Efficacy and Safety of Vagus Nerve Stimulation in Lennox–Gastaut Syndrome: A Scoping Review
Abstract
:1. Introduction
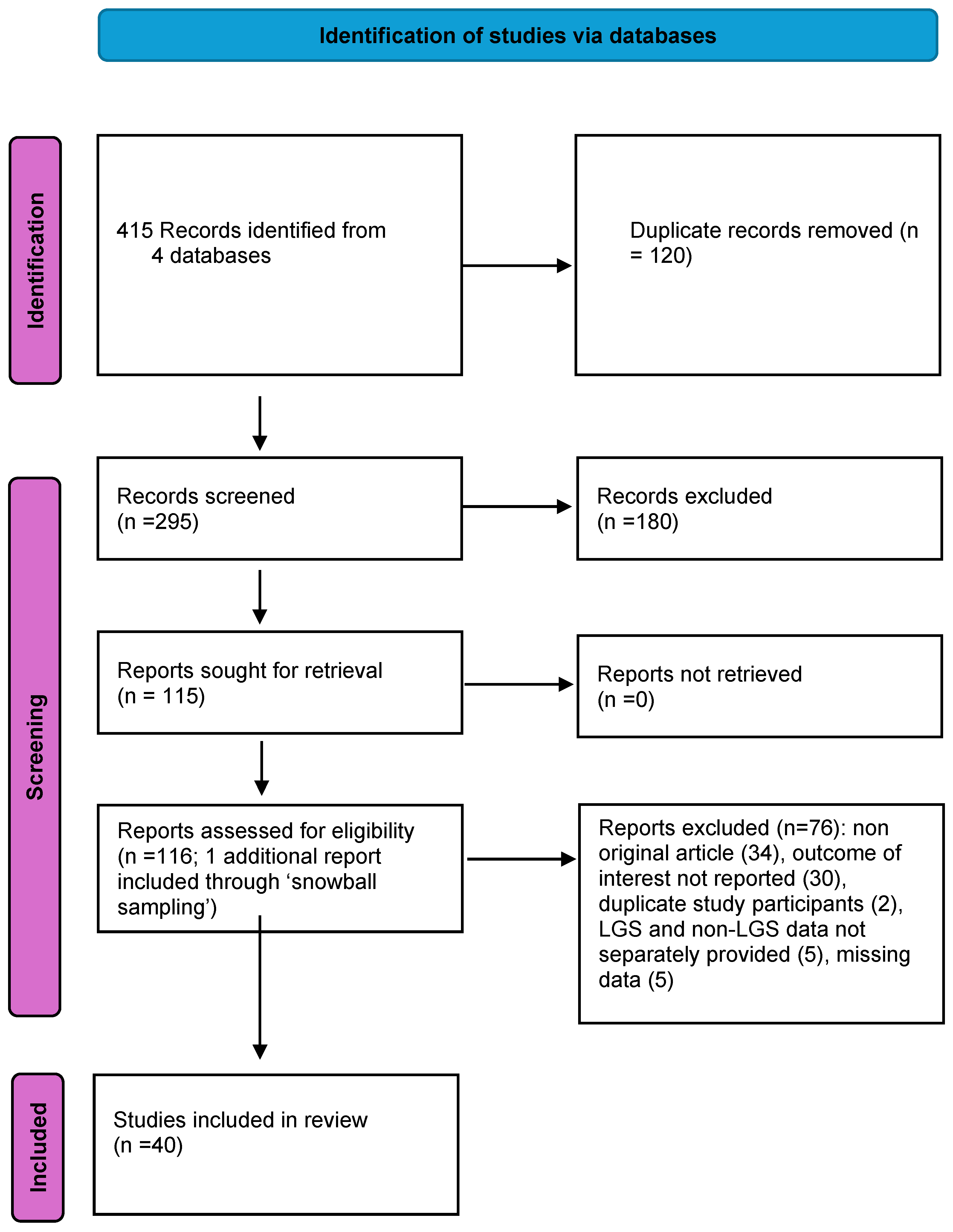
2. Materials and Methods
3. Results
3.1. Study Characteristics
3.2. Seizure Outcomes
3.2.1. Seizure Reduction
3.2.2. Seizure Types and Responsiveness to VNS
3.2.3. Variables Affecting Responsiveness
3.2.4. Immediate and Long-Term Efficacy
3.2.5. Impact on Seizure Severity and Status Epilepticus
3.2.6. Standard vs. Rapid Cycling, Magnet Stimulation, and Autostimulation
3.2.7. Cognitive and Adaptive Functioning, Quality of Life Outcomes
3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asadi-Pooya, A.A. Lennox-Gastaut syndrome: A comprehensive review. Neurol. Sci. 2018, 39, 403–414. [Google Scholar] [CrossRef]
- Samanta, D. Management of Lennox-Gastaut syndrome beyond childhood: A comprehensive review. Epilepsy Behav. 2021, 114, 107612. [Google Scholar] [CrossRef] [PubMed]
- Specchio, N.; Wirrell, E.C.; Scheffer, I.E.; Nabbout, R.; Riney, K.; Samia, P.; Guerreiro, M.; Gwer, S.; Zuberi, S.M.; Wilmshurst, J.M.; et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1398–1442. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Auvin, S.; Falip, M.; Striano, P.; Arzimanoglou, A. Expert Opinion on the Management of Lennox-Gastaut Syndrome: Treatment Algorithms and Practical Considerations. Front. Neurol. 2017, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Benítez, A.; Roth, J.; Andrews, J.S.; Shah, D.; Butcher, E.; Jones, A.; Sullivan, J. A comprehensive systematic literature review of the burden of illness of Lennox–Gastaut syndrome on patients, caregivers, and society. Epilepsia 2024, 65, 1224–1239. [Google Scholar] [CrossRef]
- Schachter, S.C. Vagus nerve stimulation therapy summary: Five years after FDA approval. Neurology 2002, 59, S15–S29. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.L., III; Gloss, D.; Buchhalter, J.; Mack, K.J.; Nickels, K.; Harden, C. Evidence-based guideline update: Vagus nerve stimulation for the treatment of epilepsy: Report of the guideline development subcommittee of the american academy of neurology. Epilepsy. Curr. 2013, 13, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Majoie, H.J.; Berfelo, M.W.; Aldenkamp, A.P.; Evers, S.M.; Kessels, A.G.; Renier, W.O. Vagus nerve stimulation in children with therapy-resistant epilepsy diagnosed as Lennox-Gastaut syndrome: Clinical results, neuropsychological effects, and cost-effectiveness. J. Clin. Neurophysiol. 2001, 18, 419–428. [Google Scholar] [CrossRef] [PubMed]
- You, S.J.; Kang, H.-C.; Ko, T.-S.; Kim, H.D.; Yum, M.-S.; Hwang, Y.S.; Lee, J.-K.; Kim, D.S.; Park, S.K. Comparison of corpus callosotomy and vagus nerve stimulation in children with Lennox-Gastaut syndrome. Brain Dev. 2008, 30, 195–199. [Google Scholar] [CrossRef]
- Kostov, K.; Kostov, H.; Taubøll, E. Long-term vagus nerve stimulation in the treatment of Lennox-Gastaut syndrome. Epilepsy Behav. 2009, 16, 321–324. [Google Scholar] [CrossRef]
- Cersósimo, R.O.; Bartuluchi, M.; De Los Santos, C.; Bonvehi, I.; Pomata, H.; Caraballo, R.H. Vagus nerve stimulation: Effectiveness and tolerability in patients with epileptic encephalopathies. Child’s Nerv. Syst. 2011, 27, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, J.S.; Mathern, G.W. Vagal nerve stimulation for pharmacoresistant epilepsy in children. Surg. Neurol. Int. 2012, 3, S269–S274. [Google Scholar] [PubMed]
- Thirunavu, V.; Du, R.; Wu, J.Y.; Berg, A.T.; Lam, S.K. The role of surgery in the management of Lennox-Gastaut syndrome: A systematic review and meta-analysis of the clinical evidence. Epilepsia 2021, 62, 888–907. [Google Scholar] [CrossRef]
- Lancman, G.; Virk, M.; Shao, H.; Mazumdar, M.; Greenfield, J.P.; Weinstein, S.; Schwartz, T.H. Vagus nerve stimulation vs. corpus callosotomy in the treatment of Lennox-Gastaut syndrome: A meta-analysis. Seizure 2013, 22, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dibué, M.; Greco, T.; Spoor, J.K.H.; Tahir, Z.; Specchio, N.; Hänggi, D.; Steiger, H.; Kamp, M.A. Vagus nerve stimulation in patients with Lennox-Gastaut syndrome: A meta-analysis. Acta Neurol. Scand. 2021, 143, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.B.; Samanta, D.; Willis, E. Options for pharmacoresistant epilepsy in children: When medications don’t work. Pediatr. Ann. 2015, 44, e43–e48. [Google Scholar] [CrossRef]
