The Legacy of the COVID-19 Pandemic: Impact on Infant and Maternal and Health from an Appalachian Academic Medical Center
Abstract
1. Introduction
2. Materials and Methods
| MS-DRG | Description | MS-DRG Weight | Research-DRG Weight |
|---|---|---|---|
| 795 | Normal newborn | 0.2024 | 0.2 |
| 794 | Newborn with significant problems | 1.4946 | 1.5 |
| 793 | Newborn with major problems | 4.2225 | 4.2 |
| 792 | Preterm newborn without major problems | 2.4804 | 2.5 |
| 791 | Preterm newborn with major problems | 4.1107 | 4.1 |
| 790 | Preterm less than 26 week gestation or respiratory distress syndrome (surfactant deficiency) | 6.0189 | 6.0 |
| 789 | Newborn less than 28 days that died or was transferred to another acute care facility | 1.8252 | 1.8 |
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, P.; Reddy, S.Y.; Manuel, S.; Dwivedi, A.K. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: An updated systematic review and meta-analysis. EJOG 2020, 52, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. AJOG MFM 2020, 2, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Novoa, R.H.; Quintana, W.; Llancarí, P.; Urbina-Quispe, K.; Guevara-Ríos, E.; Ventura, W. Maternal clinical Characteristics, and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review. Travel Med. Infect. Di. 2021, 39, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, K.R. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy—SET-NET, 16 Jurisdictions, March 29–October 14, 2020. MMWR 2020, 69, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Navér, L.; Söderling, J.; Ahlberg, M.; Hervius Askling, H.; Aronsson, B.; Byström, E.; Jonsson, J.; Sengpiel, V.; Ludvigsson, J.F.; et al. Association of Maternal SARS-CoV-2 Infection in Pregnancy with Neonatal Outcomes. JAMA 2021, 325, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Calvert, C.; Brockway, M.; Zoega, H.; Miller, J.E.; Been, J.V.; Amegah, A.K.; Racine-Poon, A.; Oskoui, S.E.; Abok, I.I.; Aghaeepour, N.; et al. Changes in preterm birth and stillbirth during COVID-19 lockdowns in 26 countries. Nat. Hum. Behav. 2023, 7, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Wei, S.Q.; Dayan, N.; Ukah, U.V.; Quach, C.; Lewin, A.; Healy-Profitós, J.; Ayoub, A.; Chang, J.; Luu, T.M. Impact of COVID-19 on rates of gestational diabetes in a North American pandemic epicenter. Acta Diabetol. 2023, 60, 257–264. [Google Scholar] [CrossRef]
- Vicente, D.M.C.; Martínez, A.M.; García, I.G.; Toboso, R.Q.; López, I.Q.; Rey, M.D.; Vaamonde, J.G.; Alemán, M.O.; Moragrega, R.M.; Díaz, C.G.; et al. Effects of the COVID-19 pandemic on gestational diabetes in Castilla-La Mancha (Spain). Endocrinol. Diabetes Nutr. 2024, 71, 53–60. [Google Scholar] [CrossRef]
- Scifres, C.M. Short- and Long-Term Outcomes Associated with Large for Gestational Age Birth Weight. Obstet. Gynecol. Clin. North. Am. 2021, 48, 325–337. [Google Scholar] [CrossRef]
- Charles, E.; Hunt, K.A.; Harris, C.; Hickey, A.; Greenough, A. Small for gestational age and extremely low birth weight infant outcomes. J. Perinat. Med. 2019, 47, 247–251. [Google Scholar] [CrossRef]
- Campisi, S.C.; Carbone, S.E.; Zlotkin, S. Catch-Up Growth in Full-Term Small for Gestational Age Infants: A Systematic Review. Adv. Nutr. 2019, 10, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Eiden, R.D.; Epstein, L.H.; Shenassa, E.D.; Xie, C.; Wen, X. Etiological Subgroups of Small-for-Gestational-Age: Differential Neurodevelopmental Outcomes. PLoS ONE 2016, 11, e0160677. [Google Scholar] [CrossRef] [PubMed]
- Minor, K.C.; Bianco, K.; Sie, L.; Druzin, M.L.; Lee, H.C.; Leonard, S.A. Severity of small-for-gestational-age and morbidity and mortality among very preterm neonates. J. Perinatol. 2023, 43, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.; Moise, A.; Miller, A.; Burke, R.; Cottrell, L.; Haarbauer, K.; Smith, M.C.; Polak, M. Changes in Large for Gestation and Small for Gestation Births during the COVID-19 Era. WVMJ 2023, 119, 30–35. [Google Scholar] [CrossRef]
- FY 2024 IPPS Proposed Rule Home Page. Available online: https://www.cms.gov/medicare/payment/prospective-payment-systems/acute-inpatient-pps/fy-2024-ipps-proposed-rule-home-page (accessed on 10 April 2023).
