Isolated Fetal Ventriculomegaly: Diagnosis and Treatment in the Prenatal Period
Abstract
:1. Introduction
2. Diagnostic Pathway for Fetal IVM
2.1. Key Findings of the Width of the Lateral Ventricles of the Fetal Brain
2.2. Ultrasound Neuroscan for Fetal VM
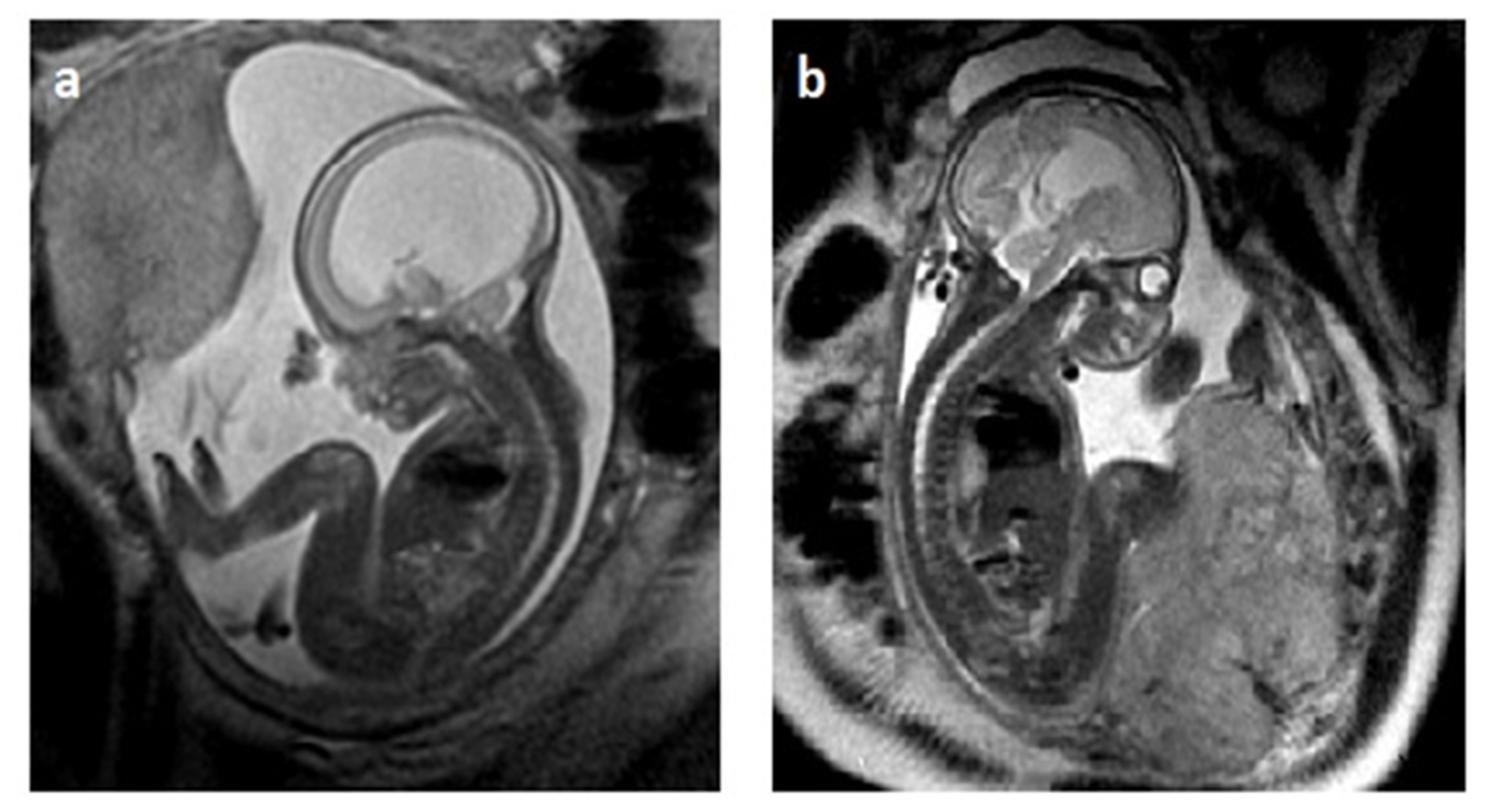
2.3. MRI in Fetal VM Diagnosis
2.4. Compatibility of Neurosonography and MRI
3. Methods of Diagnosing Genetic Disorders in VM
4. Infectious Factors in the Development of Fetal VM
5. Rationale behind Prenatal Ventriculo-Amniotic Shunting in ISVM
6. CNS Damage Caused by Fetal VM
7. Evaluation of Biomaterials and SVM Decompression Techniques during the ‘Post-Moratorium Era’
7.1. Serial Cephalocentesis and VAS
| Author | Perinatal Center | Year | Total Number of Patients Undergoing Surgery | Cephalocentesis | Percutaneous VAS/USG | OFS | EVT |
|---|---|---|---|---|---|---|---|
| Cavalheiro [92] | Sao Paulo, Brasil | 2003 | 39 | 20 | 19 | - | - |
| Bruner [95] | Nashville, TN, US | 2006 | 4 | - | - | 4 | - |
| Al-Anazi [96] | Al-Kobar, Saudi A. | 2007 | 1 | - | - | 1 | - |
| Al-Anazi [97] | Al-Kobar, Saudi A. | 2010 | 5 | - | - | 5 | - |
| Cavalheiro [9] | Sao Paulo, Brasil | 2011 | 57 | 26 | 30 | - | 1/3 attempts |
| Szaflik [89] | Łódź, Poland | 2014 | 222 | - | 222 | - | - |
| Litwińska [94] | Łódź, Poland | 2019 | 44 | - | 44 | - | - |
| Peralta [98] | Sao Paulo, Brasil | 2023 | 10 | - | - | - | 10 |
| Zamłyński [99] | Bytom, Poland | 2024 | 1 | - | - | 1 | - |
7.2. Endoscopic Third Ventriculostomy ETV
7.3. Applications of Open Fetal Surgery Techniques for SVM Decompression
7.4. FSC Criteria for VA Shunting
7.5. Model Candidates for Prenatal VA Shunting
7.6. Optimal Timing of Prenatal ISVM Shunting
7.7. Benefits and Limitations of ISVM Shunting Techniques
8. Conclusions
8.1. Current State of Knowledge about Fetal IFVM
8.2. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emery, S.P.; Narayanan, S.; Greene, S. Fetal Aqueductal Stenosis: Prenatal Diagnosis and Intervention. Prenat. Diagn. 2020, 40, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Hochstetler, A.; Raskin, J.; Blazer-Yost, B.L. Hydrocephalus: Historical Analysis and Considerations for Treatment. Eur. J. Med. Res. 2022, 27, 168. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.T.; Kulkarni, A.V.; Limbrick, D.D., Jr.; Warf, B.C. Hydrocephalus in Children. Lancet 2016, 387, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Hannon, T.; Tennant, P.W.; Rankin, J.; Robson, S.C. Epidemiology, Natural History, Progression, and Postnatal Outcome of Severe Fetal Ventriculomegaly. Obstet. Gynecol. 2012, 120, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Society for Maternal-Fetal Medicine; Fox, N.S.; Monteagudo, A.; Kuller, J.A.; Craigo, S.; Norton, M.E. Mild Fetal Ventriculomegaly: Diagnosis, Evaluation, and Management. Am. J. Obstet. Gynecol. 2018, 219, B2–B9. [Google Scholar] [CrossRef] [PubMed]