- Samanta, D.; Aungaroon, G.; Albert, G.W.; Karakas, C.; Joshi, C.N.; Singh, R.K.; Oluigbo, C.; Perry, M.S.; Naik, S.; Reeders, P.C.; et al. Advancing Thalamic neuromodulation in Epilepsy: Bridging Adult Data to Pediatric Care. Epilepsy Res. 2024, 205, 107407. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; E McKenzie, J.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; A Akl, E.; E Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Dibué, M.; Greco, T.; Spoor, J.K.H.; Senft, C.; Kamp, M.A. Does response to vagus nerve stimulation for drug-resistant epilepsy differ in patients with and without Lennox-Gastaut syndrome? Brain Behav. 2023, 13, e3025. [Google Scholar] [CrossRef]
- Karceski, S. Vagus nerve stimulation and Lennox-Gastaut syndrome: A review of the literature and data from the VNS patient registry. CNS Spectr. 2001, 6, 766–770. [Google Scholar] [CrossRef]
- Kostov, K.H.; Kostov, H.; Larsson, P.G.; Henning, O.; Aaberg, K.M.; Egge, A.; Peltola, J.; Lossius, M.I. Norwegian population-based study of effectiveness of vagus nerve stimulation in patients with developmental and epileptic encephalopathies. Epilepsia Open 2024, 9, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Orosz, I.; McCormick, D.; Zamponi, N.; Varadkar, S.; Feucht, M.; Parain, D.; Griens, R.; Vallée, L.; Boon, P.; Rittey, C.; et al. Vagus nerve stimulation for drug-resistant epilepsy: A European long-term study up to 24 months in 347 children. Epilepsia 2014, 55, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Benifla, M.; Rutka, J.T.; Logan, W.; Donner, E.J. Vagal nerve stimulation for refractory epilepsy in children: Indications and experience at The Hospital for Sick Children. Child’s Nerv. Syst. 2006, 22, 1018–1026. [Google Scholar] [CrossRef]
- Cukiert, A.; Cukiert, C.M.; Burattini, J.A.; Lima, A.M.; Forster, C.R.; Baise, C.; Argentoni-Baldochi, M. Long-term outcome after callosotomy or vagus nerve stimulation in consecutive prospective cohorts of children with Lennox-Gastaut or Lennox-like syndrome and non-specific MRI findings. Seizure 2013, 22, 396–400. [Google Scholar] [CrossRef]
- Zamponi, N.; Passamonti, C.; Cesaroni, E.; Trignani, R.; Rychlicki, F. Effectiveness of vagal nerve stimulation (VNS) in patients with drop-attacks and different epileptic syndromes. Seizure 2011, 20, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.S.; Mirsattari, S.M.; MacDougall, K.; Steven, D.A.; Parrent, A.; de Ribaupierre, S.; Andrade, A.; Diosy, D.; McLachlan, R.S.; Burneo, J.G. Vagus nerve stimulation in patients with therapy-resistant generalized epilepsy. Epilepsy Behav. 2020, 111, 107253. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.D.; Lee, J.S.; Heo, K.; Kim, D.S.; Kang, H.C. Long-term prognosis of patients with Lennox--Gastaut syndrome in recent decades. Epilepsy Res. 2015, 110, 10–19. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J.C.; Singh, H.W.; Phillips, J.; Murphy, K.; Doherty, C.P.; Delanty, N. Outcome measurement after vagal nerve stimulation therapy: Proposal of a new classification. Epilepsia 2007, 48, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Hallböök, T.; Lundgren, J.; Stjernqvist, K.; Blennow, G.; Strömblad, L.G.; Rosén, I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy, its impact on cognition, quality of life, behaviour and mood. Seizure 2005, 14, 504–513. [Google Scholar] [CrossRef]
- Casazza, M.; Avanzini, G.; Ferroli, P.; Villani, F.; Broggi, G. Vagal nerve stimulation: Relationship between outcome and electroclinical seizure pattern. Seizure 2006, 15, 198–207. [Google Scholar] [CrossRef]
- Buoni, S.; Mariottini, A.; Pieri, S.; Zalaffi, A.; Farnetani, M.A.; Strambi, M.; Palma, L.; Fois, A. Vagus nerve stimulation for drug-resistant epilepsy in children and young adults. Brain Dev. 2004, 26, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, A.; Bailey, C.; Maxiner, W.; Harvey, A.S. Vagus nerve stimulation for refractory epilepsy in children: More to VNS than seizure frequency reduction. Epilepsia 2009, 50, 1220–1228. [Google Scholar] [CrossRef]
- Frost, M.; Gates, J.; Helmers, S.L.; Wheless, J.W.; Levisohn, P.; Tardo, C.; Conry, J.A. Vagus nerve stimulation in children with refractory seizures associated with Lennox-Gastaut syndrome. Epilepsia 2001, 42, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.; Vonck, K.; Van Walleghem, P.; D’havé, M.; Goossens, L.; Vandekerckhove, T.; Caemaert, J.; De Reuck, J. Programmed and magnet-induced vagus nerve stimulation for refractory epilepsy. J. Clin. Neurophysiol. 2001, 18, 402–407. [Google Scholar] [CrossRef]