- Joya, R.M.; Cottrell, L.; Kiefer, A.; Polak, M.J. Diagnosis Related Group Weight and Derived Case Mix Index to Assess the Complexity Among Twins. Am. J. Perinatol. 2022, 39, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A. Statistical power analyses using G*Power 3.1, Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/nchs/pressroom/states/westvirginia/wv.htm (accessed on 28 May 2024).
- Pineda, R.; Knudsen, K.; Breault, C.C.; Rogers, E.E.; Mack, W.J.; Fernandez-Fernandez, A. NICUs in the US: Levels of acuity, Number of Beds, and Relationships to Population Factors. J. Perinatol. 2023, 43, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Umer, A.; Loudin, S.; Maxwell, S.; Lilly, C.; Stabler, M.E.; Cottrell, L.; Hamilton, C.; Breyel, J.; Mullins, C.; John, C. Capturing the Statewide Incidence of Neonatal Abstinence Syndrome in Real Time: The West Virginia Experience. Ped Res. 2019, 85, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Grouse, G.; Ghertner, R.; Madden, E.; Radel, L. Foster Care Entry Rates Grew Faster for Infants than for Children of Other Ages, 2011–2018. 2021. Available online: https://www.aspe.hhs.gov/sites/default/files/2021-08/infant-foster-care-brief.pdf (accessed on 31 August 2021).
- Committee on Practice Bulletins—Obstetrics. Obes. Pregnancy Obstet. Gynecol. 2021, 127, e128–e143. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/diabetes-during-pregnancy (accessed on 15 May 2024).
- Khedagi, A.M.; Bello, N.A. Hypertensive Disorders of Pregnancy. Card. Clin. 2021, 39, 77–90. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/diabetes/php/data-research/ (accessed on 15 May 2024).
- Forray, A. Substance Use during Pregnancy. F1000 Res. 2016, 5, 887. [Google Scholar] [CrossRef] [PubMed]
- Regan, A.K.; Kaur, R.; Nosek, M.; Swathi, P.A.; Gu, N.Y. COVID-19 vaccine acceptance and coverage among pregnant persons in the United States. Prev. Med. Rep. 2022, 29, 101977. [Google Scholar] [CrossRef] [PubMed]
- Loveandpositiveenergy. Build like Warriors #Bigpandemicbabies. TikTok. 13 July 2021. Available online: www.tiktok.com (accessed on 13 July 2021).
- Barker, D. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 2002, 13, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.; Harding, J. Clinical Aspects of Neonatal Hypoglycemia: A Mini Review. Front. Pediatr. 2021, 8, 562251. [Google Scholar] [CrossRef]
- Woestenberg, P.J.; de Feijter, M.; Bergman, J.E.H.; Lutke, L.R.; Passier, A.J.L.M.; Kant, A.C. Maternal first trimester COVID-19 vaccination and risk of major non-genetic congenital anomalies. Birth Defects Res. 2023, 115, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Miller, M.; Branda, M.E.; Mehta, R.A.; Theiler, R.N. Maternal COVID-19 vaccination status and association with neonatal congenital anomalies. Front. Pediatr. 2024, 12, 1355502. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Liu, Y.; Pang, W.; Zhang, D.; Wang, K.; Chen, Y. Associations of COVID-19 vaccination during pregnancy with adverse neonatal and maternal outcomes: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1044031. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, R.S.; Mormol, J.; Trawick, E.; Perry, M.F.; Allen, E.C.; Millan, D.; Miller, E.S. Association of COVID-19 Vaccination During Early Pregnancy with Risk of Congenital Fetal Anomalies. JAMA Pediatr. 2022, 176, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Luteijn, J.M.; Brown, M.J.; Dolk, H. Influenza and congenital anomalies: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 809–823. [Google Scholar] [CrossRef]
- Botto, L.D.; Panichello, J.D.; Browne, M.L.; Krikov, S.; Feldkamp, M.L.; Lammer, E.; Shaw, G.M.; National Birth Defects Prevention Study. Congenital heart defects after maternal fever. Am. J. Obstet. Gynecol. 2014, 210, 359.e1–359.e11. [Google Scholar] [CrossRef]
