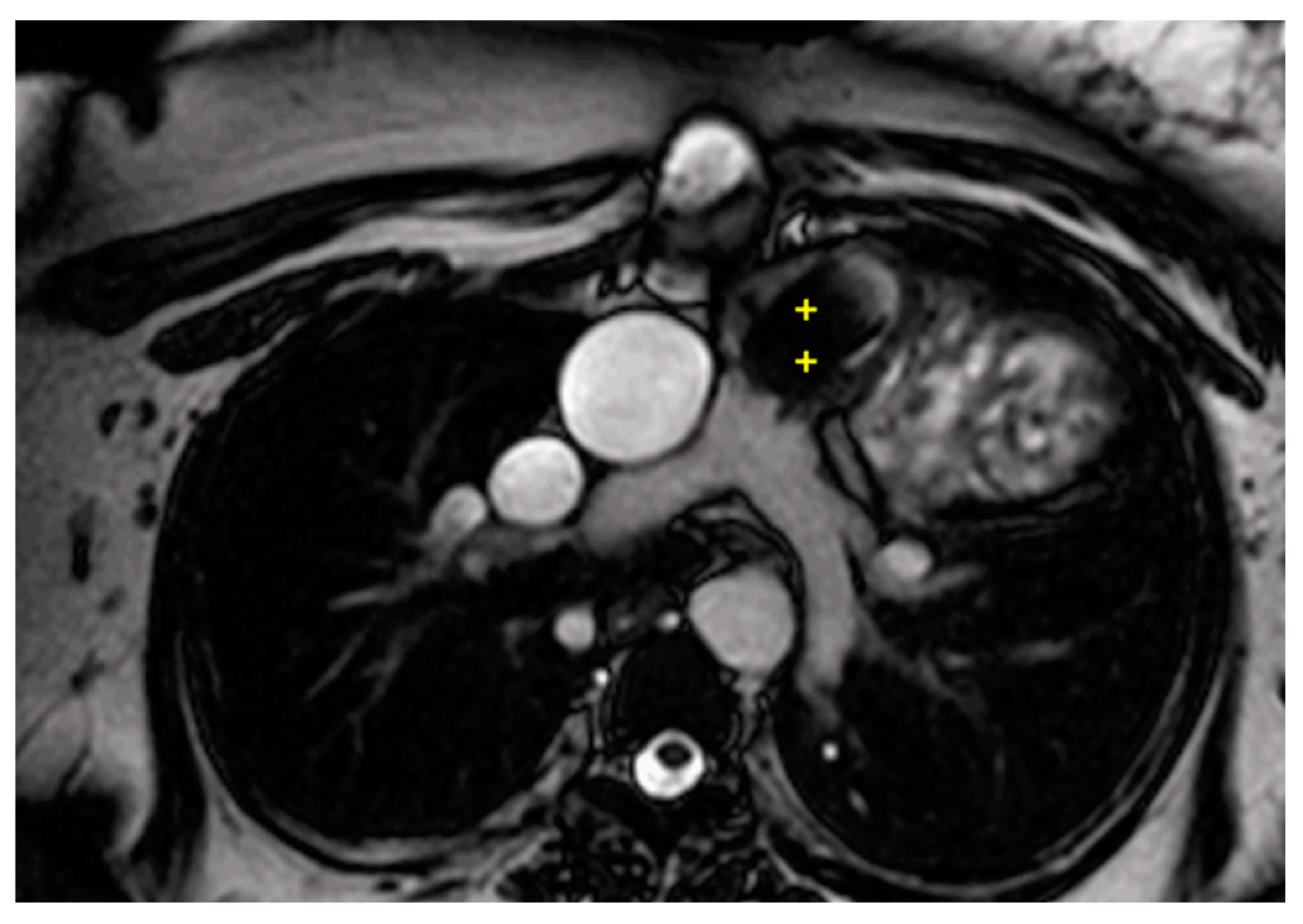
Figure 3.
Cine bSSFP image of D-TGA following an atrial switch operation using the Senning technique. This image illustrates the pathway of the systemic veins, with the superior vena cava (*) and the inferior vena cava (§) shown.
Arterial Switch Operation and the Role of CMR Imaging
Patients diagnosed with D-TGA post-1980s typically undergo the ASO, with late complications involving the great vessels, coronary arteries, and potential ventricular dilatation and dysfunction [40]. CMR imaging is crucial during long-term follow-up, particularly for assessing biventricular volumes, function, and morphology, as well as coronary artery and pulmonary artery stenosis (Figure 4) [6,12,57]. Despite normal ventricular volumes, decreased global longitudinal strain and LV torsion are noted [43,58]. CMR also evaluates myocardial perfusion, particularly in symptomatic patients, using vasodilator stress perfusion as a non-invasive test for ischemia and coronary obstruction [12]. It is essential for detecting myocardial scarring with LGE and should be repeated based on initial findings and symptoms [11,12,38,40,59,60].
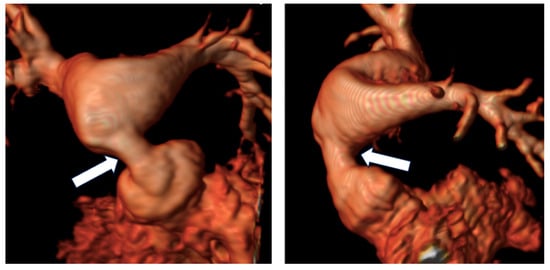
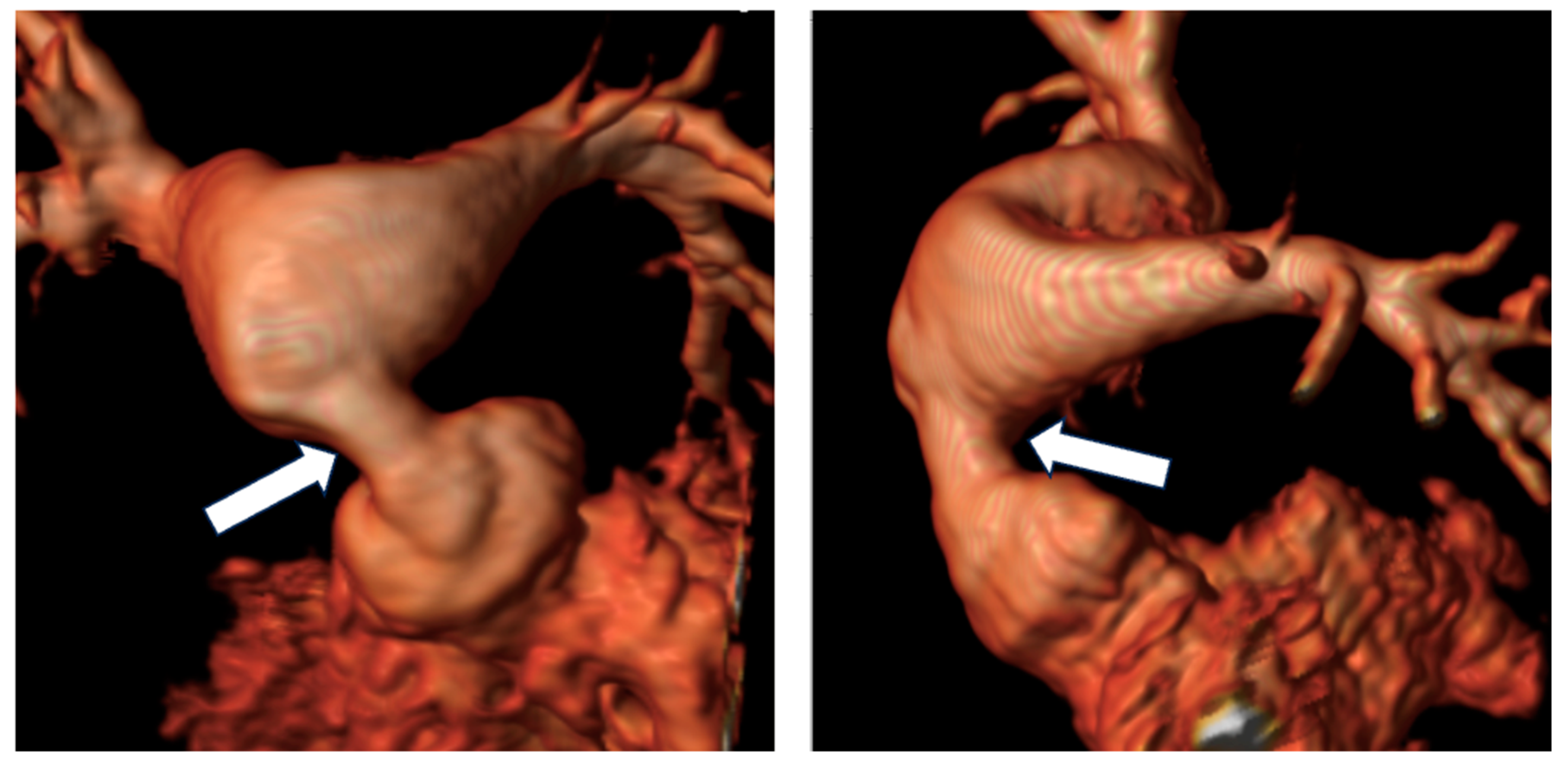
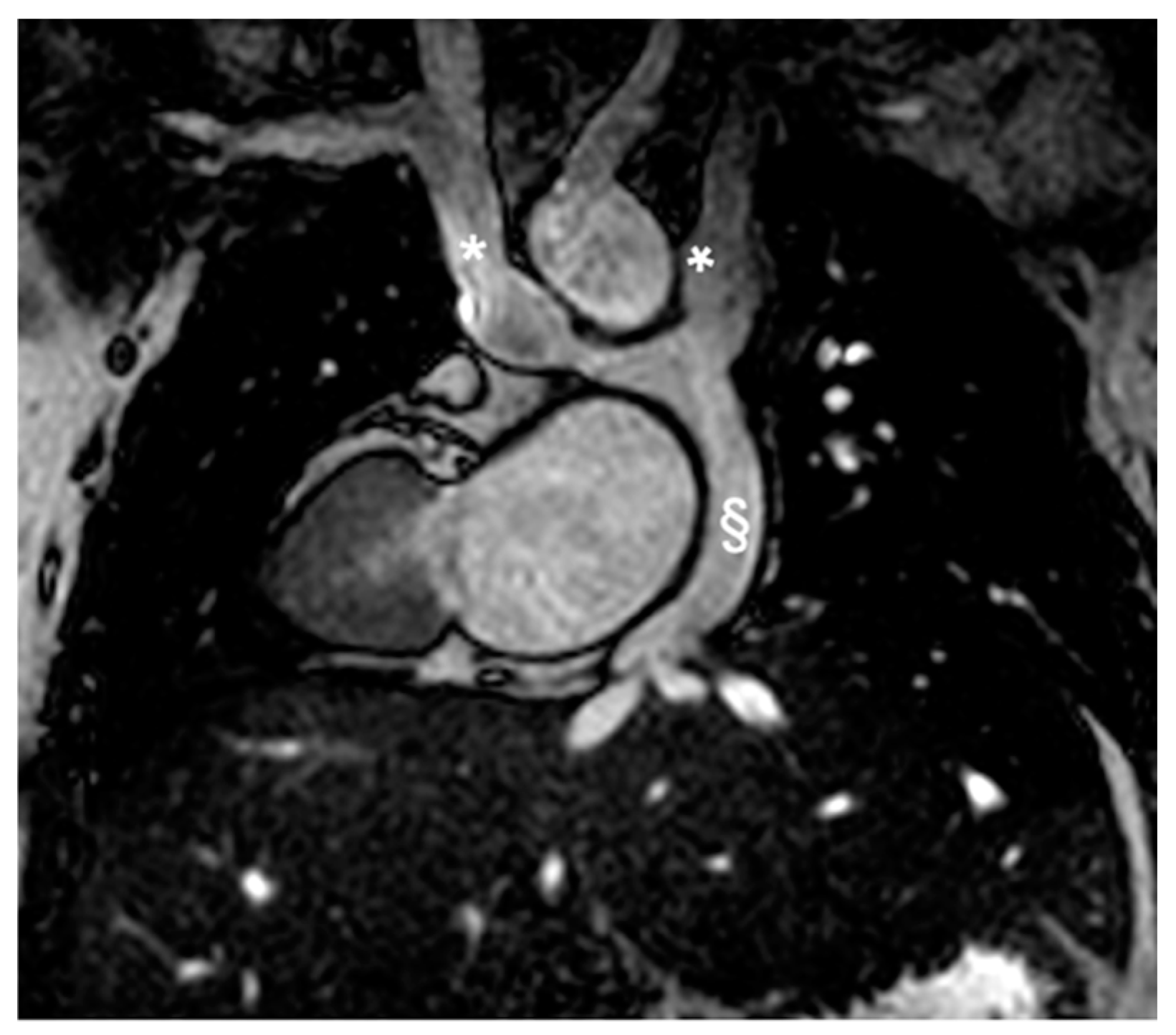
Figure 4.