- Nakken, K.O.; Henriksen, O.; Røste, G.K.; Lossius, R. Vagal nerve stimulation--the Norwegian experience. Seizure 2003, 12, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Qiabi, M.; Bouthillier, A.; Carmant, L.; Nguyen, D.K. Vagus nerve stimulation for epilepsy: The notre-dame hospital experience. Can. J. Neurol. Sci. 2011, 38, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, E.; Lortie, A.; Thomas, T.; Bouthiller, A.; Scavarda, D.; Mercier, C.; Carmant, L. Vagus nerve stimulation in pediatric epileptic syndromes. Seizure 2009, 18, 34–37. [Google Scholar] [CrossRef]
- Alanazi, G.M.; ALOsaimi, T.F.; Alwadei, A.H.; Al-Otaibi, A.D.; Jad, L.A.; Al-Attas, A.A. Efficacy and safety of corpus callosotomy versus vagus nerve stimulation as long-term adjunctive therapies in children with Lennox-Gastaut syndrome: Experience of a tertiary care center. Neurosciences 2022, 27, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Aldenkamp, A.; Majoie, H.; Berfelo, M.; Evers, S.; Kessels, A.; Renier, W.; Wilmink, J. Long-term effects of 24-month treatment with vagus nerve stimulation on behaviour in children with Lennox-Gastaut syndrome. Epilepsy Behav. 2002, 3, 475–479. [Google Scholar] [CrossRef]
- Hornig, G.W.; Murphy, J.V.; Schallert, G.; Tilton, C. Left vagus nerve stimulation in children with refractory epilepsy: An update. South. Med. J. 1997, 90, 484–488. [Google Scholar] [CrossRef]
- Elliott, R.E.; Morsi, A.; Kalhorn, S.P.; Marcus, J.; Sellin, J.; Kang, M.; Silverberg, A.; Rivera, E.; Geller, E.; Carlson, C.; et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: Long-term outcomes and predictors of response. Epilepsy Behav. 2011, 20, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rychlicki, F.; Zamponi, N.; Trignani, R.; Ricciuti, R.A.; Iacoangeli, M.; Scerrati, M. Vagus nerve stimulation: Clinical experience in drug-resistant pediatric epileptic patients. Seizure 2006, 15, 483–490. [Google Scholar] [CrossRef]
- Labar, D.; Nikolov, B.; Tarver, B.; Fraser, R. Vagus nerve stimulation for symptomatic generalized epilepsy: A pilot study. Epilepsia 1998, 39, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E.; Hellström, K.; Waldton, C.; Augustinsson, L.E. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology 1999, 52, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, M.; Iida, K.; Kagawa, K.; Hashizume, A.; Ishikawa, N.; Hanaya, R.; Arita, K.; Kurisu, K. Combined surgical intervention with vagus nerve stimulation following corpus callosotomy in patients with Lennox-Gastaut syndrome. Acta Neurochir. 2016, 158, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Cersósimo, R.O.; Bartuluchi, M.; Fortini, S.; Soraru, A.; Pomata, H.; Caraballo, R.H. Vagus nerve stimulation: Effectiveness and tolerability in 64 paediatric patients with refractory epilepsies. Epileptic Disord. 2011, 13, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Braakman, H.M.; Creemers, J.; Hilkman, D.M.; Klinkenberg, S.; Koudijs, S.M.; Hall, M.D.-V.; Cornips, E.M. Improved seizure control and regaining cognitive milestones after vagus nerve stimulation revision surgery in Lennox–Gastaut syndrome. Epilepsy Behav. Case Rep. 2018, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Hödl, S.; Olbert, E.; Mahringer, C.; Carrette, E.; Meurs, A.; Gadeyne, S.; Dauwe, I.; Goossens, L.; Raedt, R.; Boon, P.; et al. Severe autonomic nervous system imbalance in Lennox-Gastaut syndrome patients demonstrated by heart rate variability recordings. Epilepsy Res. 2021, 177, 106783. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoity, S.A.; Abdelmoity, A.A.; Riordan, S.M.; Kaufman, C.; Le Pichon, J.B.; Abdelmoity, A. The efficacy and tolerability of auto-stimulation-VNS in children with Lennox-Gastaut syndrome. Seizure 2021, 86, 168–174. [Google Scholar] [CrossRef]
- Camfield, P.R. Definition and natural history of Lennox-Gastaut syndrome. Epilepsia 2011, 52 (Suppl. 5), 3–9. [Google Scholar] [CrossRef]
- Nagarajan, L.; Walsh, P.; Gregory, P.; Lee, M. VNS therapy in clinical practice in children with refractory epilepsy. Acta Neurol. Scand. 2002, 105, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.; Amark, P.; Blennow, G.; Strömblad, L.G.; Wallstedt, L. Vagus nerve stimulation in 16 children with refractory epilepsy. Epilepsia 1998, 39, 809–813. [Google Scholar] [CrossRef]