- Graham, J.M., Jr. Update on the gestational effects of maternal hyperthermia. Birth Defects Res. 2020, 112, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.; Felkner, M.; Hendricks, K. The effect of fever, febrile illnesses, and heat exposures on the risk of neural tube defects in a Texas-Mexico border population. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, M.; Taheri, M.; Mazaheripour, Z.; Abbasi-Khameneh, F. The incidence of congenital anomalies in infants before and during the COVID-19 pandemic. Ital. J. Pediatr. 2022, 48, 174. [Google Scholar] [CrossRef] [PubMed]
- Reppucci, M.L.; Kaizer, A.M.; Prendergast, C.; Acker, S.N.; Mandell, E.W.; Euser, A.G.; Diaz-Miron, J. Incidence of congenital complications related to COVID-19 infection during pregnancy. J. Neonat. Perinat. Med. 2023, 6, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Arbour, L.; Lewin, A.; Brousseau, É.; Healy-Profitós, J.; Luu, T.M. Congenital anomalies during COVID-19: Artifact of surveillance or a real TORCH? Eur. J. Epidemiol. 2024, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Brandibur, T.E.; Kundnani, N.R.; Boia, M.; Nistor, D.; Velimirovici, D.M.; Mada, L.; Manea, A.M.; Boia, E.R.; Neagu, M.N.; Popoiu, C.M. Does COVID-19 Infection during Pregnancy Increase the Appearance of Congenital Gastrointestinal Malformations in Neonates? Biomedicines 2023, 11, 3105. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Moise, A.; Cottrell, L.; Polak, M.J. Incidence of Congenital Microcephaly is Increased With Fetal Exposure to Tobacco, Opioids, and Cannabis. WVMJ 2023, 118, 32–36. [Google Scholar]
- Pederson, A. Maternal Mortality Rates in Appalachia. 2022. The Pitt Journal. Available online: https://pitjournal.unc.edu/2023/03/22/maternal-mortality-rates-in-appalachia (accessed on 10 October 2022).
- Michelle, J.K.; Osterman, M.H.S. Changes in Primary and Repeat Cesarean Delivery: United States, 2016–2021. Available online: https://www.cdc.gov/nchs/data/vsrr/vsrr021.pdf (accessed on 21 July 2022).
- Rodolaki, K.; Pergialiotis, V.; Iakovidou, N.; Boutsikou, T.; Iliodromiti, Z.; Kanaka-Gantenbein, C. The impact of maternal diabetes on the future health and neurodevelopment of the offspring: A review of the evidence. Front. Endocrinol. 2023, 14, 1125628. [Google Scholar] [CrossRef]
- Agrawal, A.; Wenger, N.K. Hypertension During Pregnancy. Curr. Hypertens. Rep. 2020, 22, 64. [Google Scholar] [CrossRef]
- Delalić, Đ.; Jug, J.; Prkačin, I. Arterial Hypertension Following COVID-19, A Retrospective Study of Patients in a Central European Tertiary Care Center. Acta Clin. Croat. 2022, 61 (Suppl. S1), 23–27. [Google Scholar] [CrossRef]
- Zhang, V.; Fisher, M.; Hou, W.; Zhang, L.; Duong, T.Q. Incidence of New-Onset Hypertension Post-COVID-19: Comparison With Influenza. Hypertension 2023, 80, 2135–2148. [Google Scholar] [CrossRef]
- Akpek, M. Does COVID-19 Cause Hypertension? Angiology 2022, 73, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W., Jr.; LaMarca, B. Risk of cardiovascular disease, end-stage renal disease, and stroke in postpartum women and their fetuses after a hypertensive pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R521–R528. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- About Opioid Use during Pregnancy. Available online: https://www.cdc.gov/pregnancy/opioids/data (accessed on 25 April 2024).
- Shephard, H.M.; Manning, S.E.; Nestoridi, E.; Darling, A.M.; Brown, C.M.; Hatch, M.; Ahnger-Pier, K.; Pagnano, S.; Mather, D.; Yazdy, M.M. Inequities in COVID-19 Vaccination Coverage Among Pregnant Persons, by Disaggregated Race and Ethnicity—Massachusetts, May 2021–October 2022. MMWR 2023, 72, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Births and Natality. Available online: https://www.cdc.gov/nchs/fastats/births.htm (accessed on 25 April 2024).