Reconstruction following angiographic sequences in TGA post-arterial switch operation. The image highlights a suprapulmonary stenosis (white arrows) at the level of the surgical suture of the switch with post-stenotic dilation.
Rastelli Procedure and the Role of CMR Imaging
The Rastelli procedure and its variants are favored for D-TGA cases with VSD, pulmonary stenosis/atresia [61]. Common complications include RV-PA conduit deterioration (Figure 5), necessitating revisions or replacements, coronary artery and pulmonary branch stenosis, and deteriorating subpulmonary RV function due to prolonged pressure. Risks also involve subaortic obstruction and aortic valve dysfunction post-procedure [40,45]. CMR scans are essential for evaluating ventricular function, conduit and aortic baffle conditions, and coronary artery patency, using 2d bSSFP, angiography, and PC flow MRI to detect and quantify stenosis and regurgitation [40].
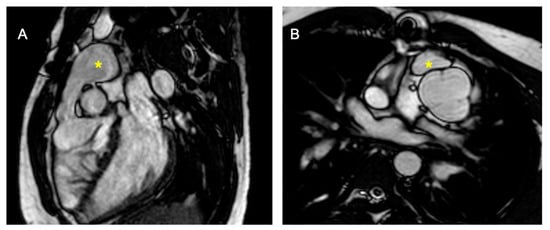
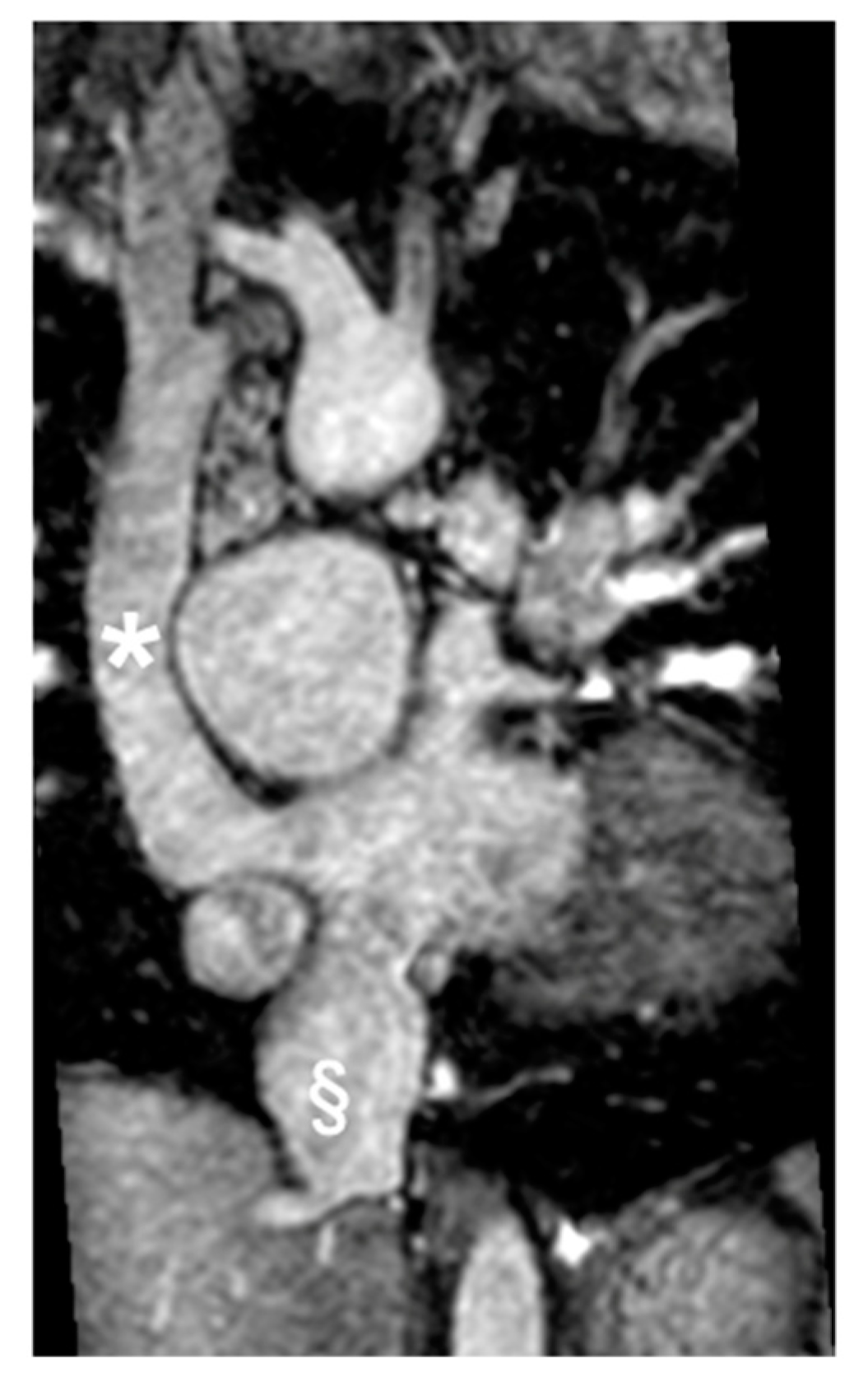
Figure 5.
D-TGA with pulmonary stenosis/atresia post-placement of the right ventricle to pulmonary artery (RV-PA) conduit. (A) shows a sagittal section, and (B) displays a transverse section, both highlighting the pulmonary conduit (marked with *).
2.2.2. Congenitally Corrected Transposition of Great Arteries (cc-TGA)
cc-TGA is a rare congenital cardiac malformation known as “double discordance”, characterized by atrio-ventricular and ventriculo-arterial discordance, representing less than 1% of all CHDs [62,63,64,65,66,67]. CMR is the preferred method for assessing the sRV [12,66] and TR for planning valve interventions, as well as for identifying myocardial fibrosis, which impacts sRV function over time [12,45,55]. The role of CMR extends to presurgical planning and monitoring post-surgical outcomes, helping to visualize ventricular function, anatomical repairs, and potential complications [40]. In 2019, Kawakubo et al. introduced the use of fractal analysis with CMR feature tracking to assess RV remodeling and myocardial strain, which could serve as indicators of systemic afterload response in adults with cc-TGA [66].
2.2.3. Tetralogy of Fallot
TOF is a key type of CHDs, comprising 5 to 7% of all CHDs and it requires ongoing comprehensive management across a patient’s life [67]. CMR is essential for the longitudinal monitoring of TOF, offering detailed insights into cardiac morphology, function, and hemodynamics, and is less invasive compared to catheterization [68]. It effectively identifies post-surgical complications like pulmonary stenosis and regurgitation, right ventricular dilatation, and residual ventricular septal defects (Figure 6) [65]. CMR is particularly crucial for accurately measuring pulmonary regurgitation, helping to decide the timing for pulmonary valve replacement and evaluating myocardial viability for surgical planning [69,70]. Recent advancements, like 4D flow imaging, enhance CMR’s utility by enabling dynamic blood flow visualization and quantification, which is vital in assessing repaired TOF patients, as shown in systematic reviews and studies focusing on valve function and myocardial fibrosis [71,72,73].
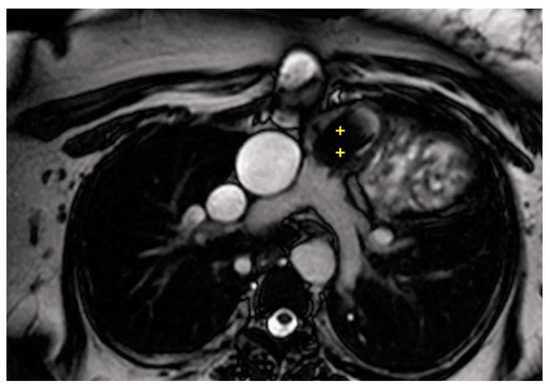
Figure 6.
