Use of Lung Ultrasound in Cystic Fibrosis: Is It a Valuable Tool?
Abstract
1. Introduction
2. Methods
3. Results
3.1. Role in Pulmonary Exacerbations and Acute Complications
3.2. Role in Follow-Up Evaluations of Stable Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CR | Chest radiograph |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| LUS | Lung ultrasound |
| PExs | Pulmonary exacerbations |
| PFTs | Pulmonary function tests |
| LCI | Lung clearance index |
| FEV1 | Forced expiratory volume in 1 s |
| FEF 25–75 | Forced expiratory flow at 25–75% |
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.S.; Prince, A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Tiddens, H.A.W.M.; Stick, S.M.; Wild, J.M.; Ciet, P.; Parker, G.J.M.; Koch, A.; Vogel-Claussen, J. Respiratory tract exacerbations revisited: Ventilation, inflammation, perfusion, and structure (VIPS) monitoring to redefine treatment: Respiratory Tract Exacerbations Revisited. Pediatr. Pulmonol. 2015, 50 (Suppl. S40), S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Hota, P.; Madan, R. Cystic Fibrosis from Childhood to Adulthood. Radiol. Clin. N. Am. 2020, 58, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Ciet, P.; Bertolo, S.; Ros, M.; Casciaro, R.; Cipolli, M.; Colagrande, S.; Costa, S.; Galici, V.; Gramegna, A.; Lanza, C.; et al. State-of-the-art review of lung imaging in cystic fibrosis with recommendations for pulmonologists and radiologists from the “iMAging managEment of cySTic fibROsis” (MAESTRO) consortium. Eur. Respir. Rev. 2022, 31, 210173. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Allinovi, M.; Giacalone, M.; Corsini, I. To B or not to B. The rationale for quantifying B-lines in pediatric lung diseases. Pediatr. Pulmonol. 2023, 58, 9–15. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Demi, L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev. Respir. Med. 2019, 13, 163–172. [Google Scholar] [CrossRef]
- Ullmann, N.; D’Andrea, M.L.; Gioachin, A.; Papia, B.; Testa, M.B.C.; Cherchi, C.; Bock, C.; Tomà, P.; Cutrera, R. Lung ultrasound: A useful additional tool in clinician’s hands to identify pulmonary atelectasis in children with neuromuscular disease. Pediatr. Pulmonol. 2020, 55, 1490–1494. [Google Scholar] [CrossRef]
- Curatola, A.; Corona, F.; Squillaci, D.; Saccari, A.; Chiaretti, A.; Barbi, E.; Maschio, M. Lung ultrasound evaluation in people with cystic fibrosis: A new approach in the pulmonology outpatient clinic. Pediatr. Pulmonol. 2024, 59, 592–599. [Google Scholar] [CrossRef]
- Strzelczuk–Judka, L.; Wojsyk–Banaszak, I.; Zakrzewska, A.; Jończyk–Potoczna, K. Diagnostic value of chest ultrasound in children with cystic fibrosis—Pilot study. PLoS ONE 2019, 14, e0215786. [Google Scholar] [CrossRef]
- Ciuca, I.M.; Pop, L.L.; Dediu, M.; Stoicescu, E.R.; Marc, M.S.; Manea, A.M.; Manolescu, D.L. Lung Ultrasound in Children with Cystic Fibrosis in Comparison with Chest Computed Tomography: A Feasibility Study. Diagnostics 2022, 12, 376. [Google Scholar] [CrossRef]