- Mikati, M.A.; Ataya, N.F.; El-Ferezli, J.C.; Baghdadi, T.S.; Turkmani, A.H.; Comair, Y.G.; Kansagra, S.; Najjar, M.W. Quality of life after vagal nerve stimulator insertion. Epileptic Disord. 2009, 11, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, S.; Chayasirisobhon, S.; Cahan, L.; Choi, S.; Enos, B.; Hwang, J.; Lin, M.; Schweitzer, J. Neuromodulation Therapy with Vagus Nerve Stimulation for Intractable Epilepsy: A 2-Year Efficacy Analysis Study in Patients under 12 Years of Age. Epilepsy Res. Treat. 2016, 2016, 9709056. [Google Scholar] [CrossRef]
- Kulju, T.; Haapasalo, J.; Rainesalo, S.; Lehtimäki, K.; Peltola, J. Autostimulation in Vagus Nerve Stimulator Treatment: Modulating Neuromodulation. Neuromodulation 2019, 22, 630–637. [Google Scholar] [CrossRef]
- Winston, G.M.; Guadix, S.; Lavieri, M.T.; Uribe-Cardenas, R.; Kocharian, G.; Williams, N.; Sholle, E.; Grinspan, Z.; Hoffman, C.E. Closed-loop vagal nerve stimulation for intractable epilepsy: A single-center experience. Seizure 2021, 88, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.P.; Polkey, C.E.; Binnie, C.D.; Madigan, C.; Ferrie, C.D.; Robinson, R.O. Vagal nerve stimulation in epileptic encephalopathies. Pediatrics 1999, 103 Pt 1, 778–782. [Google Scholar] [CrossRef]
- Hajnsek, S.; Petelin, Z.; Poljaković, Z.; Mrak, G.; Paladino, J.; Desnica, A. Vagus nerve stimulation in the treatment of patients with pharmacoresistant epilepsy: Our experiences. Coll. Antropol. 2011, 35, 755–760. [Google Scholar] [PubMed]
- Kang, B.S.; Woo, Y.S.; Lee, J.; Yi, Y.Y.; Koo, B.S.; Kang, J.W. Treatment outcomes of vagus nerve stimulation in Lennox-gastaut syndrome. Ann. Child Neurol. 2019, 27, 63–70. [Google Scholar] [CrossRef]
- Hosain, S.; Nikalov, B.; Harden, C.; Li, M.; Fraser, R.; Labar, D. Vagus nerve stimulation treatment for Lennox-Gastaut syndrome. J. Child Neurol. 2000, 15, 509–512. [Google Scholar] [CrossRef]
- Brinkley, K.M.; Ayers, M.D.; Sokol, D.K. Complete Atrioventricular Heart Block From an Epilepsy Treatment. Pediatr. Neurol. 2018, 80, 90–91. [Google Scholar] [CrossRef]
- Sivathamboo, S.; Myers, K.A.; Pattichis, A.; White, E.J.; Ku, K.N.; O’Brien, T.J.; Perucca, P.; Kwan, P. Sleep and respiratory abnormalities in adults with developmental and epileptic encephalopathies using polysomnography and video-EEG monitoring. Epilepsia Open 2023, 8, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, D.E.; Bae, H.; Song, J.Y.; Yang, K.I.; Hong, S.B. Effects of Vagus Nerve Stimulation on Sleep-Disordered Breathing, Daytime Sleepiness, and Sleep Quality in Patients with Drug-Resistant Epilepsy. J. Clin. Neurol. 2022, 18, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J. An Observational Report of Worsening Seizures with Increase in Total Charge Delivered Per Day by Vagus Nerve Stimulation in 4 Patients with Lennox-Gastaut Syndrome. Brain Stimul. 2016, 9, 310–311. [Google Scholar] [CrossRef]
- Lee, S.; Denton, A.; Ladino, L.D.; Waterhouse, K.; Vitali, A.; Tellez-Zenteno, J.F. Forced normalization after turning off vagus nerve stimulation in Lennox-Gastaut syndrome. Epilepsy Behav. Case Rep. 2019, 11, 81–83. [Google Scholar] [CrossRef]
- Dalic, L.J.; Warren, A.E.L.; Bulluss, K.J.; Thevathasan, W.; Roten, A.; Churilov, L.; Archer, J.S. DBS of Thalamic Centromedian Nucleus for Lennox-Gastaut Syndrome (ESTEL Trial). Ann. Neurol. 2022, 91, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.S.; Warren, A.E.; Jackson, G.D.; Abbott, D.F. Conceptualizing lennox-gastaut syndrome as a secondary network epilepsy. Front. Neurol. 2014, 5, 225. [Google Scholar] [CrossRef]
- Abdullahi, A.; Etoom, M.; Badaru, U.M.; Elibol, N.; Abuelsamen, A.A.; Alawneh, A.; Zakari, U.U.; Saeys, W.; Truijen, S. Vagus nerve stimulation for the treatment of epilepsy: Things to note on the protocols, the effects and the mechanisms of action. Int. J. Neurosci. 2024, 134, 560–569. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, X.; Wang, X.H.; Li, J.Y.; Qiu, H.; Yang, D.D. Transcutaneous auricular vagus nerve stimulation for epilepsy. Seizure 2024, 119, 84–91. [Google Scholar] [CrossRef]
- Pan, L.; Wang, J.; Wu, W.; Wang, Y.; Zhu, Y.; Song, Y. Transcutaneous auricular vagus nerve stimulation improves working memory in temporal lobe epilepsy: A randomized double-blind study. CNS Neurosci. Ther. 2024, 30, e14395. [Google Scholar] [CrossRef]
