The Motor Optimality Score—Revised Improves Early Detection of Unilateral Cerebral Palsy in Infants with Perinatal Cerebral Stroke
Abstract
1. Introduction
2. Aims of the Study
3. Materials and Methods
3.1. Study Design
3.2. Subjects
3.3. General Movements Assessment and MOS-R
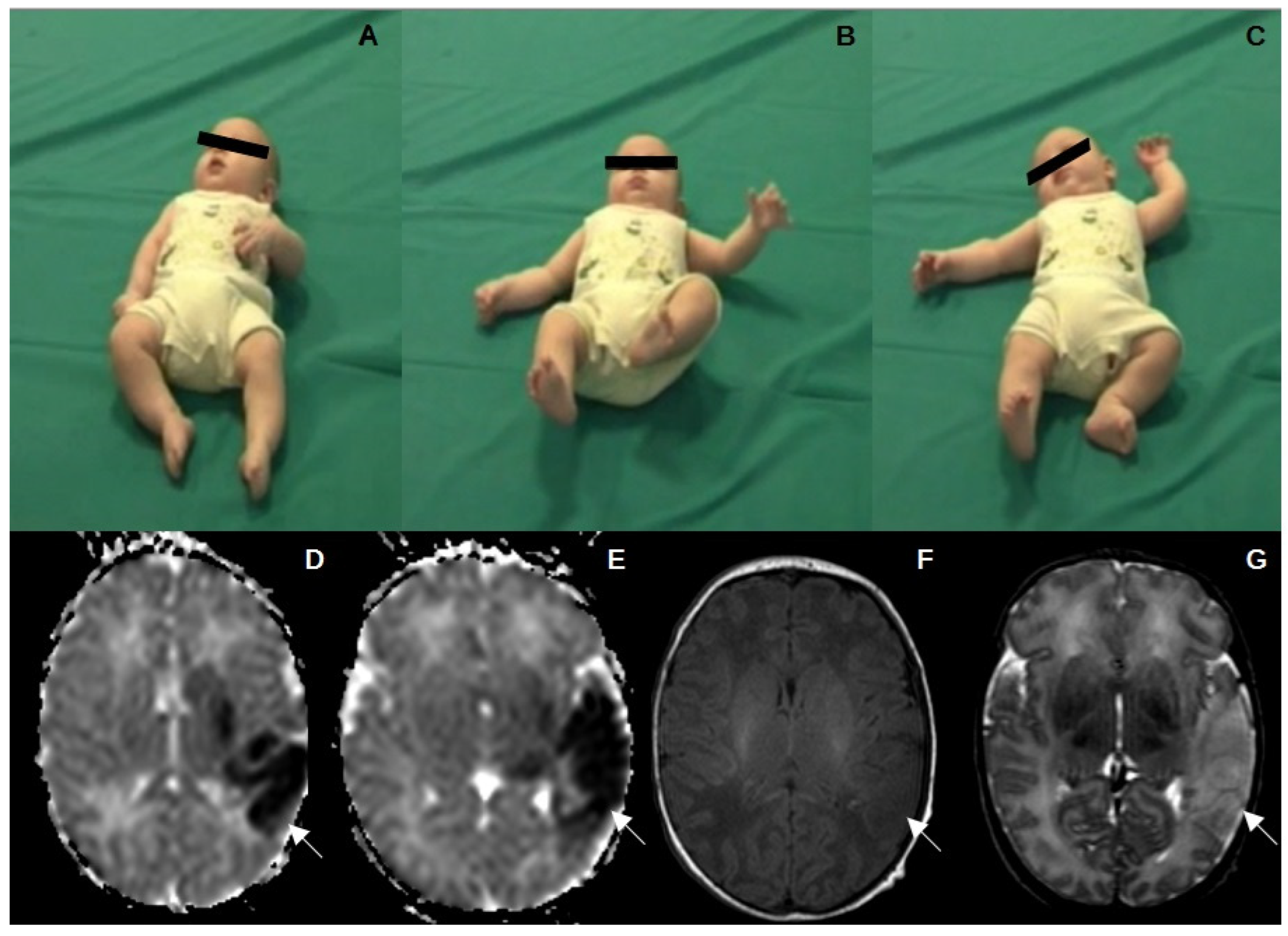
3.4. Brain Magnetic Resonance Imaging (MRI)
3.5. Outcome Assessment
4. Results
4.1. GMs Assesment
4.2. Movement Patterns
4.3. Postural Patterns
4.4. Movement Character
4.5. Motor Optimality List
4.6. Neurodevelopmental Outcome and Prognostic Factors
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitagliano, M.; Dunbar, M.; Dyck Holzinger, S.; Letourneau, N.; Dewey, D.; Oskoui, M.; Shevell, M.; Kirton, A. Perinatal arterial ischemic stroke and periventricular venous infarction in infants with unilateral cerebral palsy. Dev. Med. Child Neurol. 2022, 64, 56–62. [Google Scholar] [CrossRef]
- Dunbar, M.; Kirton, A. Perinatal Stroke. Semin. Pediatr. Neurol. 2019, 32, 100767. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.; Kirton, A. Perinatal stroke: Mechanisms, management, and outcomes of early cerebrovascular brain injury. Lancet Child Adolesc. Health 2018, 2, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Govaert, P.; Matthys, E.; Zecic, A.; Roelens, F.; Oostra, A.; Vanzieleghem, B. Perinatal cortical infarction within middle cerebral artery trunks. Arch. Dis. Child Fetal Neonatal. Ed. 2000, 82, F59–F63. [Google Scholar] [CrossRef] [PubMed]
- Prechtl, H.F.R. Developmental neurology of the fetus. Baillieres Clin. Obstet. Gynaecol. 1988, 2, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Cioni, G.; Ferrari, F.; Prechtl, H.F. Posture and spontaneous motility in fullterm infants. Early Hum. Dev. 1989, 18, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Cioni, G.; Prechtl, H.F. Qualitative changes of general movements in preterm infants with brain lesions. Early Hum. Dev. 1990, 23, 193–231. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Crowle, C.; Goyen, T.A.; Hradman, C.; Jackman, M.; Novak, I.; Badawi, N. Sensitivity and specificity of general movement assessment for diagnostic accuracy of detecting cerebral palsy early in an Australian context. J. Paediatr. Child Health 2016, 52, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef]
- Morgan, C.; Fahey, M.; Roy, B.; Novak, I. Diagnosing cerebral palsy in full-term infants. J. Paediatr. Child Health 2018, 54, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Prechtl, H.F.R.; Einspieler, C.; Cioni, G.; Ferrari, F.; Sontheimer, D. An early marker for neurological deficits after perinatal brain lesions. Lancet 1997, 349, 1361–1363. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, A.; Mercuri, E.; Rapisardi, G.; Ferrari, F.; Roversi, M.F.; Cowan, F.; Rutherford, M.; Paolicelli, P.B.; Einspieler, C.; Boldrini, A.; et al. General movements detect early signs of hemiplegia in term infants with neonatal cerebral infarction. Neuropediatrics 2003, 34, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, A.; Pizzardi, A.; Belmonti, V.; Boldrini, A.; Carotenuto, M.; D’Acunto, G.; Ferrari, F.; Fiori, S.; Gallo, C.; Ghirri, P.; et al. Hand movements at 3 months predict later hemiplegia in term infants with neonatal cerebral infarction. Dev. Med. Child Neurol. 2010, 52, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Cioni, G.; Bos, A.F.; Einspieler, C.; Ferrari, F.; Martijn, A.; Paolicelli, P.B.; Rapisardi, G.; Roversi, M.F.; Prechtl, H.F.R. Early neurological signs in preterm infants with unilateral intraparenchymal echodensity. Neuropediatrics 2000, 31, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Bos, A.F.; Krieber-Tomantschger, M.; Alvarado, E.; Barbosa, V.M.; Bertoncelli, N.; Burger, M.; Chorna, O.; Del Secco, S.; DeRegnier, R.A.; et al. Cerebral Palsy: Early Markers of Clinical Phenotype and Functional Outcome. Clin. Med. 