- Querdasi, F.R.; Vogel, S.C.; Thomason, M.E.; Callaghan, B.L.; Brito, N.H. A comparison of the infant gut microbiome before versus after the start of the COVID-19 pandemic. Sci. Rep. 2023, 13, 13289. [Google Scholar] [CrossRef]
- Giesbrecht, G.F.P.; Lebel, C.; Dennis, C.-L.; Silang, K.B.; Xie, E.B.M.; Tough, S.; McDonald, S.; Tomfohr-Madsen, L.P. Risk for Developmental Delay Among Infants Born During the COVID-19 Pandemic. JDBP 2023, 44, e412–e420. [Google Scholar] [CrossRef]
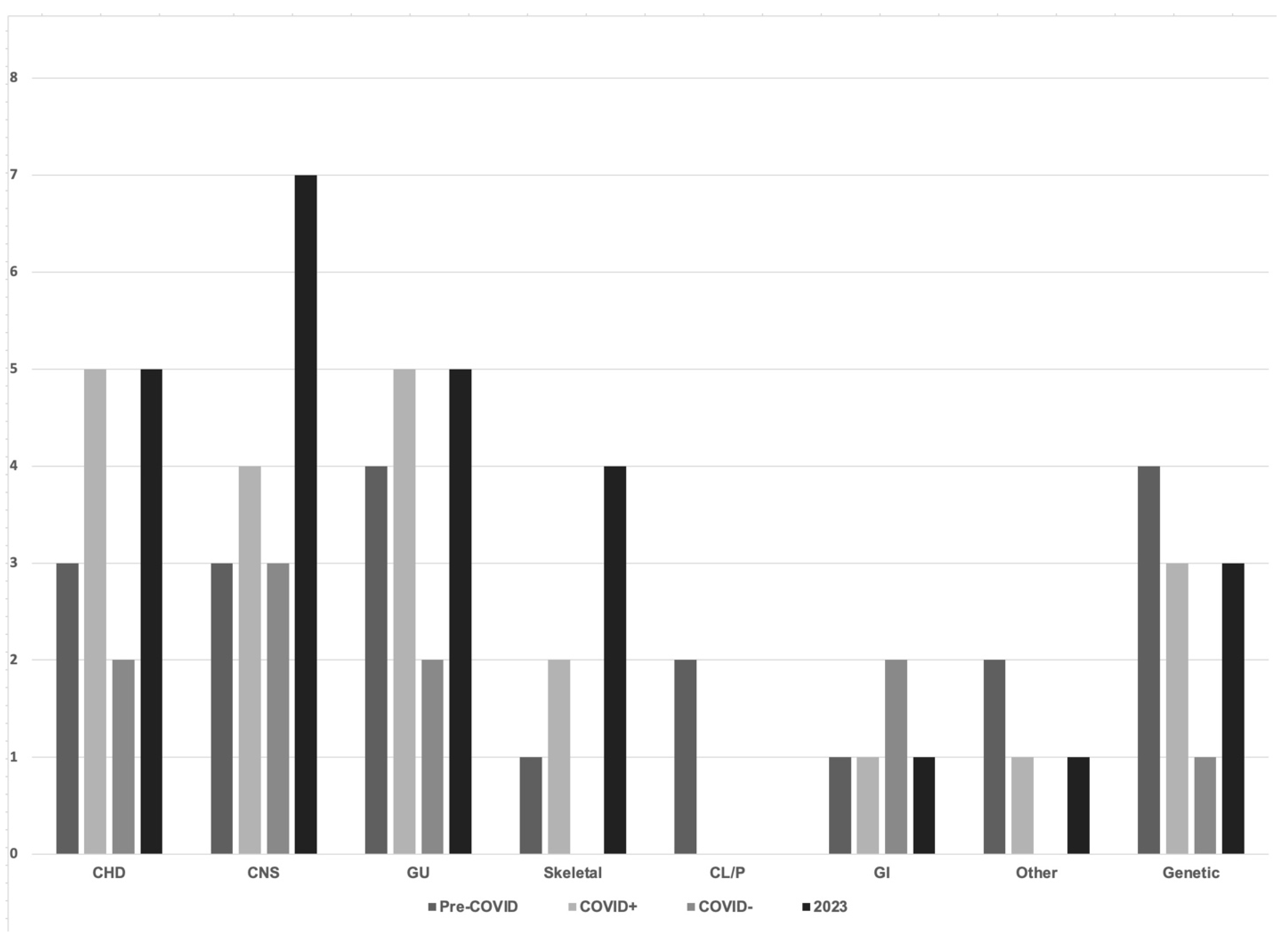
| Pre-COVID 2018–2019 (n = 300) | COVID-19 Period Infected Mothers 2020–2022 (n = 305) | COVID-19 Period Noninfected Mothers 2020–2022 (n = 300) | Post COVID-19 2023 (n = 300) | p < 0.05 | |
|---|---|---|---|---|---|
| Gestation (weeks) | 37 ± 3 | 38 ± 2 | 38 ± 3 | 37 ± 3 | NS |
| Prematurity < 36 6/7 wk. | 28% | 19% * | 19% * | 23% * | 0.011 |
| Male | 56% | 53% | 51% | 53% | NS |
| C-section | 37% | 36% | 39% | 37% | NS |
| Instrumented vaginal deliveries | 1% * | 4% | 8% * | 1% * | 0.014 |
| LOS (days) | 10 ± 24 * | 7 ± 20 | 7 ± 18 | 6 ± 15 * | 0.036 |
| Apgar 1 min | 7 ± 2 | 8 ± 2 | 8 ± 2 | 7 ± 2 | NS |
| Apgar 5 min | 9 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 1 | NS |
| Z score for weight | −0.04 ± 0.83 | 0.07 ± 0.94 | 0.10 ± 0.92 | 0.04 ± 0.98 | NS |
| Large for gestational age (LGA) | 6% * | 10% | 13% * | 10% | 0.041 |
| Small for gestational age (SGA) | 7% | 6% | 4% * | 13% * | 0.02 |
| Hypoglycemia | 18% | 16% | 19% | 18% | NS |
| Hypoglycemia +IV fluids | 72.2% | 62.5% | 47.4% | 50% | NS |
| NICU Admission | 29% | 23% | 26% | 30% | NS |