Cine bSSFP image from a 15-year-old male with TOF, post complete correction and Melody valve implantation. The image shows significant migration of the Melody valve into the infundibulum (++).
2.2.4. Double Outlet Right Ventricle
Double outlet right ventricle (DORV) is characterized by both great arteries primarily arising from the right ventricle, representing 1–3% of all CHDs with an incidence of 3–9 per 100,000 live births [74,75,76]. Its classification hinges on the VSD location, arterial positioning, and potential outflow tract obstructions [77,78,79]. Transthoracic echocardiography initially assesses these anatomies, while preoperative CMR is invaluable for detailed visualizations of VSD and the spatial relationships necessary for surgical planning [77,79,80]. Post-surgery, CMR is critical for evaluating late-stage complications in older children and adults, helping assess structural and functional integrity across multiple cardiac components [80,81]. 4D flow imaging has proven effective in estimating right ventricular outflow tract (RVOT) diameters and characterizing cardiac flow dynamics, while computational fluid dynamics provides a deep analysis of cardiovascular dynamics, crucial for optimizing treatment and predicting patient outcomes [79,82,83].
2.3. Coarctation of the Aorta
Coarctation of the aorta (CoA) can be difficult to diagnose in utero, even with the advancements in fetal echocardiography, which can sometimes result in excessive false positives. Fetal CMR is emerging as a potent tool to accurately predict severe neonatal CoA issues before birth [84,85,86].
CMR is highly recommended for comprehensive aortic assessment in adolescents and adults, especially for evaluating the extent and severity of aortic narrowing, post-repair complications, and other critical aortic features. Current guidelines suggest regular CMR examinations post-intervention, with intervals of three to five years depending on the underlying condition. For structural and functional analysis, CMR uses 2D bSSFP cine sequences to assess cardiac volumes, mass, and the hypertrophic effects of long-standing hypertension in coarctation cases [87]. CMR angiography helps delineate the cardiovascular anatomy and identify any abnormalities such as constrictions or collateral circulation [88]. The 3D whole heart sequence, which does not require contrast, also contributes to this, and flow analysis can quantify the collaterals [89,90].
Predictive models based on CMR findings suggest that the minimum aortic cross-sectional area, heart rate-corrected deceleration time, and percentage of flow increase are critical predictors of outcomes in CoA patients [91,92].
2.4. Cardiovascular Magnetic Resonance Application in the “Univentricular Heart”
The term “univentricular heart” refers to hearts unable to undergo biventricular repair, typically due to having one functional ventricle or two ventricles unable to support separate pulmonary and systemic circulations consecutively. Examples include conditions such as pulmonary or aortic atresia, severe stenosis with a hypoplastic ventricle, hypoplastic left heart syndrome (HLHS), as well as rare conditions like large intramural cardiac tumors and Ebstein anomaly with extensive atrialization of the right ventricular cavity [93].
Surgical intervention for these cases involves univentricular repair through a Total Cavopulmonary Connection (TCPC) operation, which bypasses the ventricular mass in three stages [93,94]. Cardiac magnetic resonance imaging (CMR) plays a crucial role throughout these stages. Following the Norwood procedure, the decision to proceed to a bidirectional Glenn operation has traditionally relied on echocardiography and diagnostic cardiac catheterization. However, a retrospective study by Muthurangu et al. involving 37 HLHS patients demonstrated that CMR can effectively define ventricular and valvular function, as well as vascular anatomy, aiding in the planning of subsequent surgical interventions [95,96].
Furthermore, Brown et al. conducted a prospective, randomized, single-center trial comparing CMR to catheterization in infants’ post-Norwood procedure, showing CMR to be a safe and cost-effective alternative in appropriately selected patients. However, further research is necessary to determine the generalizability of these findings to other centers [97].
Further on, in the lead-up to TCPC completion, CMR aids in patient selection and preoperative assessment of critical information before the final surgery. Currently, there is no consensus on a standardized diagnostic protocol pre-TCPC—some centers rely on cardiac catheterization, despite the associated risks, while others favor CMR or a combination of both. Pujia Banka et al. in their cohort found that catheterization added little clinical value for about half of the patients, with echocardiography often providing incomplete information, suggesting a need for complementary imaging modalities like CMR [98]. Harris’s group highlighted CMR’s non-invasive assessment capabilities, particularly in predicting outcomes based on branch pulmonary area size and flow before the TCPC operation, potentially indicating patients at risk of prolonged hospitalization [99]. In summary, existing literature suggests that cardiac catheterization may be avoidable in select patients with single ventricle physiology before TCPC [100,101].
Lastly, CMR plays a crucial role in post-TCPC completion (Figure 7) by providing comprehensive information on anatomy, function, and hemodynamics, essential for identifying and understanding various complications. Routinely used in follow-up, CMR is performed every three to five years, with additional scans conducted when clinically indicated or during emergencies [102,103]. 2D bSSFP cine images facilitate the assessment of wall motion abnormalities, systolic impairment, and volume calculations [104]. Atrioventricular valve regurgitation, a common TCPC complication, is detectable and quantifiable through flow sequences. Moreover, CMR aids in identifying ventricular obstructions and stenosis in pulmonary arteries, systemic veins, and pulmonary veins. Flow sequences present flow distribution patterns of caval flows and pulmonary arteries, providing valuable information supporting potential transcatheter or surgical reinterventions [104,105,106]. Thromboembolic complications are assessed using EGE sequences, particularly important in TCPC patients with atrial arrhythmias. Desaturation can stem from conduit fenestration, pulmonary-to-systemic venous collaterals, or arterial venous malformations (Figure 8). CMR allows the precise calculation of collateral flow contribution to systemic cardiac output, guiding interventions if necessary. Additionally, CMR can investigate TCPC-associated liver disease and lymphatic dysfunction, though specialized protocols may be required. As awareness of the long-term effects grows, further studies will be needed to comprehensively understand TCPC’s impact on other systems [104,105,106].
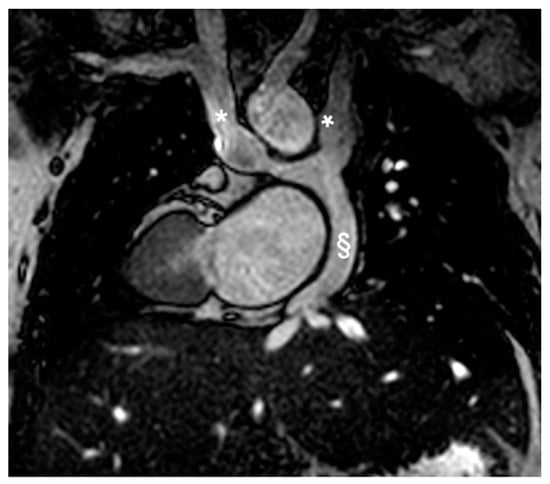
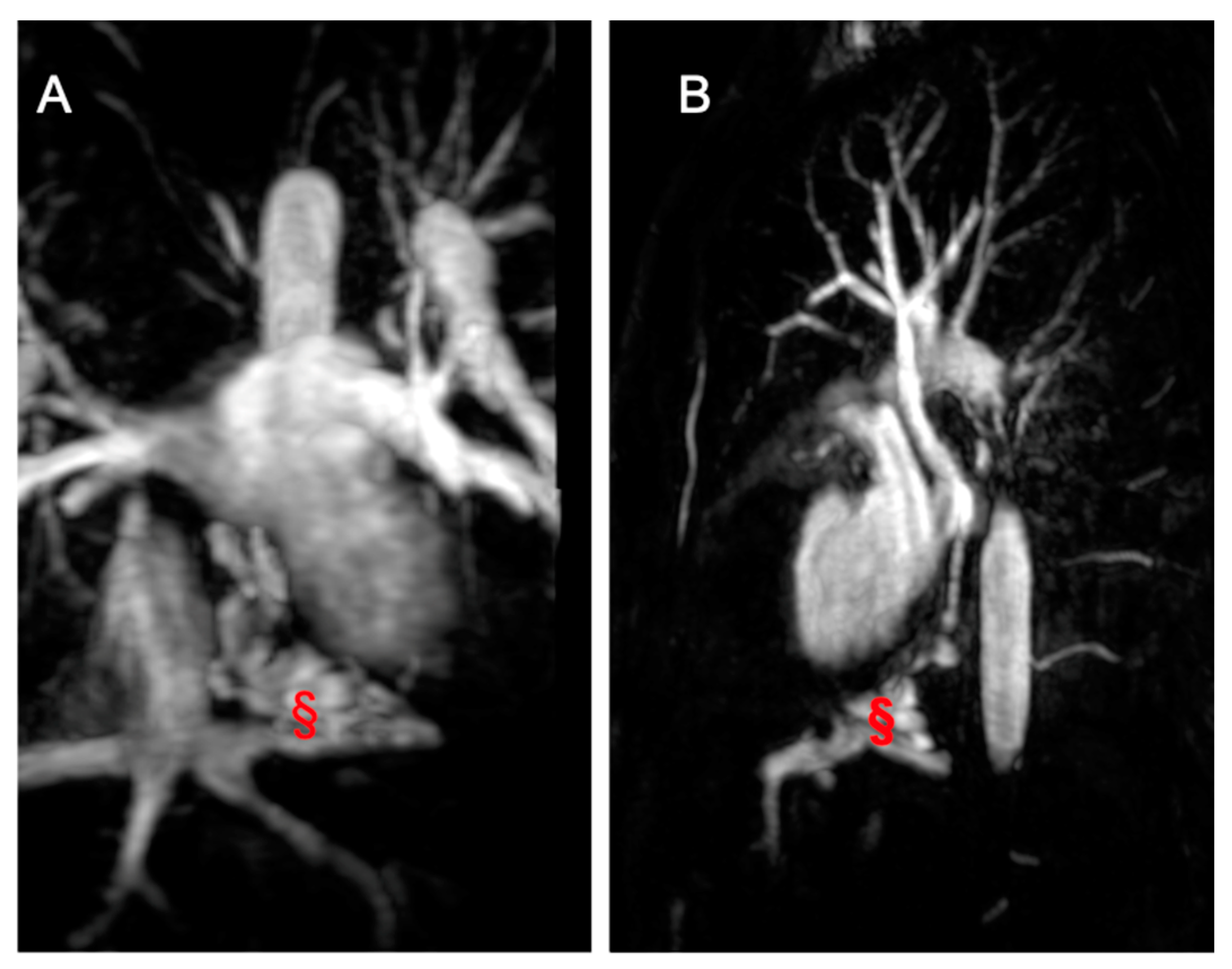
Figure 7.