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Prechtl, H.F. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Örtqvist, M.; Einspieler, C.; Marschik, P.B.; Ådén, U. Movements and posture in infants born extremely preterm in comparison to term-born controls. Early Hum. Dev. 2021, 154, 105304. [Google Scholar] [CrossRef] [PubMed]
- Örtqvist, M.; Einspieler, C.; Ådén, U. Early prediction of neurodevelopmental outcomes at 12 years in children born extremely preterm. Pediatr. Res. 2022, 91, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Örtqvist, M.; Marschik, P.B.; Toldo, M.; Zhang, D.; Fajardo-Martinez, V.; Nielsen-Saines, K.; Ådén, U.; Einspieler, C. Reliability of the Motor Optimality Score-Revised: A study of infants at elevated likelihood for adverse neurological outcomes. Acta Paediatr. 2023, 112, 1259–1265. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Lugli, L.; Guidotti, I.; Pugliese, M.; Roversi, M.F.; Bedetti, L.; Della Casa Muttini, E.; Cavalleri, F.; Todeschini, A.; Genovese, M.; Ori, L.; et al. Polygraphic EEG Can Identify Asphyxiated Infants for Therapeutic Hypothermia and Predict Neurodevelopmental Outcomes. Children 2022, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Lugli, L.; Bariola, M.C.; Guidotti, I.; Pugliese, M.; Roversi, M.F.; Bedetti, L.; Della Casa Muttini, E.; Miselli, F.; Ori, L.; Lucaccioni, L.; et al. Neurodevelopmental outcome of neonatal seizures: A longitudinal study. Eur. J. Paediatr. Neurol. 2024, 49, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lugli, L.; Pugliese, M.; Plessi, C.; Berardi, A.; Guidotti, I.; Ancora, G.; Grandi, S.; Gargano, G.; Braibanti, S.; Sandri, F.; et al. Neuroprem: The Neuro-developmental outcome of very low birth weight infants in an Italian region. Ital. J. Pediatr. 