- Goynumer, G.; Yayla, M.; Arisoy, R.; Turkmen, O. The Criterion Value of Fetal Cerebral Lateral Ventricular Atrium Width for Diagnosis of Ventriculomegaly. Clin. Exp. Obstet. Gynecol. 2014, 41, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D.; Reeves, M.J.; Morris, J.E.; Mason, G.; Russell, S.A.; Paley, M.N.; Whitby, E.H. A Prospective Study of Fetuses with Isolated Ventriculomegaly Investigated by Antenatal Sonography and in Utero Mr Imaging. AJNR Am. J. Neuroradiol. 2010, 31, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Wyldes, M.; Watkinson, M. Isolated Mild Fetal Ventriculomegaly. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F9–F13. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, S.; Moron, A.F.; Almodin, C.G.; Suriano, I.C.; Hisaba, V.; Dastoli, P.; Barbosa, M.M. Fetal Hydrocephalus. Childs Nerv. Syst. 2011, 27, 1575–1583. [Google Scholar] [CrossRef]
- Letouzey, M.; Chadie, A.; Brasseur-Daudruy, M.; Proust, F.; Verspyck, E.; Boileau, P.; Marret, S. Severe Apparently Isolated Fetal Ventriculomegaly and Neurodevelopmental Outcome. Prenat. Diagn. 2017, 37, 820–826. [Google Scholar] [CrossRef]
- Peiro, J.L.; Fabbro, M.D. Fetal Therapy for Congenital Hydrocephalus-Where We Came from and Where We Are Going. Childs Nerv. Syst. 2020, 36, 1697–1712. [Google Scholar] [CrossRef] [PubMed]
- Society for Maternal-Fetal Medicine; Norton, M.E.; Fox, N.S.; Monteagudo, A.; Kuller, J.A.; Craigo, S. Fetal Ventriculomegaly. Am. J. Obstet. Gynecol. 2020, 223, B30–B33. [Google Scholar]
- Paladini, D.; Malinger, G.; Birnbaum, R.; Monteagudo, A.; Pilu, G.; Salomon, L.J.; Timor-Tritsch, I.E. Isuog Practice Guidelines (Updated): Sonographic Examination of the Fetal Central Nervous System. Part 2: Performance of Targeted Neurosonography. Ultrasound Obstet. Gynecol. 2021, 57, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, J.M.; Sinha, S.; Zarnow, D.M.; Johnson, M.P.; Heuer, G.G. Fetal Ventriculomegaly: Diagnosis, Treatment, and Future Directions. Childs Nerv. Syst. 2017, 33, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Scelsa, B.; Rustico, M.; Righini, A.; Parazzini, C.; Balestriero, M.A.; Introvini, P.; Spaccini, L.; Mastrangelo, M.; Lista, G.; Zuccotti, G.V.; et al. Mild Ventriculomegaly from Fetal Consultation to Neurodevelopmental Assessment: A Single Center Experience and Review of the Literature. Eur. J. Paediatr. Neurol. 2018, 22, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Sonographic Examination of the Fetal Central Nervous System: Guidelines for Performing the ‘Basic Examination’ and the ‘Fetal Neurosonogram’. Ultrasound Obstet. Gynecol. 2007, 29, 109–116. [CrossRef] [PubMed]
- Emery, S.P.; Greene, S.; Hogge, W.A. Fetal Therapy for Isolated Aqueductal Stenosis. Fetal Diagn. Ther. 2015, 38, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Enso Working Group. Role of Prenatal Magnetic Resonance Imaging in Fetuses with Isolated Mild or Moderate Ventriculomegaly in the Era of Neurosonography: International Multicenter Study. Ultrasound Obstet. Gynecol. 2020, 56, 340–347. [Google Scholar] [CrossRef]