- Abel, T.J.; Remick, M.; Welch, W.C.; Smith, K.J. One-year cost-effectiveness of callosotomy vs vagus nerve stimulation for drug-resistant seizures in Lennox-Gastaut Syndrome: A decision analytic model. Epilepsia Open 2022, 7, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Piazza, M.G.; Kellogg, R.J.; Wisniewski, S.; Abel, T.J. A survey of preferences and expectations for surgical interventions targeting atonic seizures in Lennox-Gastaut syndrome. Child’s Nerv. Syst 2024, 40, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- Apantaku, G.O.; McDonald, P.J.; Aguiar, M.; Cabrera, L.Y.; Chiong, W.; Connolly, M.B.; Hrincu, V.; Ibrahim, G.M.; Kaal, K.J.; Lawson, A.; et al. Clinician preferences for neurotechnologies in pediatric drug-resistant epilepsy: A discrete choice experiment. Epilepsia 2022, 63, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Cukiert, A.; Cukiert, C.M.; Burattini, J.A.; Guimaraes, R.B. Combined Neuromodulation (Vagus Nerve Stimulation and Deep Brain Stimulation) in Patients with Refractory Generalized Epilepsy: An Observational Study. Neuromodulation 2023, 26, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Suresh, H.; Mithani, K.; Warsi, N.; Ochi, A.; Otsubo, H.; Drake, J.M.; Rutka, J.T.; Kerr, E.; Smith, M.L.; Breitbart, S.; et al. Add-On Deep Brain Stimulation versus Continued Vagus Nerve Stimulation for Childhood Epilepsy (ADVANCE): A Partially Randomized Patient Preference Trial. Ann. Neurol. 2024, 96, 405–411. [Google Scholar] [CrossRef]
| PICO Element | Details |
|---|---|
| Patient Population | Patients diagnosed with Lennox–Gastaut syndrome (LGS), all ages. Exclusion: studies not specifically identifying LGS patients for any outcome measures. |
| Intervention | Vagus nerve stimulation (VNS) therapy |
| Control | Not required |
| Outcome Measures |
|
| |
| |
| |
| |
| Inclusion Criteria | Studies reporting on VNS outcomes in LGS patients, regardless of publication date. English abstracts are required. |
| Exclusion Criteria | Studies where LGS patient numbers are not specifically identified. Non-English language studies without available translations. |
| Author | Country/Setting | Type of Study | N | Key Findings |
|---|---|---|---|---|
| Abdelmoity | Single center in the USA | Retrospective Cohort | 71 | A total of 55% of patients achieved greater than 50% seizure reduction at six months, 67.7% at 12 months, and 65% at 24 months. At 12 months, 11% of the patients were completely seizure free, and at 24 months, 17% were seizure free. Most families reported at least a 50% improvement in one or more quality-of-life measures. Common adverse events were dysphonia, paresthesia, and shortness of breath, which subsided by 24 months. |
| Alanazi | Single center in Saudi Arabia | Retrospective Cohort | 4 | Mean age of 13.8 ± 3.9 years; 2 (50%) patients achieved a reduction in seizure frequency of greater than 75% and 2 (50%) greater than 25%; ASM was unchanged in 3 and decreased in 1; swallowing difficulty ¼. |
| Aldenkamp | Single center in the Netherlands | Prospective Cohort | 19 | In 13 patients, LGS was confirmed; 5 had Lennox-like syndromes. Average age was 11.2 years. Average seizure reduction was 20.6% at 24 months. No significant deterioration in cognitive or quality of life measures. Mental age showed mild positive changes. |
| Benifla | Single center in Canada | Retrospective Cohort | 10 | A total of 4 out of 10 had a >50% seizure reduction. Subgroup analysis for non-seizure outcomes and complications were not provided. |
| Ben-Menachem | Single center in Sweden | Prospective Cohort | 8 | Average treatment time was 20 months. Five patients were responders in all seizure types, particularly GTCS and absences. Some patients had significant reductions in atonic seizures. Seizure reduction and severity improvement varied. Subgroup analysis for non-seizure outcome and complication were not provided. |
| Boon | Single center in Belgium | Prospective | 3 | All 3 responders had no side effects. All 3 had additional benefit from magnet stimulation (used by caregivers). |
| Buoni | Single center in Italy | Prospective Cohort | 7 | Age range was 6–28 years (median 17 years). A total of 38.4% had a ≥50% reduction in seizures. Some patients reported improved alertness, hyperactivity, appetite, and social communication. None had major complication; one had intractable headaches with increasing stimulation requiring decreased stim. |
| Casazza | Single center in Italy | Prospective cohort | 4 | None of them had >50% seizure reduction; fecal and urinary incontinence, associated with diarrhea, when the output current was increased. These side effects disappeared when there was a reduction in the intensity of the output current. |
| Cersósimo | Single center in Italy | Retrospective Cohort | 46 | Mean age at implant was 13 years, with a mean follow-up of 30 months. A total of 65% showed a ≥50% reduction in seizures. There were no significant differences between symptomatic and cryptogenic LGS patients or those with prior West syndrome. Improvement in behavior, cognition, and quality of life noted. |