2020, 46, 26. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, J.; Gahagan, S.; Amiel-Tison, C. The Amiel-Tison neurological assessment at term: Conceptual and methodological continuity in the course of follow-up. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Amiel-Tison, C.; Gosselin, J. Neurologic Development from Birth to Six Years; Johns Hopkins University Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Griffiths, R. Griffiths Mental Developmental Scale-Revised: Birth to 2 Years (GMDS-R); Hogrefe: Firenze, Italy, 1996. [Google Scholar]
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr. Dis. Treat. 2020, 16, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Cioni, G.; D’Acunto, G.; Guzzetta, A. Perinatal brain damage in children: Neuroplasticity, early intervention, and molecular mechanisms of recovery. Prog. Brain Res. 2011, 189, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Pietruszewski, L.; Nelin, M.A.; Batterson, N.; Less, J.; Moore-Clingenpeel, M.; Lewandowski, D.; Levengood, K.; Maitre, N.L. Hammersmith Infant Neurological Examination Clinical Use to Recommend Therapist Assessment of Functional Hand Asymmetries. Pediatr. Phys. Ther. 2021, 33, 200–206. [Google Scholar] [CrossRef]
- Maitre, N.L.; Chorna, O.; Romeo, D.M.; Guzzetta, A. Implementation of the Hammersmith Infant Neurological Examination in a high-risk infant follow-up program. Pediatr. Neurol. 2016, 65, 31–38. [Google Scholar] [CrossRef]
- Yin, H.; Wang, X.; Yang, H.; Zhu, X.; Wang, J.; Li, Z. A pilot study of the General Movement Optimality Score detects early signs of motor disorder in neonates with arterial ischemic stroke. Early Hum. Dev. 2021, 163, 105484. [Google Scholar] [CrossRef] [PubMed]
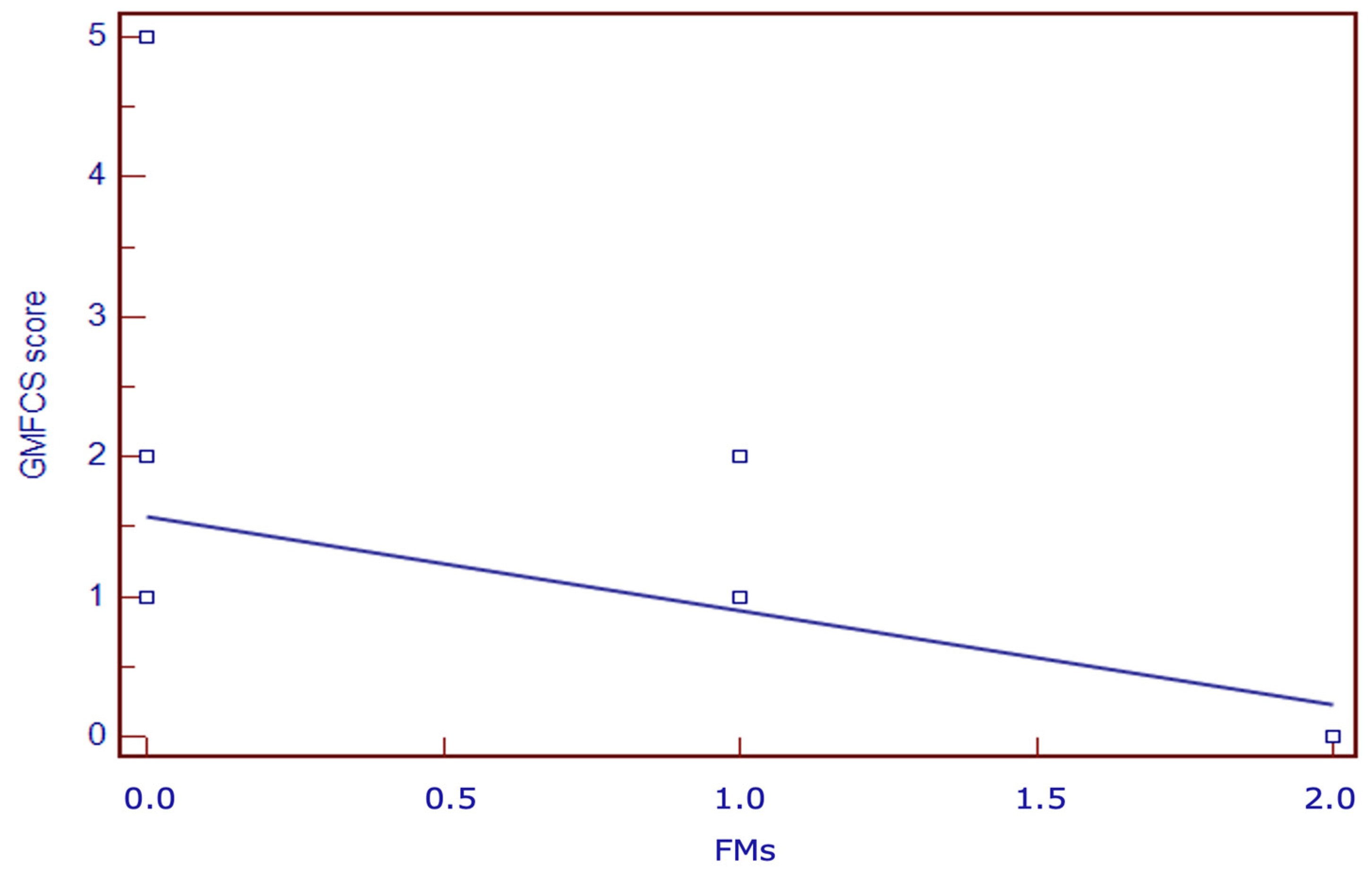