Coronal Cine bSSFP image from a 12-year-old male TCPC with dextrocardia. The image illustrates two superior vena cava (*) and the external conduit (§).
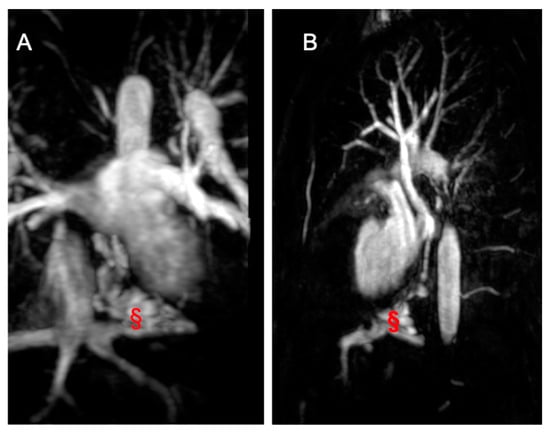
Figure 8.
Imaging from a 17-year-old male TCPC. (A) shows a coronal angiography image, while (B) presents a sagittal angiography image displaying a veno-venous fistula (marked with §) between the suprahepatic veins and the pulmonary veins.
2.5. Evaluation of Coronary Anatomy and Stress Perfusion Imaging
Coronary artery abnormalities (CAA) are uncommon congenital defects, with an estimated prevalence of 1%, involving either anomalous locations of the coronary ostium or abnormalities in the coronary course [107]. These can occur alone or alongside complex CHDs [108,109]. Clinical presentations in children vary significantly, ranging from no symptoms to severe complications like chest pain, ventricular dysfunction, and sudden cardiac death. Post-surgical scenarios, such as after ASO operations for TGA, may necessitate CA evaluations due to complications, likewise coronary allograft vasculopathy (CAV), a notable risk following heart transplantation that negatively impacts long-term outcomes. While invasive coronary angiography remains the gold standard, CMR with vasodilator-infused perfusion has proven effective for detecting anomalies and inducible myocardial ischemia [110,111].
CA aneurysms (CAA), a complication in 15–25% of untreated Kawasaki Disease cases, can progress to rupture, thrombosis, or stenosis, potentially leading to myocardial infarction [112,113]. CT scans provide detailed visualization of CA’s origin and course with excellent spatial resolution, although radiation concerns persist, especially in children [114]. CMR offers a valuable alternative, enabling comprehensive assessments of cardiac structures and functions without radiation. It can identify myocardial edema with T2-weighted images (STIR and T2 mapping) and detect fibrosis with T1-weighted images (LGE), differentiating between ischemic and non-ischemic damage [115].
Despite its advantages, CMR’s longer acquisition times and difficulty distinguishing artifacts from true pathological changes limit its clinical use [116,117]. However, according to European guidelines (Class I, Level C), and a recent American Heart Association statement, CMR is recommended over CT for non-invasive assessment of CAA in young patients, avoiding ionizing radiation [118,119]. Stress sequences using physical or pharmacological agents enhance CMR’s diagnostic capabilities, enabling detailed visualization of myocardial perfusion and ischemia under stress conditions [14,120,121,122]. Although challenges remain in visualizing distal coronary segments and acquiring cooperative patient behavior without sedation, CMR’s comprehensive capabilities make it a preferred modality in pediatric cardiology.
3. Challenges and Limitations
There are some considerations that need to be done regarding CMR imaging in the pediatric population. The smaller body size of these patients may require voxel size optimization to maintain an adequate spatial resolution. Technical adjustments to increase signal-to-noise ratio may require longer acquisition time, often not tolerated in pediatric population, particularly under anesthesia. Similarly, the higher heart rates may hamper temporal resolution and require specific adjustments in several sequences at a cost of an increase in scan time. Young children may also require anesthesia or sedation; this is generally safe when performed by experienced staff, but nevertheless it requires additional coordination between different departments and may be unavailable in smaller centers. In addition, risks of adverse events still exist, particularly in patients with cardiomyopathies, severe CHDs, and pulmonary hypertension. Acquiring CMR cine images usually requires appropriate breath hold, which can be addressed by using free-breathing techniques [123]. Concern arises about repeated use of GBCA, often required in follow-up scanning, due to the evidence of gadolinium deposition within the brain [124]. More recently, ferumoxytol, a superparamahnetic iron oxide particle, has emerged as an alternative to GBCA with encouraging safety data also in the pediatric population [125]. Finally, limitations to the use of CMR may stem from its relatively high cost compared to other cardiac imaging techniques, as well as from the limited availability of the technology and of the specialized training required for its application in CHD and in the prenatal diagnosis [126].
4. Conclusions and Future Directions
CMR is an advanced cardiovascular imaging tool crucial for diagnosing and managing CHDs. It enables precise assessments of cardiac anatomy, function, hemodynamics, and tissue characteristics, and is particularly effective for complex cases due to its 3D capabilities [11,13,18,19,110]. The leading role of CMR in the challenging management of CHD is confirmed by both the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC), which emphasize the use of CMR in the initial evaluation of patients with particularly complex anatomical structures and for the serial evaluation of patients at risk of RV enlargement and dysfunction [127,128]
These statements are also supported by two expert consensus documents from radiologists and cardiologists that outline the appropriateness criteria for the use of CMR use in various clinical contexts, including CHD [129,130].
Looking ahead, CMR is poised to integrate further with technologies like artificial intelligence (AI), which enhances the automation of image analysis and the development of predictive models to optimize personalized treatments and outcomes, despite some existing limitations. The application of deep-learning in CMR imaging acquisition appears very promising, as it enables automated localization and detection of the heart, thereby reducing the long acquisition times. Furthermore, AI helps in shortening the time needed for exam evaluation by facilitating image reconstruction with advanced reconstruction function, and improving the post-processing phase, particularly in the CMR segmentation and the automatic characterization of myocardial tissue [131,132,133,134,135,136,137,138,139,140,141,142].
Additionally, the growing application of CMR in prenatal cardiology suggests its future integration into routine prenatal screening for high-risk pregnancies, potentially revolutionizing early CHDs detection and management [117,118,119]. With advancements in 3D modeling and virtual reality, CMR will continue to enhance presurgical planning and educational tools in cardiology, making it an indispensable resource in the evolving landscape of congenital cardiac care [81,82,138,139,140,141,142].
Author Contributions
Writing—original draft preparation, S.M., A.P., I.L., J.I., A.S. and S.P., Image Editing: A.S., E.R. and A.C.; writing—review and editing, S.M., A.P. and G.D.S.; supervision, G.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 2D | Two-Dimensional |
| 3D | Tri-Dimensional |
| 4DFlow CMR | Four-Dimensional Flow CMR Resonance |
| AI | Artificial Intelligence |
| ASDs | Atrial Septal Defects |
| ASO | Arterial Switch Operation |
| AtSO | Atrial Switch Operation |
| AVSDs | Atrioventricular Septal Defects |
| BSA | Body Surface Area |
| bSSFP | balances Steady-State Free Precession |
| BT | Blalock-Taussig |
| CA | Coronary Arteries |
| CAA | Coronary Artery Abnormalities |
| CAV | Coronary Allograft Vasculopathy |
| cc-TGA | Congenitally Corrected Transposition of Great Arteries |
| CoA | Coarctation of the aorta |
| CtA | Conotruncal Anomalies |
| CFD | Computational Fluid Dynamics |
| CCHD | cyanotic CHD |
| CHD | Congenital Heart Diseases |
| CS | Circumferential Strain |
| CMR | Cardiovascular Magnetic Resonance |
| CCT | Cardiovascular Computed Tomography |
| D-TGA | Dextro-Transposition of Great Arteries |
| DORV | Double outlet right ventricle |
| ECG | Electrocardiogram |
| EF | Ejection Fraction |
| EGE | Early Gadolinium Enhancement |
| FT-GLS | Feature Tracking-Global Longitudinal Strain |
| GBCA | Gadolinium-Based Contrast Agents |
| HLHS | Hypoplastic Left Heart Syndrome |
| HFpEF | Heart Failure with preserved Ejection Fraction |
| IAA | Interrupted Aortic Arch |
| IVC | Inferior Vena Cava |
| KD | Kawasaki Disease |
| LGE | Late Gadolinium Enhancement |
| LV | Left Ventricle |
| LV-EDV | left ventricular end diastolic volume |
| NYHA | New York Heart Association |
| MRA | Magnetic Resonance Angiogram |
| MRI | Magnetic Resonance Imaging |
| PA | Pulmonary Artery |
| PDA | Patent Ductus Arteriosus |
| PC | Phase Contrast |
| Qp/Qs | pulmonary-to-systemic circulation flow ratio |
| r-TOF | repaired Tetralogy of Fallot |
| RV | Right Ventricle |
| RVOT | Right Ventricle Outflow Tract |
| sRV | Systemic Right Ventricle |
| SVASD | Sinus Venosus Atrial Septal Defect |
| SVC | Superior Vena Cava |
| SV | Stroke Volume |
| TA | Truncus Arteriosus |
| TCPC | Total Cavopulmonary Connection |
| TEE | Transesophageal Echocardiography |
| TGA | Transposition of Great Arteries |
| TOF | Tetralogy of Fallot |
| TTE | Transthoracic Echocardiography |
| VSDs | Ventricular Septal Defects |
References