| RDS [P22.0] | 11% | 7% | 8% | 7% | NS |
| Neonatal COVID-19 | - | 0.6% | 0 | 0 | |
| Congenital anomaly | 5% | 7% | 3% * | 8% * | 0.022 |
| Microcephaly (<3% for gestational age) | 1% | 1% | 0% | 1% | NS |
| NOWS | 2% | 2% | 3% | 2% | NS |
| Foster care | 5% | 4% | 3% | 3% | NS |
| Twin | 10% | 8% | 9% | 6% | NS |
| Survival | 99% | 99.3% | 99.7% | 99% | NS |
| Pre-COVID 2018–2019 (n = 284) | COVID-19 Period Infected Mothers 2020–2022 (n = 298) | COVID-19 Period Noninfected Mothers 2020–2022 (n = 288) | Post COVID-19 2023 (n = 289) | p < 0.05 | |
|---|---|---|---|---|---|
| Maternal age (years) | 28 ± 6 | 27± 5 | 28 ± 5 | 28 ± 5 | NS |
| Survival | 100% | 99% | 100% | 100% | NS |
| BMI | 33.29 ± 14.39 | 34.85 ± 8.44 | 34.49 ± 8.12 | 34.57 ± 8.45 | NS |
| BMI > 30 | 61% | 70% | 67% | 69% | NS |
| Tobacco exposure | 14% | 13% | 16% | 15% | NS |
| Cannabis exposure | 5% | 3% | 9% | 7% | NS |
| Opioid exposure | 6% | 2% | 6% | 4% | NS |
| Cocaine exposure | 2% | 0 | 1% | 0 | NS |
| Methamphetamine exposure | 1% | 0 | 2% | 2% | NS |
| Hepatitis C. positive | 4% | 3% | 2% | 4% | NS |
| Hypertension (+ preeclampsia and HELLP) | 21% * | 32% | 32% | 38% * | 0.001 |
| Diabetes (including GDM, type 1, and type 2) | 7% * | 13% * | 12% | 11% | 0.02 |
| COVID-19 infection | - | 100% | - | 4% | NS |
| Days from COVID-19 infection to delivery | - | 66 ± 68 | - | 50–260 | NS |
| Symptomatic COVID-19 infection | - | 59% | - | 50% | NS |
| COVID-19 antibody treatment | - | 6.2% | - | 10% | NS |
| NCPAP/vent support (without ECMO) | - | 0.6% | - | 0 | NS |
| ECMO + vent support | - | 1.7% | - | 0 | NS |