- Ciuca, I.; Pop, L.; Marc, M.; Oancea, C. How useful is the lung ultrasound in cystic fibrosis? Eur. Respir. J. 2016, 48, PA1261. [Google Scholar]
- Barakat, M. B lines: Role of chest sonography in assessment of bronchiectasis. Eur. Respir. J. 2016, 48, PA3797. [Google Scholar]
- Ghany, M.F.A. Transthoracic ultrasound in the diagnosis of bronchiectasis: Is it valuable? Egypt. J. Bronchol. 2019, 13, 303–308. [Google Scholar] [CrossRef]
- Ciuca, I.M.; Dediu, M.; Pop, L.L. Lung clearance index and lung ultrasound in cystic fibrosis children. Eur. Respir. J. 2018, 52 (Suppl. S62), OA4988. [Google Scholar]
- Peixoto, A.O.; Marson, F.A.L.; Souza, T.H.; Fraga, A.D.M.A.; Ribeiro, J.D. Lung ultrasound assessment of response to antibiotic therapy in cystic fibrosis exacerbations: A study of two cases. J. Bras. Pneumol. 2019, 45, e20190128. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.O.; Marson, F.A.; Dertkigil, S.S.; Dertkigil, R.P.; Souza, T.H.; Fraga, A.M.; Ribeiro, A.F.; Toro, A.A.; Ribeiro, J.D. The Use of Ultrasound as a Tool to Evaluate Pulmonary Disease in Cystic Fibrosis. Respir. Care 2020, 65, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Hassanzad, M.; Kiani, A.; Abedini, A.; Ghaffaripour, H.; Emami, H.; Alizadeh, N.; Zoghi, G.; Hashemi, S.; Velayati, A.A. Lung ultrasound for the diagnosis of cystic fibrosis pulmonary exacerbation. BMC Pulm. Med. 2021, 21, 353. [Google Scholar] [CrossRef]
- Jaworska, J.; Buda, N.; Kwaśniewicz, P.; Komorowska-Piotrowska, A.; Sands, D. Lung Ultrasound in the Evaluation of Lung Disease Severity in Children with Clinically Stable Cystic Fibrosis: A Prospective Cross-Sectional Study. J. Clin. Med. 2023, 12, 3086. [Google Scholar] [CrossRef]
- Ouyang, L.; Grosse, S.D.; Amendah, D.D.; Schechter, M.S. Healthcare expenditures for privately insured people with cystic fibrosis. Pediatr. Pulmonol. 2009, 44, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.J.; Borowitz, D.S.; Christiansen, D.H.; Morris, E.M.; Nash, M.L.; Ramsey, B.W.; Rosenstein, B.J.; Smith, A.L.; Mary Ellen Wohl, for the Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N. Engl. J. Med. 1994, 331, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.; Emerson, J.; Williams-Warren, J.; Pepe, M.; Smith, A.; Montgomery, A.B.; Ramsey, B. Defining a pulmonary exacerbation in cystic fibrosis. J. Pediatr. 2001, 139, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; Mogayzel, P.J.; Robinson, K.A.; Goss, C.H.; Rosenblatt, R.L.; Kuhn, R.J.; Marshall, B.C.; the Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic Fibrosis Pulmonary Guidelines: Treatment of Pulmonary Exacerbations. Am. J. Respir. Crit. Care Med. 2009, 180, 802–808. [Google Scholar] [CrossRef]
- Belanger, A.R.; Nguyen, K.; Osman, U.; Gilbert, C.R.; Allen, K.; Al Rais, A.F.; Yarmus, L.; Akulian, J.A. Pleural effusions in non-transplanted cystic fibrosis patients. J. Cyst. Fibros. 2017, 16, 499–502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Musolino, A.M.; Tomà, P.; De Rose, C.; Pitaro, E.; Boccuzzi, E.; De Santis, R.; Morello, R.; Supino, M.C.; Villani, A.; Valentini, P.; et al. Ten Years of Pediatric Lung Ultrasound: A Narrative Review. Front. Physiol. 2022, 12, 721951. [Google Scholar] [CrossRef]