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.; Li, N.; Keavney, B.D. Global Birth Prevalence of Congenital Heart Defects 1970–2017: Updated Systematic Review and Meta-Analysis of 260 Studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Avverte, C.A.; Liberthson, R.; Danielson, G.K.; Adorare, L.; Harris, J.I.; Hoffmann, J.; Somerville, R.G.; Williams, G.D. Task force 1: The changing profile of congenital heart disease in adult life. J. Am. Coll. Cardiol. 2001, 37, 1170–1175. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Rosengren, A.; Skoglund, K.; Lappas, G.; Eriksson, P.; Dellborg, M. Survivorship in Children and Young Adults with Congenital Heart Disease in Sweden. JAMA Intern. Med. 2017, 177, 224. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Bovijn, L.; Budts, W.; Belmans, A.; Gewillig, M. Temporal Trends in Survival to Adulthood among Patients Born with Congenital Heart Disease from 1970 to 1992 in Belgium. Circulation 2010, 122, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.D.; Menashe, V.D. 25-Year Mortality after Surgical Repair of Congenital Heart Defect in Childhood. A Population-Based Cohort Study. JAMA 1991, 266, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Powell, A.J.; Geva, T. Multimodality Noninvasive Imaging for Assessment of Congenital Heart Disease. Circ. Cardiovasc. Imaging 2010, 3, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Kilner, P.J. Imaging congenital heart disease in adults. Br. J. Radiol. 2011, 84, S258–S268. [Google Scholar] [CrossRef] [PubMed]
- Uretsky, S.; Gillam, L.; Lang, R.; Chaudhry, F.A.; Argulian, E.; Supariwala, A.; Gurram, S.; Jain, K.; Subero, M.; Jang, J.J.; et al. Discordance between Echocardiography and MRI in the Assessment of Mitral Regurgitation Severity. J. Am. Coll. Cardiol. 2015, 65, 1078–1088. [Google Scholar] [CrossRef]
- Moscatelli, S.; Bianco, F.; Cimini, A.; Panebianco, M.; Leo, I.; Bucciarelli-Ducci, C.; Perrone, M.A. The Use of Stress Cardiovascular Imaging in Pediatric Population. Children 2023, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Fratz, S.; Chung, T.; Greil, G.F.; Samyn, M.M.; Taylor, A.M.; Valsangiacomo Buechel, E.R.; Yoo, S.-J.; Powell, A.J. Guidelines and Protocols for Cardiovascular Magnetic Resonance in Children and Adults with Congenital Heart Disease: SCMR Expert Consensus Group on Congenital Heart Disease. J. Cardiovasc. Magn. Reson. 2013, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the Use of Cardiovascular Magnetic Resonance in Pediatric Congenital and Acquired Heart Disease. J. Cardiovasc. Magn. Reson. 2022, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Secinaro, A.; Ait-Ali, L.; Curione, D.; Clemente, A.; Gaeta, A.; Giovagnoni, A.; Alaimo, A.; Esposito, A.; Tchana, B.; Sandrini, C.; et al. Recommendations for Cardiovascular Magnetic Resonance and Computed Tomography in Congenital Heart Disease: A Consensus Paper from the CMR/CCT Working Group of the Italian Society of Pediatric Cardiology (SICP) and the Italian College of Cardiac Radiology Endorsed by the Italian Society of Medical and Interventional Radiology (SIRM) Part I. Radiol. Med. 2022, 127, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Ntsinjana, H.N.; Tann, O.; Hughes, M.; Derrick, G.; Secinaro, A.; Schievano, S.; Muthurangu, V.; Taylor, A.M. Utility of Adenosine Stress Perfusion CMR to Assess Paediatric Coronary Artery Disease. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Kilner, P.J.; Geva, T.; Kaemmerer, H.; Trindade, P.T.; Schwitter, J.; Webb, G.D. Recommendations for Cardiovascular Magnetic Resonance in Adults with Congenital Heart Disease from the Respective Working Groups of the European Society of Cardiology. Eur. Heart J. 2010, 31, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D. Clinical Indications for Cardiovascular Magnetic Resonance (CMR): Consensus Panel Report? Eur. Heart J. 2004, 25, 1940–1965. [Google Scholar] [CrossRef] [PubMed]
- Westenberg, J.J.M.; Roes, S.D.; Ajmone Marsan, N.; Binnendijk, N.M.J.; Doornbos, J.; Bax, J.J.; Reiber, J.H.C.; De Roos, A.; Van Der Geest, R.J. Mitral Valve and Tricuspid Valve Blood Flow: Accurate Quantification with 3D Velocity-Encoded MR Imaging with Retrospective Valve Tracking. Radiology 2008, 249, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Isorni, M.-A.; Moisson, L.; Moussa, N.B.; Monnot, S.; Raimondi, F.; Roussin, R.; Boet, A.; Van Aerschot, I.; Fournier, E.; Cohen, S.; et al. 4D Flow Cardiac Magnetic Resonance in Children and Adults with Congenital Heart Disease: Clinical Experience in a High Volume Center. Int. J. Cardiol. 2020, 320, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.M.; Raimondi, F.; Ait Ali, L.; Allen, B.D.; Barker, A.J.; Bolger, A.; Burris, N.; Carhäll, C.-J.; Collins, J.D.; Ebbers, T.; et al. 4D Flow Cardiovascular Magnetic Resonance Consensus Statement: 2023 Update. J. Cardiovasc. Magn. Reson. 2023, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Bonnemains, L.; Raimondi, F.; Odille, F. Specifics of Cardiac Magnetic Resonance Imaging in Children. Arch. Cardiovasc. Dis. 2016, 109, 143–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ntsinjana, H.N.; Tann, O.; Taylor, A.M. Trends in Pediatric Cardiovascular Magnetic Resonance Imaging. Acta Radiol. 2013, 54, 1063–1074. [Google Scholar] [CrossRef]
- Lederlin, M.; Thambo, J.-B.; Latrabe, V.; Corneloup, O.; Cochet, H.; Montaudon, M.; Laurent, F. Coronary Imaging Techniques with Emphasis on CT and MRI. Pediatr. Radiol. 2011, 41, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Bonnemains, L.; Odille, F.; Cherifi, A.; Marie, P.-Y.; Pasquier, C.; Felblinger, J. Free-Breathing with Motion-Correction and Video Projection during Cardiac MRI: A Paediatric Design! J. Cardiovasc. Magn. Reson. 2014, 16, P319. [Google Scholar] [CrossRef]
- Pushparajah, K. Non-Invasive Imaging in the Evaluation of Cardiac Shunts for Interventional Closure. Front. Cardiovasc. Med. 2021, 8, 651726. [Google Scholar] [CrossRef] [PubMed]
- Scatteia, A.; Silverio, A.; Padalino, R.; De Stefano, F.; America, R.; Cappelletti, A.M.; Dalla Vecchia, L.A.; Guarini, P.; Donatelli, F.; Caiazza, F.; et al. Non-Invasive Assessment of Left Ventricle Ejection Fraction: Where Do We Stand? J. Pers. Med. 2021, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.L.; Lalude, O.O.; Schoenhagen, P.; Lerakis, S. Cardiovascular Magnetic Resonance Imaging for Structural and Valvular Heart Disease Interventions. JACC Cardiovasc. Interv. 2016, 9, 399–425. [Google Scholar] [CrossRef] [PubMed]
- Festa, P.; Ait-Ali, L.; Cerillo, A.G.; De Marchi, D.; Murzi, B. Magnetic Resonance Imaging Is the Diagnostic Tool of Choice in the Preoperative Evaluation of Patients with Partial Anomalous Pulmonary Venous Return. Int. J. Cardiovasc. Imaging 2006, 22, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kafka, H.; Mohiaddin, R.H. Cardiac MRI and Pulmonary MR Angiography of Sinus Venosus Defect and Partial Anomalous Pulmonary Venous Connection in Cause of Right Undiagnosed Ventricular Enlargement. AJR Am. J. Roentgenol. 2009, 192, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.E.; Kaplan, S. The Incidence of Congenital Heart Disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Cozijnsen, M.A.; Cozijnsen, L.; Maas, A.C.P.; Bakker-de Boo, M.; Bouma, B.J. A Ventricular Septal Defect with a Giant Appendiform Aneurysm of the Membranous Septum. Neth. Heart J. 2013, 21, 152–154. [Google Scholar] [CrossRef][Green Version]
- Schneider, D.J.; Moore, J.W. Patent Ductus Arteriosus. Circulation 2006, 114, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Broadhouse, K.M.; Price, A.N.; Durighel, G.; Cox, D.J.; Finnemore, A.E.; Edwards, A.D.; Hajnal, J.V.; Groves, A.M. Assessment of PDA Shunt and Systemic Blood Flow in Newborns Using Cardiac MRI. NMR Biomed. 2013, 26, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Craig, B. Atrioventricular septal defect: From fetus to adult. Heart 2006, 92, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Calkoen, E.E.; Westenberg, J.J.; Kroft, L.J.; Blom, N.A.; Hazekamp, M.G.; Rijlaarsdam, M.E.; Jongbloed, M.R.; de Roos, A.; Roest, A.A. Characterization and quantification of dynamic eccentric regurgitation of the left atrioventricular valve after atrioventricular septal defect correction with 4D Flow cardiovascular magnetic resonance and retrospective valve tracking. J. Cardiovasc. Magn. Reason. 2015, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, A.; Geva, T. Magnetic Resonance Imaging Evaluation of Congenital Heart Disease: Conotruncal Anomalies. J. Cardiovasc. Magn. Reson. 2006, 8, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R. Conotruncal Cardiac Defects: A Clinical Imaging Perspective. Pediatr. Cardiol. 2010, 31, 430–437. [Google Scholar] [CrossRef]
- Harris, M.A.; Avitabile, C.M.; Fu, G.L.; Kim, D.W.; Kim, T.S.; Gillespie, M.J.; Keller, M.S.; Fogel, M.A.; Whitehead, K.K. Accuracy and Internal Consistency of Cardiac Magnetic Resonance Imaging in Measuring Branch Pulmonary Artery Flows in Patients with Conotruncal Anomalies and Branch Pulmonary Artery Stents. Am. J. Cardiol. 2016, 117, 1160–1166. [Google Scholar] [CrossRef]
- Frank, L.; Dillman, J.R.; Parish, V.; Mueller, G.C.; Kazerooni, E.A.; Bell, A.; Attili, A.K. Cardiovascular MR Imaging of Conotruncal Anomalies. Radiographics 2010, 30, 1069–1094. [Google Scholar] [CrossRef] [PubMed]