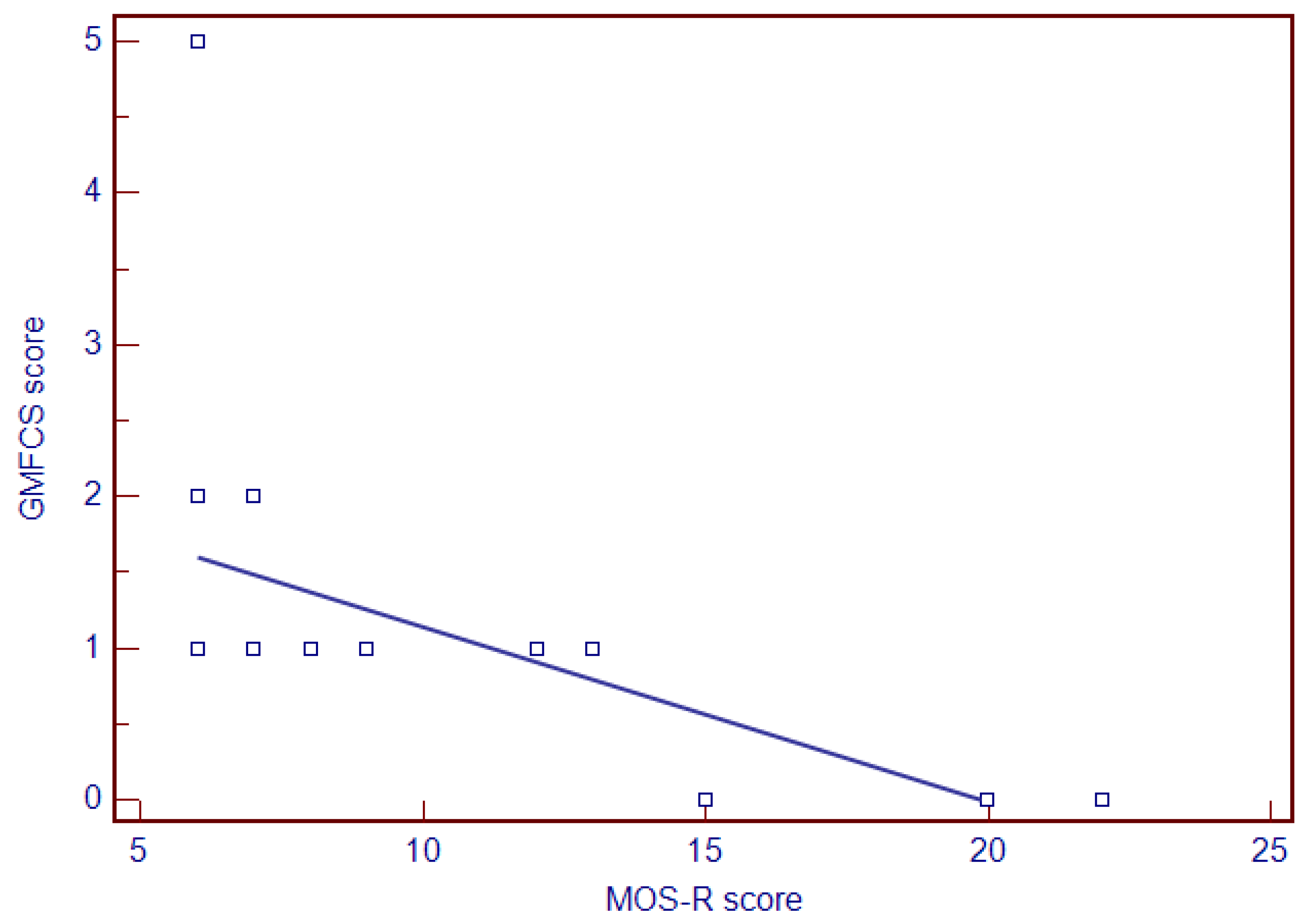
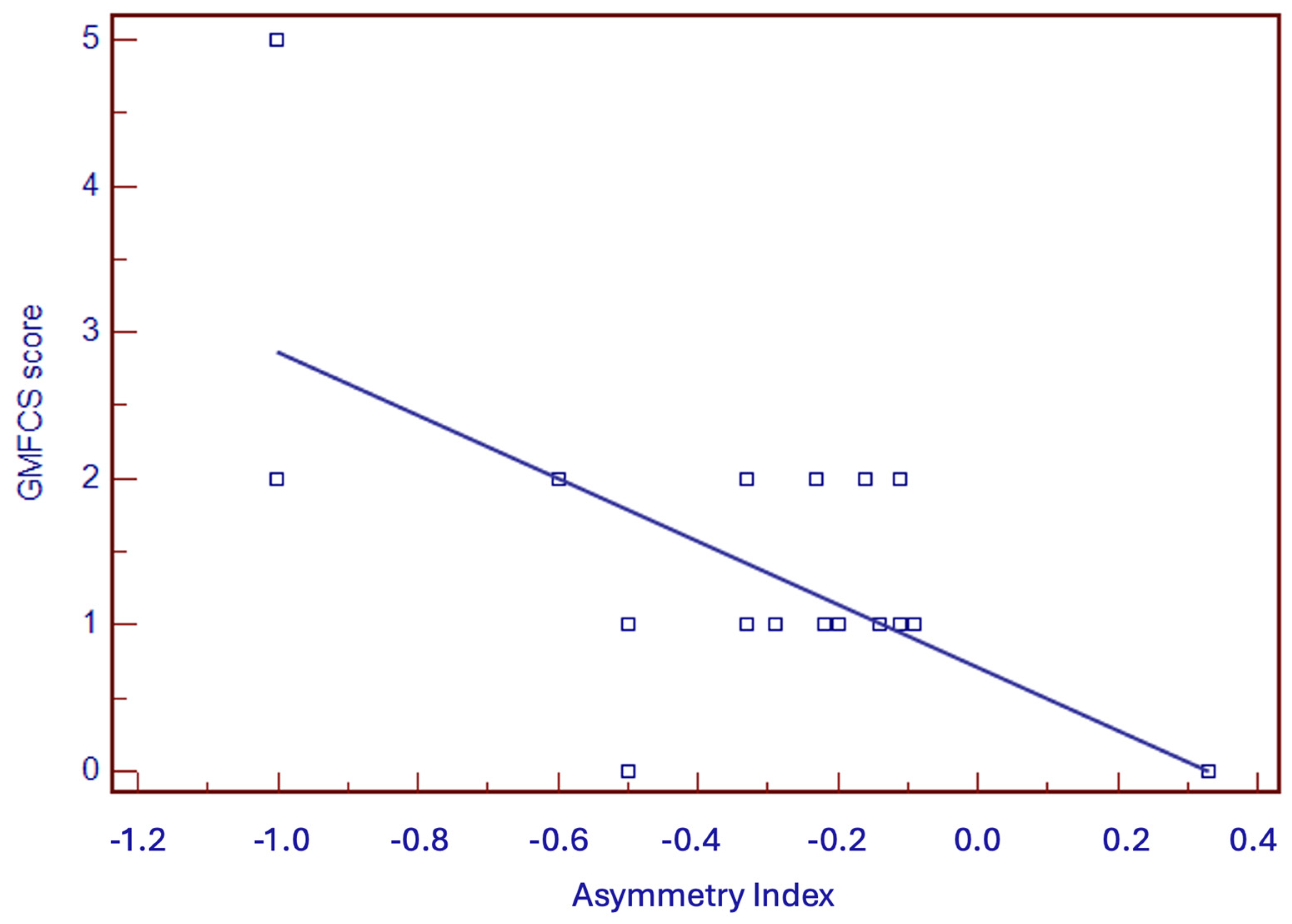
| Infants | Type of Lesion | Outcome | Fidgety Movements | MOS-R Score | Asymmetry Index | Griffiths DQ | GMFCS Score |
|---|---|---|---|---|---|---|---|
| 1 | Right PCA | Left UCP | Absent | 6 | −1 | 69 | 2 |
| 2 | Right PCA | Left UCP | Sporadic | 13 | −0.29 | 94 | 1 |
| 3 | Left MCA (post) | Right UCP | Absent | 9 | −0.33 | 97 | 1 |
| 4 | Left MCA (ant) | Right UCP | Absent | 6 | −0.33 | 115 | 2 |
| 5 | Right MCA (ant) | Left UCP | Absent | 6 | −0.23 | 103 | 2 |
| 6 | Left MCA + ACA | Right UCP | Absent | 6 | −0.11 | 49 | 2 |
| 7 | Right MCA_(ant) | Normal | Normal | 15 | −0.5 | 106 | 0 |
| 8 | Left MCA (ant) | Right UCP | Absent | 7 | −0.2 | 115 | 1 |
| 9 | Left MCA | Right UCP | Absent | 6 | −1 | 48 | 5 |
| 10 | Right MCA (ant) | Left UCP | Absent | 6 | −0.33 | 103 | 1 |
| 11 | Left MCA (post) | Right UCP | Absent | 6 | −0.22 | 105 | 1 |
| 12 | Right MCA (ant) | Left UCP | Absent | 8 | −0.2 | 94 | 1 |
| 13 | Left MCA (ant) | Right UCP | Sporadic | 6 | −0.6 | 105 | 2 |
| 14 | Right MCA (post) | Left UCP | Absent | 6 | −0.5 | 89 | 1 |
| 15 | Left MCA | Right UCP | Absent | 7 | −0.16 | 110 | 2 |
| 16 | Left MCA | Normal | Normal | 22 | 0.33 | 104 | 0 |
| 17 | Right MCA/PCA | Left UCP | Absent | 12 | −0.09 | 105 | 1 |
| 18 | Left MCA (post) | Right UCP | Absent | 6 | −0.2 | 112 | 1 |
| 19 | Left MCA | Right UCP | Absent | 12 | −0.29 | 80 | 1 |
| 20 | Right PCA | Normal | Normal | 20 | 0.33 | 112 | 0 |
| 21 | Right PCA | Left UCP | Absent | 6 | −0.14 | 100 | 1 |
| 22 | Right MCA + ACA | Left UCP | Sporadic | 6 | −0.14 | 93 | 1 |
| 23 | Left PCA | Right UCP | Absent | 6 | −0.11 | 88 | 1 |
| 24 | Right PCA | Left UCP | Sporadic | 6 | −0.14 | 105 | 1 |
| Movement Patterns | Cases | Controls | Total | p-Value | ||
|---|---|---|---|---|---|---|