- Birnbaum, R.; Parodi, S.; Donarini, G.; Meccariello, G.; Fulcheri, E.; Paladini, D. The Third Ventricle of the Human Fetal Brain: Normative Data and Pathologic Correlation. A 3d Transvaginal Neurosonography Study. Prenat. Diagn. 2018, 38, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, D.A.; Richter, B.; Andrews, E.; Xu, C.; Emery, S.P.; Greene, S. Clinical Outcomes of Isolated Congenital Aqueductal Stenosis. World Neurosurg. 2018, 114, e976–e981. [Google Scholar] [CrossRef]
- Melchiorre, K.; Bhide, A.; Gika, A.D.; Pilu, G.; Papageorghiou, A.T. Counseling in Isolated Mild Fetal Ventriculomegaly. Ultrasound Obstet. Gynecol. 2009, 34, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arriaga, P.I.; Nunez, N.; Zamora, B.; Villalain, C.; Risco, B.; Liebana, C.; Herraiz, I.; Galindo, A. Natural History and Mid-Term Neurodevelopmental Outcome of Fetuses with Isolated Mild Ventriculomegaly Diagnosed in the Second Half of Pregnancy. J. Matern. Fetal Neonatal Med. 2023, 36, 2214836. [Google Scholar] [CrossRef]
- Powers, A.M.; White, C.; Neuberger, I.; Maloney, J.A.; Stence, N.V.; Mirsky, D. Fetal Mri Neuroradiology: Indications. Clin. Perinatol. 2022, 49, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Masselli, G.; Notte, M.R.V.; Zacharzewska-Gondek, A.; Laghi, F.; Manganaro, L.; Brunelli, R. Fetal Mri of Cns Abnormalities. Clin. Radiol. 2020, 75, 640.e1–640.e11. [Google Scholar] [CrossRef]
- Prayer, D.; Malinger, G.; De Catte, L.; De Keersmaecker, B.; Goncalves, L.F.; Kasprian, G.; Laifer-Narin, S.; Lee, W.; Millischer, A.E.; Platt, L.; et al. Isuog Practice Guidelines (Updated): Performance of Fetal Magnetic Resonance Imaging. Ultrasound Obstet. Gynecol. 2023, 61, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, U.D.; Kline-Fath, B.M. Clinical Applications of Fetal Mri in the Brain. Diagnostics 2022, 12, 764. [Google Scholar] [CrossRef]
- Epstein, K.N.; Kline-Fath, B.M.; Zhang, B.; Venkatesan, C.; Habli, M.; Dowd, D.; Nagaraj, U.D. Prenatal Evaluation of Intracranial Hemorrhage on Fetal Mri: A Retrospective Review. AJNR Am. J. Neuroradiol. 2021, 42, 2222–2228. [Google Scholar] [CrossRef]
- Van Doorn, M.; Rengerink, K.O.; Newsum, E.A.; Reneman, L.; Majoie, C.B.; Pajkrt, E. Added Value of Fetal Mri in Fetuses with Suspected Brain Abnormalities on Neurosonography: A Systematic Review and Meta-Analysis. J. Matern. Fetal Neonatal Med. 2016, 29, 2949–2961. [Google Scholar] [CrossRef]
- Mufti, N.; Sacco, A.; Aertsen, M.; Ushakov, F.; Ourselin, S.; Thomson, D.; Deprest, J.; Melbourne, A.; David, A.L. What Brain Abnormalities Can Magnetic Resonance Imaging Detect in Foetal and Early Neonatal Spina Bifida: A Systematic Review. Neuroradiology 2022, 64, 233–245. [Google Scholar] [CrossRef]
- Heaphy-Henault, K.J.; Guimaraes, C.V.; Mehollin-Ray, A.R.; Cassady, C.I.; Zhang, W.; Desai, N.K.; Paldino, M.J. Congenital Aqueductal Stenosis: Findings at Fetal Mri That Accurately Predict a Postnatal Diagnosis. AJNR Am. J. Neuroradiol. 2018, 39, 942–948. [Google Scholar] [CrossRef]