| Cukiert | Single center in Brazil | Prospective Cohort | 20 | Age range was 5–12 years (mean 8.4 years). Mean follow-up was 32 months. A total of 17 out of 20 had a ≥50% seizure reduction. Quality of life and health measures improved in up to 50% of children. Attention improved in most children. |
| Cukiert (2022) | Single center in Brazil | Retrospective Cohort | 10 | Follow-up time after VNS ranged from 18 to 132 months (mean 52 months). Four patients initially responded to VNS. |
| Dibue | USA | Registry Analysis | 564 | Responder rates at 24 months were 57.5% in the LGS group and 61.5% in the non-LGS group. Median seizure frequency reduction at 24 months was 64.3% vs. 66.7% in the LGS vs. non-LGS group, respectively. VNS was most effective at reducing focal aware seizures, “other” seizures, generalized-onset non-motor seizures, and drop attacks. Significant regression from bilateral tonic–clonic response in LGS group. Within the 24 months of follow-up, there were 4 deaths in the LGS group and 5 deaths in the non-LGS group. |
| Elliott | Single center in the USA | Retrospective (prospective data collection) | 24 | Mean seizure reduction was 57.6% (43.0–72.2%). Median seizure reduction was 52.1%. Not different from the majority of the study group who had unidentified TRE causes (mean reduction: 57.0%). Non-seizure outcomes, etc., were not given for specific LGS groups. |
| Frost | 6 centers in the USA | Retrospective Cohort | 50 (46 had evaluable data at 1 month; 43 after 3 months, and 24 after 6 months) | Median age at implant was 13 years. At 1 month, median seizure reduction was 42%. At 3 and 6 months, reductions were 58.2% and 57.9%, respectively. Drop-attack seizures significantly decreased. QOL assessment (simple, invalidated, 5-point rating scale, which featured ratings of much worse, worse, same, better, and much better) was performed at 3 and 6 months. Improvement in alertness and verbal abilities in more patients than memory and mood. The least effect was shown on ambulation. The most common adverse events reported were voice alteration and coughing during stimulation. Other uncommon adverse events included increased drooling and behavioral changes. In total, 4% of patients had superficial wound infection; 10% had transient pain at the incision site; 44% had voice alteration/hoarseness. Moreover, 30% reported coughing, neck tingling (8%), nonspecific pain sensation (8%), shortness of breath during exertion (4%), decreased appetite (4%), hiccups (4%), and dyspepsia (4%). One (2%) patient reported dysphagia and insomnia, and another (2%) reported vomiting after stimulation started. One patient reported both ear pain and jaw pain as stimulation. Increased salivation (8%), worsening behavior or hyperactivity (6%). |
| Gurubani | Single center in the USA | Retrospective (prospective data collection) | 13 | Responder, 38.5%, partial responder(e (≥50% seizure reduction in 2 or less study periods) 23%, non-responder 38.5% in 24 months. |
| Hajnsek | Single center in Croatia | Retrospective | 3 | Mean age of 26.67 ± 5.77 13.34 ± 2.88; p = 0.02 (follow-up duration unknown); improvement of mood and the general quality of life was reported in all patients. |
| Hallbook | Single center in Sweden | Case Series | 4 | A total of 1 patient had a >25% seizure reduction. No changes in cognitive scales (BSID) in all 4 patients. |
| Hödl | Single center in Belgium | Prospective cohort | 7 | A total of 2/7 patients had >50% seizure reduction; no difference in pre-ictal HRV parameters between VNS responders and VNS non-responders could be found, but high frequency (HF) power, reflecting the parasympathetic tone increased significantly in the pre-ictal epoch in both VNS responders and VNS non-responders (p = 0.017, p = 0.004). |
| Hornig | Single center in the USA | Retrospective Cohort | 6 | A total of 5 out of 6 had a >90% seizure reduction. Assessed with a global evaluation score (10 cm horizontal line: central point no change; extreme left, considerable worse; extreme right, considerable improvement. Most (no specific n was given) had improvements in alertness, independence, and learning. |