| N (%) | A (%) | N (%) | A (%) | 22 | ||
| Swipes | 8/9 (89) | 1/9 (11) | 13/13 (100) | 0 | 26 | 0.8499 |
| Wiggling–Oscillating | 5/7 (71.4) | 2/7 (28.6) | 19/19 (100) | 0 | 22 | 0.1106 |
| Kicking | 2.6 (33.3) | 4/6 (66.7) | 15/16 (93.8) | 1/16 (6.2) | 8 | 0.0147 |
| Excitement Bursts | 0 | 1/1 (100) | 77 (100) | 0 | 20 | 0.2254 |
| Smiles | 5/8 (62.5) | 3/8 (37.5) | 12/12 (100) | 0 | 39 | 0.0966 |
| Mouth Movements | 4/17 (23.5) | 13/13 (76.5) | 22/22 (100) | 0 | 12 | <0.0001 |
| Tongue Movements | 0 | 11/11 (100) | 1/1 (100) | 5 | 0.0094 | |
| Side-to-side Movement of the Head | 0 | 5/5 (100) | 0 | 0 | NA | NA |
| Hand-to-Mouth Contact | 3/3 (100) | 0 | 1/1 (100) | 0 | 4 | 0.6171 |
| Hand-to hand Contact | 3/3 (100) | 0 | 5/5 (100) | 0 | 8 | 0.7237 |
| Fiddling | 5/7 (71.4) | 2/7 (28.6) | 16/16 (100) | 0 | 23 | 0.1517 |
| Reaching | 0 | 0 | 0 | 0 | 0 | NA |
| Foot-to-Foot Contact | 7/14 (50) | 7/14 (50) | 5/6 (83.3) | 1/6 (16.7) | 20 | 0.37 |
| Legs lift | 0 | 0 | 1/1 (100) | 0 | 1 | NA |
| Hand-to-toe Contact | 0 | 0 | 1/1 (100) | 1 | NA | |
| Segmental Movement of Wrists | 22/22 (100) | 0 | 22 | NA | ||
| Arching | 2/3 (66.7) | 1/3 (33.3) | 3/3 (100) | 0 | 6 | 1 |
| Rolling to side | 0 | 2/2 (100) | 2/2 (100) | 0 | 4 | 0.3173 |
| Visual Exploration | 7/7 (100) | 0 | 17/17 (100) | 0 | 24 | 0.0662 |
| Hand Regard | 0 | 5/5 (100) | 5 | NA | ||
| Head Anteflexion | 0 | 0 | 0 | 0 | 0 | NA |
| Circular Arm Movements | 0 | 0 | 0 | NA | ||
| Almost no Leg Movements | 0 | 0 | 0 | NA | ||
| Postural Patterns | Cases | Controls | Total | p-Value | ||
|---|---|---|---|---|---|---|
| N (%) | A (%) | N (%) | A (%) | |||
| Head-Centered | 6/24 (25) | 18/24 (75) | 20/24 (83.3) | 4/24 (16.7) | 48 | 0.0002 |
| Body Symmetry | 4/24 (16.7) | 18/24 (83.3) | 3/24 (12.5) | 21/24 (87.5) | 48 | 1 |