- Brady, D.; Schlatterer, S.D.; Whitehead, M.T. Fetal Brain Mri: Neurometrics, Typical Diagnoses, and Resolving Common Dilemmas. Br. J. Radiol. 2023, 96, 20211019. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Pilu, G.; Rizzo, G.; Caulo, M.; Liberati, M.; Giancotti, A.; Lees, C.; Volpe, P.; Buca, D.; et al. Role of Prenatal Magnetic Resonance Imaging in Fetuses with Isolated Severe Ventriculomegaly at Neurosonography: A Multicenter Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Van der Knoop, B.J.; Zonnenberg, I.A.; Verbeke, J.; de Vries, L.S.; Pistorius, L.R.; van Weissenbruch, M.M.; Vermeulen, R.J.; de Vries, J.I.P. Additional Value of Advanced Neurosonography and Magnetic Resonance Imaging in Fetuses at Risk for Brain Damage. Ultrasound Obstet. Gynecol. 2020, 56, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D.; Bradburn, M.; Mandefield, L.; Mooney, C.; Jarvis, D. The Rate of Brain Abnormalities on in Utero Mri Studies in Fetuses with Normal Ultrasound Examinations of the Brain and Calculation of Indicators of Diagnostic Performance. Clin. Radiol. 2019, 74, 527–533. [Google Scholar] [CrossRef]
- Scelsa, B. Fetal Neurology: From Prenatal Counseling to Postnatal Follow-Up. Diagnostics 2022, 12, 3083. [Google Scholar] [CrossRef] [PubMed]
- Hadjidekov, G.; Haynatzki, G.; Chaveeva, P.; Nikolov, M.; Masselli, G.; Rossi, A. Concordance between Us and Mri Two-Dimensional Measurement and Volumetric Segmentation in Fetal Ventriculomegaly. Diagnostics 2023, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Sileo, F.G.; Di Mascio, D.; Rizzo, G.; Caulo, M.; Manganaro, L.; Bertucci, E.; Masmejan, S.; Liberati, M.; D’Amico, A.; Nappi, L.; et al. Role of Prenatal Magnetic Resonance Imaging in Fetuses with Isolated Agenesis of Corpus Callosum in the Era of Fetal Neurosonography: A Systematic Review and Meta-Analysis. Acta Obstet. Gynecol. Scand. 2021, 100, 7–16. [Google Scholar] [CrossRef]
- D’Addario, V. Diagnostic Approach to Fetal Ventriculomegaly. J. Perinat. Med. 2023, 51, 111–116. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Brackley, K.; Bradburn, M.; Connolly, D.J.A.; Gawne-Cain, M.L.; Griffiths, D.I.; Kilby, M.D.; Mandefield, L.; Mooney, C.; Robson, S.C.; et al. Anatomical Subgroup Analysis of the Meridian Cohort: Ventriculomegaly. Ultrasound Obstet. Gynecol. 2017, 50, 736–744. [Google Scholar] [CrossRef]
- Lok, W.Y.; Kong, C.W.; Hui, S.Y.A.; Shi, M.M.; Choy, K.W.; To, W.K.; Leung, T.Y. Chromosomal Abnormalities and Neurological Outcomes in Fetal Cerebral Ventriculomegaly: A Retrospective Cohort Analysis. Hong Kong Med. J. 2021, 27, 428–436. [Google Scholar] [CrossRef]
- Giorgione, V.; Haratz, K.K.; Constantini, S.; Birnbaum, R.; Malinger, G. Fetal Cerebral Ventriculomegaly: What Do We Tell the Prospective Parents? Prenat. Diagn. 2022, 42, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Pauta, M.; Martinez-Portilla, R.J.; Borrell, A. Diagnostic Yield of Exome Sequencing in Fetuses with Multisystem Malformations: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2022, 59, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Mellis, R.; Oprych, K.; Scotchman, E.; Hill, M.; Chitty, L.S. Diagnostic Yield of Exome Sequencing for Prenatal Diagnosis of Fetal Structural Anomalies: A Systematic Review and Meta-Analysis. Prenat. Diagn. 2022, 42, 662–685. [Google Scholar] [CrossRef]