| COVID-19 vaccine (after 15 April 2021) | - | 13% | 14% | 43% | NS |
| Maternal COVID-19 Infections | COVID-19 Asymptomatic (n = 120) | COVID-19 Symptomatic (n = 185) | Significance p ≤ 0.05 | COVID-19 Severe Symptoms (n = 7) |
|---|---|---|---|---|
| Gestation (weeks) | 38 ± 2 | 38 ± 2 | NS | 32 ± 3 |
| Prematurity < 36 6/7 wk. | 18% | 20% | NS | 100% |
| Male | 55% | 50% | NS | 57 |
| C-section | 31% | 41% | NS | 86% |
| Instrumented vaginal deliveries | 4% | 4% | NS | 0 |
| LOS (days) | 6 ± 18 | 7 ± 21 | NS | 27 ± 25 |
| Apgar 1 minute | 8 ± 2 | 7 ± 2 | NS | 6 ± 2 |
| Apgar 5 minute | 9 ± 1 | 9 ± 1 | NS | 8 ± 1 |
| Z score for weight | 0.00 ± 0.93 | 0.13 ± 0.89 | NS | 0.73 ± 0.36 |
| Large for gestational age (LGA) | 9% | 11% | NS | 14% |
| Small for gestational age (SGA) | 7% | 5% | NS | 0 |
| Hypoglycemia | 13% | 18% | NS | 86% |
| Hypoglycemia + IV fluids | 46% | 66.6% | p = 0.05 | 74% |
| NICU Admission | 19% | 26% | p = 0.05 | 100% |
| RDS [P22.0] | 4% | 8% | NS | 71% |
| Neonatal COVID-19 | 0% | 0.5% | NS | 0 |
| Congenital anomaly | 6% | 8% | NS | 0 |
| NALS/NOWS | 3% | 1% | NS | 0 |
| Survival | 99.2% | 99% | NS | 100% |
| Pre-COVID 2018–2019 (n = 300) | COVID-19 Period Infected Mothers 2020–2022 (n = 305) | COVID-19 Period Noninfected Mothers 2020–2022 (n = 300) | Post COVID-19 2023 (n = 300) | |
|---|---|---|---|---|
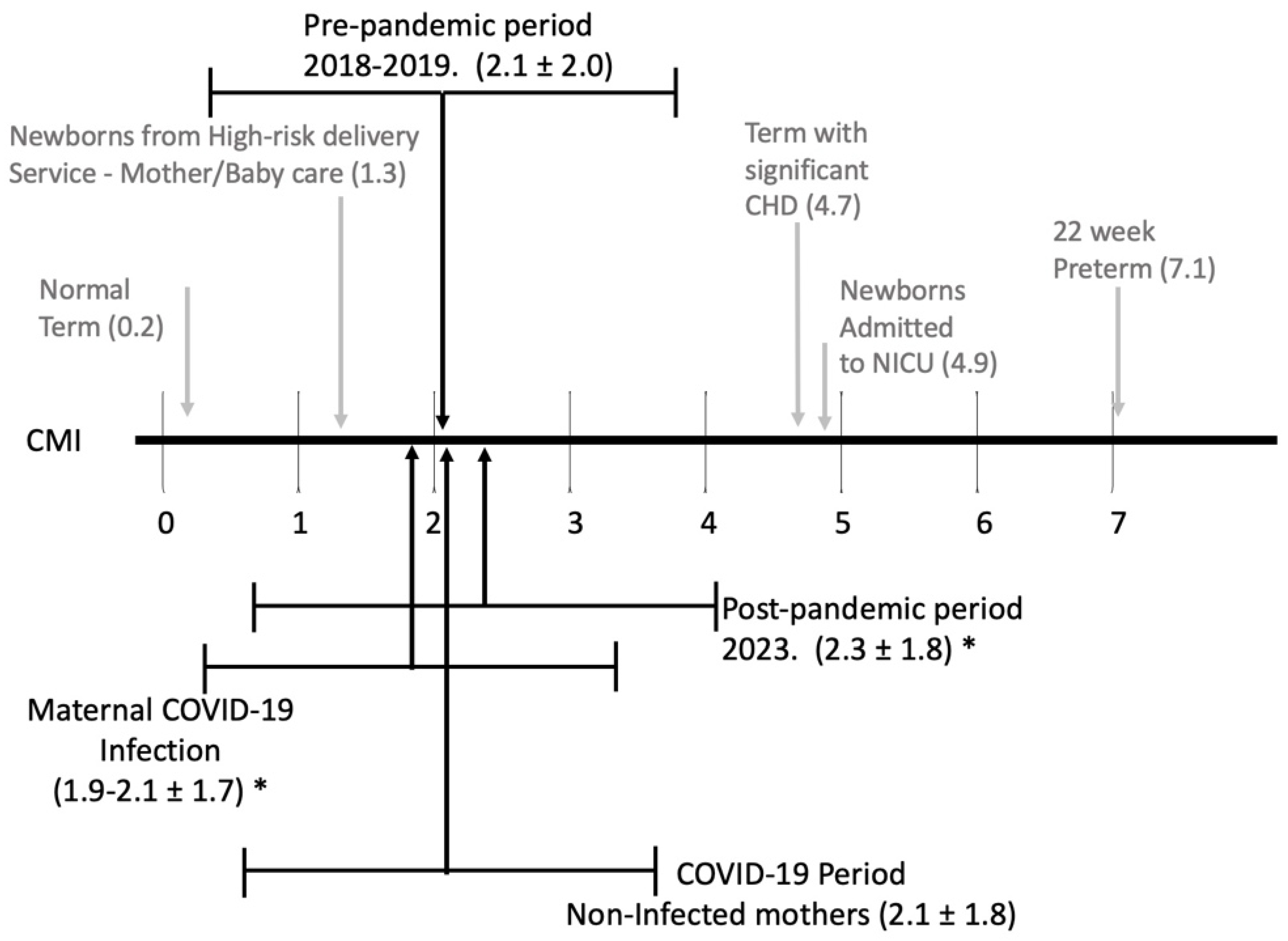
| Case Mix Index CMI | 2.1 ± 2.0 | 1.9 ± 1.7 * | 2.1± 1.8 | 2.3 ± 1.8 * |
| Research-DRG 795 Weight = 0.2 | 35% * | 31% | 28% | 22% * |
| Research-DRG 794 Weight = 1.5 | 28% * § ¶ | 42% § | 39% ¶ | 42% * |