| Hosain | Single center in the USA | Prospective Cohort | 13 | Age range 4–44 years. An amount of 52% seizure reduction at 6 months. Six patients had a >50% seizure reduction. No cognitive, behavioral, motor, or coordination worsening. A total of 3/13 had hoarseness; 3 had excessive coughing, 1 surgical debridement and antibiotics for incisional infection. |
| Kang | Single center in Korea | Retrospective cohort | 7 | A total of 6/7 were responders; the seizure frequency at the last follow-up (mean follow-up 38.8 months; range-29.7–49.7) showed a decrease of 57.2% (0% to 100%) on average. One patient achieved seizure free status. One patient with seizure worsening. There was no mortality or complications related to the VNS therapy except one case requiring ICU admission due to pneumonia. Comparing the results before and after VNS surgery, the VNS therapy also had a tendency to have a positive effect on quality of life (p = 0.066). Severity of illness: 4 improved, 3 unchanged; CGI-I: 2 marked improvement, 2 moderate, 4 minimal, and 1 unchanged. |
| Karceski | VNS patient registry | Retrospective | 167 | A total of 107 had >50% seizure reduction. |
| Katagiri | Single center in Japan | Retrospective | 10 | Age 10 years 8 months (range, 3–30 years); 6 had >50% seizure reduction in all residual seizure types after CC over 1 year follow-up; 2 were seizure free. Tonic seizures were the most common seizure type that was residual after CC. Responses were observed in five of the ten (50%) patients after VNS. Atypical absence seizures were less likely to be controlled with VNS (25% response rate). Myoclonic seizure was observed in four patients, two of whom were responders. None of the patients with residual drop attacks caused by atonic seizures responded to VNS. Patients who responded to VNS after CC could have conversations with others, while non-responders could not. After VNS, transient hoarseness was observed in two patients, and coughing only with magnet activation was observed in one patient. |
| Kim | Single center in Korea | Retrospective | 14 | The mean age 18.1 ± 4.9 years, and mean follow-up duration was 5.4 ± 2.2 years; one year after the procedure, 2 of the 14 patients showed a reduction in seizure frequency of more than 90%. |
| Kostov 2009 | Single center in Norway | Retrospective Cohort | 30 | Median observation time was 52 months. Median seizure reduction was 60.6%. A total of 20/30 were responders. Best effects on atonic and tonic seizures. Additional improvements in postictal phase (16/30) and alertness (76.7%). Decrease in seizure duration from magnet use in 73.3% of the patients. Adverse effects 20/30, drooling and voice alteration 6/30. V cord paralysis in 1 patient (reverse following stimulator explanted). One with enuresis, etc. Most side effects transient and stimulation dependent. |
| Kostov 2023 | Norwegian VNS quality registry | Retrospective Cohort | 62 | Median follow-up was 88 months. Seizure reduction at 6, 12, 24, 36, and 60 months was 18.8%, 31.3%, and 30.6%. Median seizure reduction for LGS was 40.6%. Highest reduction for atonic seizures (64.6%). Improved alertness and seizure severity noted. More DEE patients (total 105 patients; 62 of them had LGS) were reported to have greater improvement in ictal or postictal severity (43.8% vs. 28.3%, p = 0.006) and alertness (62.9% vs. 31.6%, p < 0.001) than patients without ID. |
| Labar | Single center in the USA | Prospective | 5 | Median seizure reduction 41% comparison of the rates of all seizures for the baseline month, with the rates of all seizures for the first 9 months of VNS treatment (2 out of 5 were responders) Variable reduction in different seizure types. Adverse events reported in one patient each were incisional infection, choking sensation and voice change, and coughing (noted by two patients). One patient discontinued VNS due to coughing. |
| Lundgren | Single center in Sweeden | Case Series | 4 | Two patients had a >50% seizure reduction. Improvement noted in seizure severity score and quality of life (linear visual analog scale: −100 to +100). Two patients had up to +50 at 16–18 months, one patient had 0, and one patient had QOL assessment up to 10–12 months, which was +10; premature current failure 2/4, aspiration ¼, tiredness ¼, increased salivation 2/4. |
| Mikati | Single center in Lebanon | Prospective cohort | 6 | Mean age 23 years; over mean follow-up of 1 year 11 months, 3 had >50% seizure reduction (−175% to 85% reduction). Seizure severity: 3/6 milder, 2/6 same, 1/6 stronger; 2/6 seizure duration decreased. For post-vagal nerve stimulation, the total group scored significantly higher in the social domain (p = 0.039) but LGS specific information was not available. |