- Santirocco, M.; Plaja, A.; Rodo, C.; Valenzuela, I.; Arevalo, S.; Castells, N.; Abuli, A.; Tizzano, E.; Maiz, N.; Carreras, E. Chromosomal Microarray Analysis in Fetuses with Central Nervous System Anomalies: An 8-Year Long Observational Study from a Tertiary Care University Hospital. Prenat. Diagn. 2021, 41, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Toren, A.; Alpern, S.; Berkenstadt, M.; Bar-Yosef, O.; Pras, E.; Katorza, E. Chromosomal Microarray Evaluation of Fetal Ventriculomegaly. Isr. Med. Assoc. J. 2020, 22, 639–644. [Google Scholar] [PubMed]
- Van den Veyver, I.B.; Chandler, N.; Wilkins-Haug, L.E.; Wapner, R.J.; Chitty, L.S.; Ispd Board of Directors. International Society for Prenatal Diagnosis Updated Position Statement on the Use of Genome-Wide Sequencing for Prenatal Diagnosis. Prenat. Diagn. 2022, 42, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Li, R.; Yu, Q.; Wang, D.; Deng, Q.; Li, L.; Lei, T.; Chen, G.; Nie, Z.; Yang, X.; et al. Application of Exome Sequencing for Prenatal Diagnosis of Fetal Structural Anomalies: Clinical Experience and Lessons Learned from a Cohort of 1618 Fetuses. Genome Med. 2022, 14, 123. [Google Scholar] [CrossRef]
- Petrovski, S.; Aggarwal, V.; Giordano, J.L.; Stosic, M.; Wou, K.; Bier, L.; Spiegel, E.; Brennan, K.; Stong, N.; Jobanputra, V.; et al. Whole-Exome Sequencing in the Evaluation of Fetal Structural Anomalies: A Prospective Cohort Study. Lancet 2019, 393, 758–767. [Google Scholar] [CrossRef]
- Lord, J.; McMullan, D.J.; Eberhardt, R.Y.; Rinck, G.; Hamilton, S.J.; Quinlan-Jones, E.; Prigmore, E.; Keelagher, R.; Best, S.K.; Carey, G.K.; et al. Prenatal Exome Sequencing Analysis in Fetal Structural Anomalies Detected by Ultrasonography (Page): A Cohort Study. Lancet 2019, 393, 747–757. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Kurolap, A.; Ilivitzki, A.; Mory, A.; Paperna, T.; Genetics, C.R.; Kedar, R.; Gonzaga-Jauregui, C.; Peleg, A.; Feldman, H.B. A Novel Heterozygous Loss-of-Function Dcc Netrin 1 Receptor Variant in Prenatal Agenesis of Corpus Callosum and Review of the Literature. Am. J. Med. Genet. A 2020, 182, 205–212. [Google Scholar] [CrossRef]
- Yaron, Y.; Glassner, V.O.; Mory, A.; Henig, N.Z.; Kurolap, A.; Shira, A.B.; Goldstein, D.B.; Marom, D.; Sira, L.B.; Feldman, H.B.; et al. Exome Sequencing as First-Tier Test for Fetuses with Severe Central Nervous System Structural Anomalies. Ultrasound Obstet. Gynecol. 2022, 60, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ying, J.; Jing, J. Hydrocephalus Caused by L1cam Gene Mutation in the Newborn. Asian J. Surg. 2024, 47, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Kundishora, A.J.; Singh, A.K.; Allington, G.; Duy, P.Q.; Ryou, J.; Alper, S.L.; Jin, S.C.; Kahle, K.T. Genomics of Human Congenital Hydrocephalus. Childs Nerv. Syst. 2021, 37, 3325–3340. [Google Scholar] [CrossRef] [PubMed]
- Bramall, A.N.; Anton, E.S.; Kahle, K.T.; Fecci, P.E. Navigating the Ventricles: Novel Insights into the Pathogenesis of Hydrocephalus. eBioMedicine 2022, 78, 103931. [Google Scholar] [CrossRef] [PubMed]