- Garcia, B.; Flume, P.A. Pulmonary Complications of Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2019, 40, 804–809. [Google Scholar] [CrossRef]
- Heuvelings, C.C.; Bélard, S.; Familusi, M.A.; Spijker, R.; Grobusch, M.P.; Zar, H.J. Chest ultrasound for the diagnosis of paediatric pulmonary diseases: A systematic review and meta-analysis of diagnostic test accuracy. Br. Med. Bull. 2019, 129, 35–51. [Google Scholar] [CrossRef]
- Scialanga, B.; Buonsenso, D.; Scateni, S.; Valentini, P.; Schingo, P.M.; Boccuzzi, E.; Mesturino, M.A.; Ferro, V.; Chiaretti, A.; Villani, A.; et al. Lung Ultrasound to Detect Pneumothorax in Children Evaluated for Acute Chest Pain in the Emergency Department: An Observational Pilot Study. Front. Pediatr. 2022, 10, 812246. [Google Scholar] [CrossRef]
- Goyal, V.; Chang, A.B. Bronchiectasis in Childhood. Clin. Chest Med. 2022, 43, 71–88. [Google Scholar] [CrossRef]
- La Regina, D.P.; Bloise, S.; Pepino, D.; Iovine, E.; Laudisa, M.; Cristiani, L.; Nicolai, A.; Nenna, R.; Mancino, E.; Di Mattia, G.; et al. Lung ultrasound in bronchiolitis. Pediatr. Pulmonol. 2021, 56, 234–239. [Google Scholar] [CrossRef]
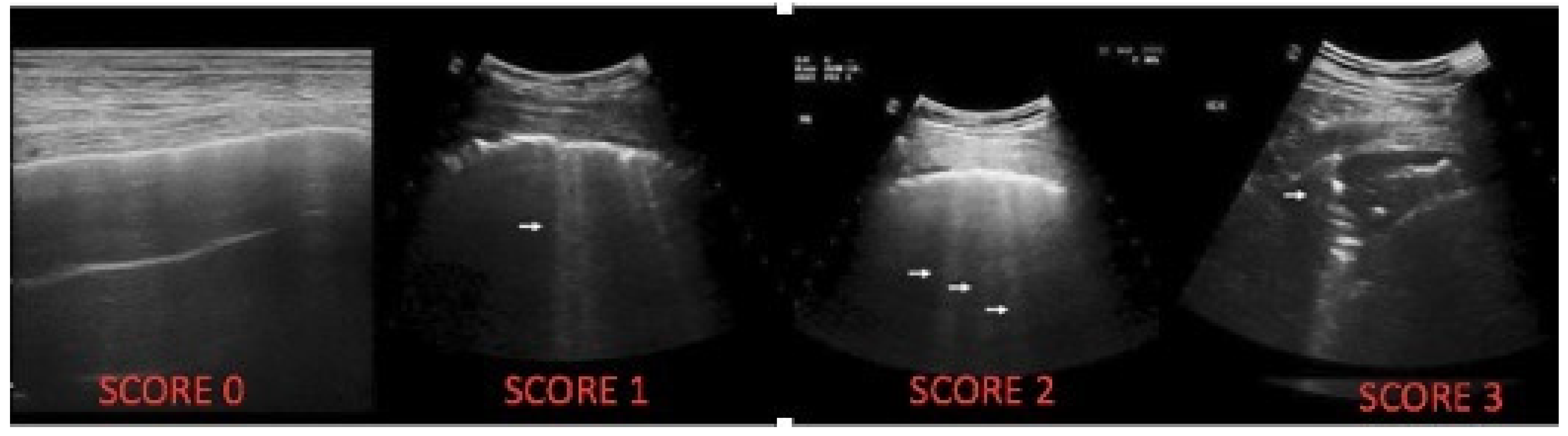

| LUS Artefact | Lung CF Score |
|---|---|
| Presence of A lines-normal aspect; distinctive B-lines < 3/ic space | 0 |
| Distinctive B-lines > 3/space or 1 coalescent B-line | 1 |
| Coalescent B-lines > 2/ic space | 2 |
| Consolidation < 1 cm | 3 |
| Consolidation > 1 cm, with bronchogram | 4 |
| Atelectasis/consolidation without bronchogram, >1 cm | 5 |
| Country | Population Size | Age Categories | Setting | Main Results | |
|---|---|---|---|---|---|
| Curatola et al., 2023 [9] | Italy | 29 | Pediatric and adults | Outpatient | Correlation between LUS score and spirometric values |
| Strzelczuk–Judka L et al., 2019 [10] | Poland | 48 | Pediatric | Outpatient | Correlation of LUS with CT, detection of subpleural consolidation higher than CR |
| Ciuca et al., 2022 [11] | Romania | 98 | Pediatric | Outpatient | Correlation of LUS with CT, higher sensitivity and specificity to detect atelectasis and consolidations |
| Ciuca et al., 2016 [12] | Romania | 82 | Pediatric and adults | Outpatient | Correlation of LUS with CT |
| Barakat M et al., 2016 [13] | Egypt | 91 | Not specified | Outpatient | Correlation between number of B-lines with type and extent of bronchiectasis |
| Ghany MFA, 2019 [14] | Egypt | 61 | Adult | Outpatient | Correlation of LUS with severity of bronchiectasis CT score |
| Ciuca et al., 2018 [15] | Romania | 42 | Not specified | Outpatient | Correlation of LUS with LCI |
| Peixoto AO et al., 2019 [16] | Brazil | 2 | Adults | Hospitalization | Use of LUS score to monitor end of antibiotic intravenous therapy |