| Nagarajan | Single center in Australia | Retrospective Cohort | 6 | A total of 5 out of 6 had a >50% seizure reduction. Reduction in seizure duration and severity, but the magnet was not useful. Total group outcome (n = 16) was given. On a three point scale, 12 of the 16 parents (entire group rather than LGS specific subgroup) reported that quality of life was definitely better and this correlated with improved seizure control. |
| Nakken | Single center in Norway | Retrospective | 3 | A total of 1/3 were responders. No other LGS-specific outcomes were available. |
| Orosz | Multiple centers in the European Union | Retrospective Cohort | 123 (6 months), 146 (12 months), 87 (24 months) | At 6, 12, and 24 months, ≥50% seizure reduction in 28.5%, 32.9%, and 39.1% of patients, respectively. Higher responder rate with stable AEDs. Significant correlation between VNS total charge and response rate. |
| Parker | Single center in the UK | Retrospective Cohort | 9 | A total of 16 patients with epileptic encephalopathies. Median reduction for 9 children with LGS was 34% between 6 and 12 months. No significant improvement in EEG before and after implant. No significant difference in communication, living, or socialization domains for Vineland adaptive behavior scale. Significant improvement in perceived treatment side effects and general behavior. |
| Qiabi | Single center in Canada | Retrospective | 5 | A total of 3/5 were responders at 24 months. Non-seizure outcomes and adverse effects were not given. |
| Rossignol | Single center in Canada | Prospective | 5 | A total of 3/5 responders, 1 seizure free, maximal seizure effect 3/5 myoclonic and 1 with tonic. |
| Rychlicki | Italy | Prospective Cohort | 9 | A total of 34 patients. Significant better results in partial epilepsy, with and without drop attacks, than in LGS. Mean reduction in seizure frequency in LGS: 8% at 3 months, 33% at 12 months, and 50% at 2 years (not significant). |
| Shahwan | Single Center in Australia | Retrospective Cohort | 9 | Seven (77.7%) had a ≥50% seizure reduction. Significant reduction in daytime drop attacks and tonic seizures. Decrease in hospitalization for SE in some patients. Improved alertness in all responders. |
| Suller Marti | Single center in Canada | Retrospective Cohort | 29 | A total of 46 patients. Of the LGS group, 41.7% had an overall seizure reduction of 50% or more. Seizure reduction rate was 59% in LGS group and 64.7% in GGE group. A total of 96.6% of LGS patients required seizure-related hospital admissions before VNS implantation, 51.7% after implantation. Number of patients with >75% seizure reduction significantly higher in GGE group. GTC seizures reduced post-VNS, but no improvement in atypical absence seizures. Frequency of side effects similar in LGS vs. Non-LGS groups (62.1% vs 64.7%). |
| You | Multiple centers in South Korea | Retrospective Cohort | 10 | A total of 70% had a >50% seizure reduction; 20% had a >75% reduction. One patient experienced dyspnea while sleeping and one patient suffered from drooling. |
| Zamponi | Single center in Italy | Retrospective Cohort | 14 | A total of 3 out of 14 had a >50% seizure reduction. Improvement in seizure frequency and quality of life in some patients. Compared to baseline, after one year and three years of stimulation, no significant changes in cognitive level and adaptive behavior scores were observed in group level but 4 had clinically relevant improvement in adaptive behavior (at least 5 point increase in standard scores) mainly due to increased alertness and a better social reciprocity. Six patients had better QoL; this was independent of seizure outcome. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samanta, D. Efficacy and Safety of Vagus Nerve Stimulation in Lennox–Gastaut Syndrome: A Scoping Review. Children 2024, 11, 905. https://doi.org/10.3390/children11080905
Samanta D. Efficacy and Safety of Vagus Nerve Stimulation in Lennox–Gastaut Syndrome: A Scoping Review. Children. 2024; 11(8):905. https://doi.org/10.3390/children11080905
Chicago/Turabian StyleSamanta, Debopam. 2024. "Efficacy and Safety of Vagus Nerve Stimulation in Lennox–Gastaut Syndrome: A Scoping Review" Children 11, no. 8: 905. https://doi.org/10.3390/children11080905
APA StyleSamanta, D. (2024). Efficacy and Safety of Vagus Nerve Stimulation in Lennox–Gastaut Syndrome: A Scoping Review. Children, 11(8), 905. https://doi.org/10.3390/children11080905