- Škorić-Milosavljević, D.; Tadros, R.; Bosada, F.M.; Tessadori, F.; Van Weerd, J.H.; Woudstra, O.I.; Tjong, F.V.Y.; Lahrouchi, N.; Bajolle, F.; Cordell, H.J.; et al. Common Genetic Variants Contribute to Risk of Transposition of the Great Arteries. Circ. Res. 2022, 130, 166–180. [Google Scholar] [CrossRef]
- Canan, A.; Ashwath, R.; Agarwal, P.P.; François, C.; Rajiah, P. Multimodality Imaging of Transposition of the Great Arteries. Radiographics 2021, 41, 338–360. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Frescura, C. Anatomical and Pathophysiological Classification of Congenital Heart Disease. Cardiovasc. Pathol. 2010, 19, 259–274. [Google Scholar] [CrossRef]
- Nakajima, Y. Mechanism Responsible for D-transposition of the Great Arteries: Is This Part of the Spectrum of Right Isomerism? Congenit. Anom. 2016, 56, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Avesani, M.; Borrelli, N.; Sabatino, J.; Pergola, V.; Leo, I.; Montanaro, C.; Contini, F.V.; Gaudieri, G.; Ielapi, J.; et al. Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach. Children 2024, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, J.A.A.E.; Eindhoven, J.A.; Slager, M.A.; Opi, P.; Utens, E.M.W.J.; Helbing, W.A.; Witsenburg, M.; Van Den Bosch, A.E.; Ouhlous, M.; Van Domburg, R.T.; et al. The Natural and Unnatural History of the Mustard Procedure: Long-Term Outcome up to 40 Years. Eur. Heart J. 2014, 35, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Miller, O.; Babu Narayan, S.; Li, W.; Budts, W.; Valsangiacomo Buechel, E.R.; Frigiola, A.; Van Den Bosch, A.E.; Bonello, B.; Mertens, L.; et al. Imaging the Adult with Congenital Heart Disease: A Multimodality Imaging Approach—Position Paper from the EACVI. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Grothoff, M.; Fleischer, A.; Abdul-Khaliq, H.; Hoffmann, J.; Lehmkuhl, L.; Luecke, C.; Gutberlet, M. The Systemic Right Ventricle in Congenitally Corrected Transposition of the Great Arteries Is Different from the Right Ventricle in Dextro-Transposition after Atrial Switch: A Cardiac Magnetic Resonance Study. Cardiol. Young 2013, 23, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lipczyńska, M.; Szymański, P.; Kumor, M.; Klisiewicz, A.; Mazurkiewicz, Ł.; Hoffman, P. Global Longitudinal Strain May Identify Preserved Systolic Function of the Systemic Right Ventricle. Can. J. Cardiol. 2015, 31, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, B.N.; Arabi, T.Z.; Shafqat, A.; Abdul Rab, S.; Razak, A.; Albert-Brotons, D.C. Heart Failure in Systemic Right Ventricle: Mechanisms and Therapeutic Options. Front. Cardiovasc. Med. 2023, 9, 1064196. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.S.; Kalisz, K.; Broncano, J.; Goerne, H.; Collins, J.D.; François, C.J.; Ibrahim, E.-S.; Agarwal, P.P. Myocardial Strain Evaluation with Cardiovascular MRI: Physics, Principles, and Clinical Applications. Radiographics 2022, 42, 968–990. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Castro, S.A.; Santangeli, P.; Nucifora, G. Clinical Applications of Feature-Tracking Cardiac Magnetic Resonance Imaging. WJC 2018, 10, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Shiina, Y.; Inai, K.; Takahashi, T.; Taniguchi, K.; Watanabe, E.; Fukushima, K.; Niwa, K.; Nagao, M. Inter- and Intra-Ventricular Dyssynchrony in the Systemic Right Ventricle Is a Surrogate Marker of Major Cardiac Events in Mildly Symptomatic Patients. Heart Vessel. 2018, 33, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Szymański, P.; Klisiewicz, A.; Lubiszewska, B.; Lipczyńska, M.; Konka, M.; Kuśmierczyk, M.; Hoffman, P. Functional Anatomy of Tricuspid Regurgitation in Patients with Systemic Right Ventricles. J. Am. Soc. Echocardiogr. 2010, 23, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.; Avesani, M.; Houyel, L.; Thambo, J.-B.; Iriart, X. The Pivotal Role of Tricuspid Regurgitation in the Failing Systemic Right Ventricle: The “Chicken and Egg Story”. Arch. Cardiovasc. Dis. 2022, 115, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Santens, B.; Helsen, F.; Van De Bruaene, A.; De Meester, P.; Budts, A.-L.; Troost, E.; Moons, P.; Claus, P.; Rega, F.; Bogaert, J.; et al. Adverse Functional Remodelling of the Subpulmonary Left Ventricle in Patients with a Systemic Right Ventricle Is Associated with Clinical Outcome. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 680–688. [Google Scholar] [CrossRef] [PubMed]
- DiLorenzo, M.P.; Grosse-Wortmann, L. Myocardial Fibrosis in Congenital Heart Disease and the Role of MRI. Radiol. Cardiothorac. Imaging 2023, 5, e220255. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.; Lam, W.W.M.; So, E.K.F.; Chow, P. Differential Myocardial Fibrosis of the Systemic Right Ventricle and Subpulmonary Left Ventricle after Atrial Switch Operation for Complete Transposition of the Great Arteries. IJC Heart Vasc. 2020, 30, 100612. [Google Scholar] [CrossRef] [PubMed]
- Voges, I.; Boll, C.; Caliebe, A.; Gabbert, D.; Uebing, A.; Krupickova, S. Reference Values for Ventricular Volumes and Pulmonary Artery Dimensions in Pediatric Patients with Transposition of the Great Arteries after Arterial Switch Operation. Magn. Reson. Imaging 2021, 54, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.; Fredriksen, P.-M.; Urheim, S.; Thaulow, E.; Smith, H.-J.; Smevik, B.; Smiseth, O.; Andersen, K. Ventricular Function in Patients with Transposition of the Great Arteries Operated with Arterial Switch. Am. J. Cardiol. 2009, 104, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, W.H.S.; Breur, J.M.P.J.; Westenberg, J.J.M.; Driessen, M.M.P.; Meijboom, F.J.; Driesen, B.; De Baat, E.C.; Doevendans, P.A.F.M.; Leiner, T.; Grotenhuis, H.B. Validation of Aortic Valve 4D Flow Analysis and Myocardial Deformation by Cardiovascular Magnetic Resonance in Patients after the Arterial Switch Operation. J. Cardiovasc. Magn. Reson. 2019, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J. 4D Flow MRI Applications in Congenital Heart Disease. Eur. Radiol. 2021, 31, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.S.; Herrmann, J.L.; Rodefeld, M.D.; Turrentine, M.W.; Brown, J.W. Rastelli Operation for D-Transposition of the Great Arteries, Ventricular Septal Defect, and Pulmonary Stenosis. World J. Pediatr. Congenit. Heart Surg. 2019, 10, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Wallis, G.A.; Debich-Spicer, D.; Anderson, R.H. Congenitally Corrected Transposition. Orphanet J. Rare Dis. 2011, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Silversides, C.K.; Roche, S.L. Congenitally Corrected Transposition of the Great Arteries. JACC Cardiovasc. Imaging 2022, 15, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.K.S. Congenitally Corrected Transposition of the Great Arteries. J. Thorac. Dis. 2020, 12, 1213–1218. [Google Scholar] [CrossRef]
- Ojha, V.; Pandey, N.N.; Sharma, A.; Ganga, K.P. Spectrum of Changes on Cardiac Magnetic Resonance in Repaired Tetralogy of Fallot: Imaging According to Surgical Considerations. Clin. Imaging 2021, 69, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, M.; Nagao, M.; Ishizaki, U.; Shiina, Y.; Inai, K.; Yamasaki, Y.; Yoneyama, M.; Sakai, S. Feature-Tracking MRI Fractal Analysis of Right Ventricular Remodeling in Adults with Congenitally Corrected Transposition of the Great Arteries. Radiol. Cardiothorac. Imaging 2019, 1, e190026. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, C.; Dubois, J.; Rypens, F.; Raboisson, M.-J.; Déry, J. Tetralogy of Fallot: Preoperative Assessment with MR and CT Imaging. Diagn. Interv. Imaging 2016, 97, 531–541. [Google Scholar] [CrossRef]
- Apostolopoulou, S.C.; Manginas, A.; Kelekis, N.L.; Noutsias, M. Cardiovascular Imaging Approach in Pre and Postoperative Tetralogy of Fallot. BMC Cardiovasc. Disord. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, B.; Perrone, M.; Calcaterra, G.; Sabatino, J.; Leo, I.; Aversani, M.; Bassareo, P.P.; Pozza, A.; Oreto, L.; Moscatelli, S.; et al. Repaired Tetralogy of Fallot: Have We Understood the Right Timing of PVR? J. Clin. Med. 2024, 13, 2682. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Puranik, R. Pulmonary Valve Replacement in Adults with Repaired Tetralogy of Fallot: The Role of Cardiac Magnetic Resonance Beyond Volume Measurements. Future Cardiol. 2012, 8, 801–804. [Google Scholar] [CrossRef]