| Peixoto AO et al., 2020 [17] | Brazil | 18 | Adults | Outpatient | Correlation of LUS with CT, functional test and nutritional status |
| Hassanzad M et al., 2021 [18] | Iran | 30 | Pediatric and adults | Hospitalization | LUS is superior to CR and comparable with CT in PExs |
| Jaworska J et al., 2023 [19] | Poland | 131 | Pediatric | Outpatient | Correlation of LUS with CR, pulmonary function, and microbiological status |
| Patients n | LUS vs. CXR | LUS vs. CT | Consolidations | Pleural Effusion | Interstitial Syndrome | Atelectasis | |
|---|---|---|---|---|---|---|---|
| Peixoto AO et al. [17] | 18 | / | R = 0.607 p = 0.001 | / | / | / | / |
| Strzelczuk–Judka L et al. [10] | 48 | R = 0.52 p = 0.0002 | / | / | / | / | / |
| Hassanzad M et al. [18] | 30 | [AUROC] = 0.900 vs. 0.575 | / | Specificity 90% Sensitivity 94.7% PPV 94.7% NPV 81.8% | Specificity 96.7% Sensitivity not calculated PPV 0% NPV 100% | / | Specificity 100% Sensitivity not calculated PPV 93.3% NPV not calculated |
| Ciuca, I et al. [12] | 82 | / | R = 0.79 p < 0.0001 | / | / | / | / |
| Ciuca I et al. [15] | 57 | / | R = 0.87 p = 0.000 | Specificity 93.02% Sensitivity 94.4% PPV 89.4% NPV 97.3% | / | / | Specificity 94.5% Sensitivity 83.7% PPV 92.5% NPV 72.3% |
| Patients n | LUS vs. CT | Saccular/Cystic | Tubular | Cylindrical | |
|---|---|---|---|---|---|
| Ciuca, I et al. [12] | 82 | / | PPV 100% | R = 0.14 p < 0.9 | / |
| Ciuca I et al. [15] | 57 | / | Sensitivity 68.4% Specificity 94.9% PPV 88.8% NPV 94.7% | / | Sensitivity 77.7% Specificity 9% PPV 80.7% NPV 76.9% |
| Ghany MFA et al. [14] | 91 | Correlated with severity of bronchiectasis (by modified Reiff score pattern, p < 0.000) | / | Correlated with consolidation pattern (100.0%, p = 0.044) | Correlated with B-lines pattern (57.1%, p = 0.001) |
| M. Barakat et al. [13] | 91 | Correlated with type (R = 0.729; p < 0.0001) and extent (R = 0.640; p < 0.0001) | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, A.; Cristiani, L.; Majo, F.; Ullmann, N.; Esposito, M.; Supino, M.C.; Tomà, P.; Villani, A.; Musolino, A.M.; Cutrera, R. Use of Lung Ultrasound in Cystic Fibrosis: Is It a Valuable Tool? Children 2024, 11, 917. https://doi.org/10.3390/children11080917
Boni A, Cristiani L, Majo F, Ullmann N, Esposito M, Supino MC, Tomà P, Villani A, Musolino AM, Cutrera R. Use of Lung Ultrasound in Cystic Fibrosis: Is It a Valuable Tool? Children. 2024; 11(8):917. https://doi.org/10.3390/children11080917
Chicago/Turabian StyleBoni, Alessandra, Luca Cristiani, Fabio Majo, Nicola Ullmann, Marianna Esposito, Maria Chiara Supino, Paolo Tomà, Alberto Villani, Anna Maria Musolino, and Renato Cutrera. 2024. "Use of Lung Ultrasound in Cystic Fibrosis: Is It a Valuable Tool?" Children 11, no. 8: 917. https://doi.org/10.3390/children11080917
APA StyleBoni, A., Cristiani, L., Majo, F., Ullmann, N., Esposito, M., Supino, M. C., Tomà, P., Villani, A., Musolino, A. M., & Cutrera, R. (2024). Use of Lung Ultrasound in Cystic Fibrosis: Is It a Valuable Tool? Children, 11(8), 917. https://doi.org/10.3390/children11080917