| Asymmetric Tonic Neck (ATN) Posture | 19/24 (79.2) | 5/24 (20.8) | 24/24 (100) | 48 | 0.0588 | |
| Flat Posture | 1 (100) | 0 | 1 | NA | ||
| Variability of Finger Posture | 7/24 (29.2) | 17/24 (70.8) | 21/23 (91.3) | 2/23 (8.7) | 47 | 0.0001 |
| Predominant Fisting | 6/6 (100) | 2/2 (100) | 8 | 0.2888 | ||
| Synchronized Opening and Closing of Fingers | 0 | 0 | 0 | NA | ||
| Finger Spreading | 6/6 (100) | 2/2 (100) | 8 | 0.2888 | ||
| Asymmetry of Finger Posture | 10/10 (100) | 3/3 (100) | 13 | 0.961 | ||
| Hyperextension of Neck | 0 | 0 | 0 | NA | ||
| Hyperextension of Trunk | 0 | 0 | 0 | NA | ||
| Extended Arms | 2/2 (100) | 0 | 2 | NA | ||
| Extended Legs | 0 | 0 | 0 | NA | ||
| Movement Character | Cases | Controls | Total | p-Value | ||
|---|---|---|---|---|---|---|
| Yes (%) | No (%) | Yes (%) | No (%) | |||
| Smooth and Fluent | 0 | 24/24 (100) | 22/24 (91.7) | 2/24 (8.3) | 48 | <0.0001 |
| Monotonous | 22/24 (91.7) | 2/24 (8.3) | 1/24 (4.2) | 23/24 (95.8) | 48 | <0.0001 |
| Jerky | 12/24 (50) | 12/24 (50) | 1/24 (4.2) | 23/24 (95.8) | 48 | 0.0012 |
| Stiff | 7/24 (29.2) | 17/24 (70.8) | 0 | 24/24 | 48 | 0.0141 |
| Predominantly Slow | 2/24 (8.3) | 22/24 (91.7) | 0 | 24/24 (100) (100) | 48 | 0.4701 |
| Predominantly Fast | 2/24 (8.3) | 22/24 (91.7) | 0 | 24/24 (100) | 48 | 0.4701 |
| Motor Optimality List | Cases | Controls | p-Value |
|---|---|---|---|
| Median (IC) | Median (IC) | ||
| Fidgety Movements | 1 (1–1) | 12 (12–12) | <0.0001 |
| Observed Movement Patterns | 1 (1–2) | 4 (4–4) | <0.0001 |
| Age-Adequate Movement Repertoire | 1 (1–1) | 2 (2–4) | 0.0005 |
| Observed Postural Pattern | 1 (1–1) | 4 (2–4) | 0.0001 |
| Movement Character | 2 (2–2) | 4 (4–4) | <0.0001 |
| MOS-R Score | 6 (6–9) | 26 (25–26) | <0.0001 |
| Sensitivity (%) (CI 95%) | Specificity (%) (CI 95%) | PPV (%) | NPV (%) | ROC (CI 95%) | |
|---|---|---|---|---|---|