- Shaaban, M.; Salama, M.; Alsaied, A.; Elsheikh, R.; Elmasry, M. Assessment of Flow Pattern of Right Ventricle Outflow and Pulmonary Arteries in Surgically Corrected Tetralogy of Fallot Patients by Four-Dimensional Cardiac Magnetic Resonance Flow. Egypt. Heart J. 2020, 72, 57. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.; Gilbert, K.; Scadeng, M.; Cowan, B.R.; Pushparajah, K.; Young, A.A. Four-Dimensional Flow Cardiovascular Magnetic Resonance in Tetralogy of Fallot: A Systematic Review. J. Cardiovasc. Magn. Reson. 2021, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.F.; Redington, A.; Yoo, S.-J.; Seed, M.; Greiser, A.; Grosse-Wortmann, L. Diffuse Myocardial Fibrosis Following Tetralogy of Fallot Repair: A T1 Mapping Cardiac Magnetic Resonance Study. Pediatr. Radiol. 2014, 44, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Franklin, R.C.G.; Béland, M.J.; Spicer, D.E.; Colan, S.D.; Walters, H.L.; Bailliard, F.; Houyel, L.; St. Louis, J.D.; Lopez, L.; et al. Nomenclature for Pediatric and Congenital Cardiac Care: Unification of Clinical and Administrative Nomenclature—The 2021 International Paediatric and Congenital Cardiac Code (IPCCC) and the Eleventh Revision of the International Classification of Diseases (ICD-11). World J. Pediatr. Congenit. Heart Surg. 2021, 12, E1–E18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z. Long-Term Results of Biventricular Correction for Patients with Double Outlet Right Ventricle. Cardiol. Young 2023, 33, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Semple, T.; Gu, H.; McCarthy, K.P.; Yen Ho, S.; Li, W. Double Outlet Ventricles: Review of Anatomic and Imaging Characteristics. Heart 2023, 109, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Karev, E.; Stovpyuk, O.F. Double Outlet Right Ventricle in Adults: Anatomic Variability, Surgical Treatment, and Late Postoperative Complications. J. Clin. Ultrasound 2022, 50, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Bell-Cheddar, Y.; Devine, W.A.; Diaz-Castrillon, C.-E.; Seese, L.; Castro-Medina, M.; Morales, R.; Follansbee, C.W.; Alsaied, T.; Lin, J.-H.I. Double Outlet Right Ventricle. Front. Pediatr. 2023, 11, 1244558. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.-H.; Gupta, S.K.; Mandell, J.; Schidlow, D.; Wernovsky, G.; Olivieri, L. Congenital Heart Disease Illustrated: Use of Cross-Sectional Imaging in Pediatric Cardiology. J. Thorac. Imaging 2024, 39, W19–W31. [Google Scholar] [CrossRef] [PubMed]
- Ntsinjana, H.N.; Hughes, M.L.; Taylor, A.M. The Role of Cardiovascular Magnetic Resonance in Pediatric Congenital Heart Disease. J. Cardiovasc. Magn. Reson. 2011, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on Clinical Indications for Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.A.V.; Yoo, S.-J.; Seed, M.; Lam, C.Z.; Valverde, I. Recent Advances in Multimodal Imaging in Tetralogy of Fallot and Double Outlet Right Ventricle. Curr. Opin. Cardiol. 2024, 39, 323–330. [Google Scholar] [CrossRef]
- Montalt-Tordera, J.; Pajaziti, E.; Jones, R.; Sauvage, E.; Puranik, R.; Singh, A.A.V.; Capelli, C.; Steeden, J.; Schievano, S.; Muthurangu, V. Automatic Segmentation of the Great Arteries for Computational Hemodynamic Assessment. J. Cardiovasc. Magn. Reson. 2022, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Leo, I.; Sabatino, J.; Avesani, M.; Moscatelli, S.; Bianco, F.; Borrelli, N.; De Sarro, R.; Leonardi, B.; Calcaterra, G.; Surkova, E.; et al. Non-Invasive Imaging Assessment in Patients with Aortic Coarctation: A Contemporary Review. J. Clin. Med. 2024, 13, 28. [Google Scholar] [CrossRef]
- Ryd, D.; Fricke, K.; Bhat, M.; Arheden, H.; Liuba, P.; Hedström, E. Utility of Fetal Cardiovascular Magnetic Resonance for Prenatal Diagnosis of Complex Congenital Heart Defects. JAMA Netw. Open. 2021, 4, e213538, Erratum in JAMA Netw. Open 2021, 4, e2111261; Erratum in JAMA Netw. Open 2022, 5, e225825. [Google Scholar] [CrossRef]
- Lloyd, D.F.A.; van Poppel, M.P.M.; Pushparajah, K.; Vigneswaran, T.V.; Zidere, V.; Steinweg, J.; van Amerom, J.F.P.; Roberts, T.A.; Schulz, A.; Charakida, M.; et al. Analysis of 3-Dimensional Arch Anatomy, Vascular Flow, and Postnatal Outcome in Cases of Suspected Coarctation of the Aorta Using Fetal Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2021, 14, e012411. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Celermajer, D.S.; Jolivet, O.; Buyens, F.; Herment, A.; Sidi, D.; Bonnet, D.; Mousseaux, E. Increased central aortic stiffness and left ventricular mass in normotensive young subjects after successful coarctation repair. Am. Heart J. 2008, 155, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.B.; Lantin-Hermoso, M.R.; Daniels, C.J.; Jaquiss, R.; Landis, B.J.; Marino, B.S.; Rathod, R.H.; Vincent, R.N.; Keller, B.B.; Villafane, J. Isolated Coarctation of the Aorta: Current Concepts and Perspectives. Front. Cardiovasc. Med. 2022, 9, 817866. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, C.; Bouchardy, J.; Blanche, C.; Piccini, D.; Pavon, A.G.; Monney, P.; Stuber, M.; Schwitter, J.; Rutz, T. 2D cine vs. 3D self-navigated free-breathing high-resolution whole heart cardiovascular magnetic resonance for aortic root measurements in congenital heart disease. J. Cardiovasc. Magn. Reason. 2021, 23, 65. [Google Scholar] [CrossRef]
- Doyle, C.M.; Orr, J.; Greenwood, J.P.; Plein, S.; Tsoumpas, C.; Bissell, M.M. Four-Dimensional Flow Magnetic Resonance Imaging in the Assessment of Blood Flow in the Heart and Great Vessels: A Systematic Review. J. Magn. Reson. Imaging. 2022, 55, 1301–1321. [Google Scholar] [CrossRef] [PubMed]
- Quail, M.A.; Segers, P.; Steeden, J.A.; Muthurangu, V. The aorta after coarctation repair—Effects of calibre and curvature on arterial haemodynamics. J. Cardiovasc. Magn. Reson. 2019, 21, 22, Erratum in J. Cardiovasc. Magn. Reson. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, S.; Meadows, A.K.; Ordovas, K.G.; Higgins, C.B.; Meadows, J.J. Usefulness of cardiovascular magnetic resonance imaging to predict the need for intervention in patients with coarctation of the aorta. Am. J. Cardiol. 2012, 109, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Frescura, C.; Thiene, G. The New Concept of Univentricular Heart. Front. Pediatr. 2014, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.E.; Hillman, D.G.; Gurka, M.J.; Gutgesell, H.P. Three-Stage Palliation of Hypoplastic Left Heart Syndrome in the University HealthSystem Consortium. Congenit. Heart Dis. 2010, 5, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Muthurangu, V.; Taylor, A.M.; Hegde, S.R.; Johnson, R.; Tulloh, R.; Simpson, J.M.; Qureshi, S.; Rosenthal, E.; Baker, E.; Anderson, D.; et al. Cardiac Magnetic Resonance Imaging After Stage I Norwood Operation for Hypoplastic Left Heart Syndrome. Circulation 2005, 112, 3256–3263. [Google Scholar] [CrossRef] [PubMed]
- Krupickova, S.; Muthurangu, V.; Hughes, M.; Tann, O.; Carr, M.; Christov, G.; Awat, R.; Taylor, A.; Marek, J. Echocardiographic Arterial Measurements in Complex Congenital Diseases before Bidirectional Glenn: Comparison with Cardiovascular Magnetic Resonance Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Gauvreau, K.; Powell, A.J.; Lang, P.; Colan, S.D.; Del Nido, P.J.; Odegard, K.C.; Geva, T. Cardiac Magnetic Resonance Versus Routine Cardiac Catheterization Before Bidirectional Glenn Anastomosis in Infants with Functional Single Ventricle: A Prospective Randomized Trial. Circulation 2007, 116, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Banka, P.; McElhinney, D.B.; Bacha, E.A.; Mayer, J.E.; Gauvreau, K.; Geva, T.; Brown, D.W. What Is the Clinical Utility of Routine Cardiac Catheterization before a Fontan Operation? Pediatr. Cardiol. 2010, 31, 977–985. [Google Scholar] [CrossRef]
- Harris, M.A.; Cosulich, M.T.; Gillespie, M.J.; Whitehead, K.K.; Liu, T.I.; Weinberg, P.M.; Fogel, M.A. Pre-Fontan Cardiac Magnetic Resonance Predicts Post-Fontan Length of Stay and Avoids Ionizing Radiation. J. Thorac. Cardiovasc. Surg. 2009, 138, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Khan, M.A.; Hardy, R.; Torres, A.J.; Chen, J.M.; Gersony, W.M. A New Diagnostic Algorithm for Assessment of Patients with Single Ventricle before a Fontan Operation. J. Thorac. Cardiovasc. Surg. 2009, 138, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-P.; Liang, C.-H.; Huang, M.-P.; Liu, H.; Deng, Q.-P.; Yang, M.-F. Assessment of Aortopulmonary Collateral Flow and Pulmonary Vascular Growth Using a 3.0 T Magnetic Resonance Imaging System in Patients Who Underwent Bidirectional Glenn Shunting. Eur. J. Cardio-Thorac. Surg. 2012, 41, e146–e153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moscatelli, S.; Borrelli, N.; Sabatino, J.; Leo, I.; Avesani, M.; Montanaro, C.; Di Salvo, G. Role of Cardiovascular Imaging in the Follow-Up of Patients with Fontan Circulation. Children 2022, 9, 1875. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.L.; St. Clair, N.; Powell, A.J.; Geva, T.; Rathod, R.H. Integrated Clinical and Magnetic Resonance Imaging Assessments Late after Fontan Operation. J. Am. Coll. Cardiol. 2021, 77, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, F.; Voges, I.; Gatehouse, P.; Rigby, M.; Izgi, C.; Pennell, D.J.; Krupickova, S. Performance of Cardiac MRI in Pediatric and Adult Patients with Fontan Circulation. Radiol. Cardiothorac. Imaging 2022, 4, e210235. [Google Scholar] [CrossRef] [PubMed]