| Research-DRG 792 Weight = 2.5 | 8% | 9% | 4% | 4% |
| Research-DRG 791 Weight = 4.1 | 10% | 6% § | 7% | 14% * § |
| Research-DRG 793 Weight = 4.2 | 9% * | 9% § | 13% | 16% * § |
| Research-DRG 790 Weight = 6.0 | 10% | 7% | 8% | 7% |
| Research-DRG 789 Weight = 1.8 | 1% | 0.7% | 0.3% | 0.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haarbauer, K.; Burke, R.; Smith, M.C.; Miller, A.N.; Moran, P.N.; Moise, A.A.; Cottrell, L.; Polak, M.J. The Legacy of the COVID-19 Pandemic: Impact on Infant and Maternal and Health from an Appalachian Academic Medical Center. Children 2024, 11, 924. https://doi.org/10.3390/children11080924
Haarbauer K, Burke R, Smith MC, Miller AN, Moran PN, Moise AA, Cottrell L, Polak MJ. The Legacy of the COVID-19 Pandemic: Impact on Infant and Maternal and Health from an Appalachian Academic Medical Center. Children. 2024; 11(8):924. https://doi.org/10.3390/children11080924
Chicago/Turabian StyleHaarbauer, Kelsey, Rebecca Burke, M. Cody Smith, Audrey N. Miller, Patricia N. Moran, Alicia A. Moise, Lesley Cottrell, and Mark J. Polak. 2024. "The Legacy of the COVID-19 Pandemic: Impact on Infant and Maternal and Health from an Appalachian Academic Medical Center" Children 11, no. 8: 924. https://doi.org/10.3390/children11080924
APA StyleHaarbauer, K., Burke, R., Smith, M. C., Miller, A. N., Moran, P. N., Moise, A. A., Cottrell, L., & Polak, M. J. (2024). The Legacy of the COVID-19 Pandemic: Impact on Infant and Maternal and Health from an Appalachian Academic Medical Center. Children, 11(8), 924. https://doi.org/10.3390/children11080924