| MOS-R (<13) | 100 (83.7–100) | 100 (30.5–100) | 100 | 100 | 1 (0.86–1) |
| AI (<−0.09) | 100 (83.7–100) | 66.7 (11.6–94.5) | 95.5 | 100 | 0.72 (0.50–0.88) |
| Arterial branch (MCA) | 71.4 (41.9–91.4) | 33.3 (55.5–88.4) | 83.3 | 20 | 0.52 (0.27–0.76) |
| Fidgety Movements | 100 (83.7–100) | 100 (30.5–100) | 100 | 100 | 1 (0.86–1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoncelli, N.; Corso, L.; Bedetti, L.; Della Casa, E.M.; Roversi, M.F.; Toni, G.; Pugliese, M.; Guidotti, I.; Miselli, F.; Lucaccioni, L.; et al. The Motor Optimality Score—Revised Improves Early Detection of Unilateral Cerebral Palsy in Infants with Perinatal Cerebral Stroke. Children 2024, 11, 940. https://doi.org/10.3390/children11080940
Bertoncelli N, Corso L, Bedetti L, Della Casa EM, Roversi MF, Toni G, Pugliese M, Guidotti I, Miselli F, Lucaccioni L, et al. The Motor Optimality Score—Revised Improves Early Detection of Unilateral Cerebral Palsy in Infants with Perinatal Cerebral Stroke. Children. 2024; 11(8):940. https://doi.org/10.3390/children11080940
Chicago/Turabian StyleBertoncelli, Natascia, Lucia Corso, Luca Bedetti, Elisa Muttini Della Casa, Maria Federica Roversi, Greta Toni, Marisa Pugliese, Isotta Guidotti, Francesca Miselli, Laura Lucaccioni, and et al. 2024. "The Motor Optimality Score—Revised Improves Early Detection of Unilateral Cerebral Palsy in Infants with Perinatal Cerebral Stroke" Children 11, no. 8: 940. https://doi.org/10.3390/children11080940
APA StyleBertoncelli, N., Corso, L., Bedetti, L., Della Casa, E. M., Roversi, M. F., Toni, G., Pugliese, M., Guidotti, I., Miselli, F., Lucaccioni, L., Rossi, C., Berardi, A., & Lugli, L. (2024). The Motor Optimality Score—Revised Improves Early Detection of Unilateral Cerebral Palsy in Infants with Perinatal Cerebral Stroke. Children, 11(8), 940. https://doi.org/10.3390/children11080940