- d’Udekem, Y.; Iyengar, A.J.; Cochrane, A.D.; Grigg, L.E.; Ramsay, J.M.; Wheaton, G.R.; Penny, D.J.; Brizard, C.P. The Fontan Procedure: Contemporary Techniques Have Improved Long-Term Outcomes. Circulation 2007, 116, I-157–I-164. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.M.; Restrepo, M.; Tang, E.; De Zélicourt, D.A.; Sundareswaran, K.S.; Mirabella, L.; Bethel, J.; Whitehead, K.K.; Fogel, M.A.; Yoganathan, A.P. Fontan Hemodynamics from 100 Patient-Specific Cardiac Magnetic Resonance Studies: A Computational Fluid Dynamics Analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Cecchin, F.; Jones, T.K.; Portman, M.A. Major Coronary Artery Anomalies in a Pediatric Population: Incidence and Clinical Importance. J. Am. Coll. Cardiol. 2001, 37, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.W.; Griffith, G.C. Anomalies of the Coronary Arteries and Their Clinical Significance. Circulation 1956, 14, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Dymarkowski, S.; Hamaekers, P.; Razavi, R.; Gewillig, M.; Mertens, L.; Bogaert, J. MR Coronary Angiography and Late-Enhancement Myocardial MR in Children Who Underwent Arterial Switch Surgery for Transposition of Great Arteries. Radiology 2005, 234, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Paridon, S.M.; Alpert, B.S.; Boas, S.R.; Cabrera, M.E.; Caldarera, L.L.; Daniels, S.R.; Kimball, T.R.; Knilans, T.K.; Nixon, P.A.; Rhodes, J.; et al. Clinical Stress Testing in the Pediatric Age Group: A Statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Circulation 2006, 113, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Chih, S.; Ross, H.J.; Alba, A.C.; Fan, C.S.; Manlhiot, C.; Crean, A.M. Perfusion Cardiac Magnetic Resonance Imaging as a Rule-Out Test for Cardiac Allograft Vasculopathy. Am. J. Transplant. 2016, 16, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Sugimura, T.; Akagi, T.; Sato, N.; Hashino, K.; Maeno, Y.; Kazue, T.; Eto, G.; Yamakawa, R. Long-Term Consequences of Kawasaki Disease: A 10- to 21-Year Follow-up Study of 594 Patients. Circulation 1996, 94, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Buechel, R.R.; Kaufmann, P.A.; Kwong, R.Y. Multimodality Imaging in Individuals with Anomalous Coronary Arteries. JACC Cardiovasc. Imaging 2017, 10, 471–481. [Google Scholar] [CrossRef]
- Suzuki, A.; Takemura, A.; Inaba, R.; Sonobe, T.; Tsuchiya, K.; Korenaga, T. Magnetic Resonance Coronary Angiography to Evaluate Coronary Arterial Lesions in Patients with Kawasaki Disease. Cardiol. Young 2006, 16, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W. Coronary Artery Imaging in Children. Korean J. Radiol. 2015, 16, 239. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Fenton, M.; Peel, S.A.; Wiethoff, A.J.; Taylor, A.; Muthurangu, V.; Razavi, R.; Botnar, R.M.; Burch, M.; Greil, G.F. Detection and Grading of Coronary Allograft Vasculopathy in Children with Contrast-Enhanced Magnetic Resonance Imaging of the Coronary Vessel Wall. Circ. Cardiovasc. Imaging 2013, 6, 91–98. [Google Scholar] [CrossRef]
- Greil, G.F.; Seeger, A.; Miller, S.; Claussen, C.D.; Hofbeck, M.; Botnar, R.M.; Sieverding, L. Coronary Magnetic Resonance Angiography and Vessel Wall Imaging in Children with Kawasaki Disease. Pediatr. Radiol. 2007, 37, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Bluemke, D.A.; Achenbach, S.; Budoff, M.; Gerber, T.C.; Gersh, B.; Hillis, L.D.; Hundley, W.G.; Manning, W.J.; Printz, B.F.; Stuber, M.; et al. Noninvasive Coronary Artery Imaging: Magnetic Resonance Angiography and Multidetector Computed Tomography Angiography: A Scientific Statement From the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation 2008, 118, 586–606. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized Cardiovascular Magnetic Resonance Imaging (CMR) Protocols: 2020 Update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Husain, N.; Watanabe, K.; Berhane, H.; Gupta, A.; Markl, M.; Rigsby, C.K.; Robinson, J.D. Multi-Parametric Cardiovascular Magnetic Resonance with Regadenoson Stress Perfusion Is Safe Following Pediatric Heart Transplantation and Identifies History of Rejection and Cardiac Allograft Vasculopathy. J. Cardiovasc. Magn. Reson. 2021, 23, 135. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.T.; Molossi, S.; Sachdeva, S.; Wilkinson, J.C.; Loar, R.W.; Weigand, J.D.; Schlingmann, T.R.; Reaves-O’Neal, D.L.; Pednekar, A.S.; Masand, P.; et al. Dobutamine Stress Cardiac MRI Is Safe and Feasible in Pediatric Patients with Anomalous Aortic Origin of a Coronary Artery (AAOCA). Int. J. Cardiol. 2021, 334, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Steeden, J.A.; Kowalik, G.T.; Tann, O.; Hughes, M.; Mortensen, K.H.; Muthurangu, V. Real-Time Assessment of Right and Left Ventricular Volumes and Function in Children Using High Spatiotemporal Resolution Spiral bSSFP with Compressed Sensing. J. Cardiovasc. Magn. Reson. 2018, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, E.D.; Topcuoglu, O.M.; Semiz Oysu, A.; Bukte, Y. Does Gadoterate Meglumine Cause Gadolinium Retention in the Brain of Children? A Case–Control Study. Magn. Reson. Imaging 2020, 51, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.-L.; Yoshida, T.; Kathuria-Prakash, N.; Zaki, I.H.; Varallyay, C.G.; Semple, S.I.; Saouaf, R.; Rigsby, C.K.; Stoumpos, S.; Whitehead, K.K.; et al. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI. Radiology 2019, 293, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Bonnichsen, C.; Ammash, N. Choosing between MRI and CT imaging in the adult with congenital heart disease. Curr. Cardiol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019, 139, e637–e697. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Valente, A.M.; Armstrong, A.K.; Cook, S.C.; Han, B.K.; Lopez, L.; Lui, G.K.; Pickard, S.S.; Powell, A.J.; Bhave, N.M.; et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 appropriate use criteria for multimodality imaging during the follow-up care of patients with congenital heart disease. J. Am. Coll. Cardiol. 2020, 75, 657–703. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Francone, M.; Andreini, D.; Buffa, V.; Cademartiri, F.; Carbone, I.; Clemente, A.; Guaricci, A.I.; Guglielmo, M.; Indolfi, C.; et al. SIRM-SIC appropriateness criteria for the use of Cardiac Computed Tomography. Part 1: Congenital heart diseases, primary prevention, risk assessment before surgery, suspected CAD in symptomatic patients, plaque and epicardial adipose tissue characterization, and functional assessment of stenosis. Radiol. Med. 2021, 126, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Di Cesare, E.; Castelletti, S.; De Cobelli, F.; De Lazzari, M.; Esposito, A.; Focardi, M.; Di Renzi, P.; Indolfi, C.; Lanzillo, C.; et al. Appropriate use criteria for cardiovascular magnetic resonance imaging (CMR) SIC—SIRM position paper part 1 (ischemic and congenital heart diseases, cardio-oncology, cardiac masses and heart transplant). Radiol. Med. 2021, 126, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.; Reffo, E.; Castaldi, B.; Cattapan, I.; Avesani, M.; Biffanti, R.; Cavaliere, A.; Cerutti, A.; Di Salvo, G. Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art. Diagnostics 2023, 13, 3523. [Google Scholar] [CrossRef] [PubMed]
- Arya, B. Fetal Cardiac Imaging for Congenital Heart Disease—Is Cardiac Magnetic Resonance Imaging the Future? JAMA Netw. Open 2021, 4, e214617. [Google Scholar] [CrossRef]
- Udine, M.; Loke, Y.-H.; Goudar, S.; Donofrio, M.T.; Truong, U.; Krishnan, A. The Current State and Potential Innovation of Fetal Cardiac MRI. Front. Pediatr. 2023, 11, 1219091. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Van De Bruaene, A.; Farooqi, K.; Valverde, I.; Jung, C.; Votta, E.; Sturla, F.; Diller, G.P.; Brida, M.; Sun, Z.; et al. Three-Dimensional Printing, Holograms, Computational Modelling, and Artificial Intelligence for Adult Congenital Heart Disease Care: An Exciting Future. Eur. Heart J. 2022, 43, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Armstrong, A.K.; Arnaout, R.; Grosse-Wortmann, L.; Han, B.K.; Mertens, L.; Moore, R.A.; Olivieri, L.J.; Parthiban, A.; Powell, A.J. Novel Techniques in Imaging Congenital Heart Disease. J. Am. Coll. Cardiol. 2024, 83, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Shoeibi, A.; Khodatars, M.; Ghassemi, N.; Moridian, P.; Alizadehsani, R.; Khosravi, A.; Ling, S.H.; Delfan, N.; Zhang, Y.-D.; et al. Automated Diagnosis of Cardiovascular Diseases from Cardiac Magnetic Resonance Imaging Using Deep Learning Models: A Review. Comput. Biol. Med. 2023, 160, 106998. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Orwat, S.; Vahle, J.; Bauer, U.M.M.; Urban, A.; Sarikouch, S.; Berger, F.; Beerbaum, P.; Baumgartner, H. Prediction of Prognosis in Patients with Tetralogy of Fallot Based on Deep Learning Imaging Analysis. Heart 2020, 106, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Arafati, A.; Hu, P.; Finn, J.P.; Rickers, C.; Cheng, A.L.; Jafarkhani, H.; Kheradvar, A. Artificial Intelligence in Pediatric and Adult Congenital Cardiac MRI: An Unmet Clinical Need. Cardiovasc. Diagn. Ther. 2019, 9, S310–S325. [Google Scholar] [CrossRef] [PubMed]
- Brüning, J.; Kramer, P.; Goubergrits, L.; Schulz, A.; Murin, P.; Solowjowa, N.; Kuehne, T.; Berger, F.; Photiadis, J.; Weixler, V.H.-M. 3D Modeling and Printing for Complex Biventricular Repair of Double Outlet Right Ventricle. Front. Cardiovasc. Med. 2022, 9, 1024053. [Google Scholar] [CrossRef] [PubMed]
- Jathanna, N.; Podlasek, A.; Sokol, A.; Auer, D.; Chen, X.; Jamil-Copley, S. Diagnostic Utility of Artificial Intelligence for Left Ventricular Scar Identification Using Cardiac Magnetic Resonance Imaging—A Systematic Review. Cardiovasc. Digit. Health J. 2021, 2, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Guo, N.; Ge, Y.; Zhang, L.; Oudkerk, M.; Xie, X. Development and Application of Artificial Intelligence in Cardiac Imaging. Br. J. Radiol. 2020, 93, 20190812. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.; Zanella, L.; Castaldi, B.; Di Salvo, G. How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease? J. Clin. Med. 2024, 13, 2996. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).