- Devaseelan, P.; Cardwell, C.; Bell, B.; Ong, S. Prognosis of Isolated Mild to Moderate Fetal Cerebral Ventriculomegaly: A Systematic Review. J. Perinat. Med. 2010, 38, 401–409. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.S. Viral Infections and the Neonatal Brain. Semin. Pediatr. Neurol. 2019, 32, 100769. [Google Scholar] [CrossRef] [PubMed]
- Fa, F.; Laup, L.; Mandelbrot, L.; Sibiude, J.; Picone, O. Fetal and Neonatal Abnormalities Due to Congenital Herpes Simplex Virus Infection: A Literature Review. Prenat. Diagn. 2020, 40, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.; Holmes, N.E.; Hui, L. A Systematic Review of Maternal Torch Serology as a Screen for Suspected Fetal Infection. Prenat. Diagn. 2022, 42, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.V.D.; Alexiou, G.; Laube, K.A.C.; Manfroi, G.; Rehder, R. Novel Concepts in the Pathogenesis of Hydrocephalus. Childs Nerv. Syst. 2023, 39, 1245–1252. [Google Scholar] [CrossRef]
- Duru, S.; Peiro, J.L.; Oria, M.; Aydin, E.; Subasi, C.; Tuncer, C.; Rekate, H.L. Successful Endoscopic Third Ventriculostomy in Children Depends on Age and Etiology of Hydrocephalus: Outcome Analysis in 51 Pediatric Patients. Childs Nerv. Syst. 2018, 34, 1521–1528. [Google Scholar] [CrossRef]
- McAllister, J.P., 2nd; Chovan, P. Neonatal Hydrocephalus. Mechanisms and Consequences. Neurosurg. Clin. N. Am. 1998, 9, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Glick, P.L.; Harrison, M.R.; Halks-Miller, M.; Adzick, N.S.; Nakayama, D.K.; Anderson, J.H.; Nyland, T.G.; Villa, R.; Edwards, M.S. Correction of Congenital Hydrocephalus in Utero Ii: Efficacy of in Utero Shunting. J. Pediatr. Surg. 1984, 19, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, N.; Sallout, B.; Albaqawi, B.; Al AlAali, W. The Impact of Fetal Middle Cerebral Artery Doppler on the Outcome of Congenital Hydrocephalus. J. Matern. Fetal Neonatal Med. 2018, 31, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Appelgren, T.; Zetterstrand, S.; Elfversson, J.; Nilsson, D. Long-Term Outcome after Treatment of Hydrocephalus in Children. Pediatr. Neurosurg. 2010, 46, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, M.; Parodi, S.; Ballarini, M.; Paladini, D. Comparison of Fetal and Neonatal Sonographic Measurements of Ventricular Size in Second- and Third-Trimester Fetuses with or without Ventriculomegaly: Cross-Sectional Three-Dimensional Ultrasound Study. Ultrasound Obstet. Gynecol. 2022, 60, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Laskin, M.D.; Kingdom, J.; Toi, A.; Chitayat, D.; Ohlsson, A. Perinatal and Neurodevelopmental Outcome with Isolated Fetal Ventriculomegaly: A Systematic Review. J. Matern. Fetal Neonatal Med. 2005, 18, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Del Bigio, M.R.; Di Curzio, D.L. Nonsurgical Therapy for Hydrocephalus: A Comprehensive and Critical Review. Fluids Barriers CNS 2016, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Von Koch, C.S.; Gupta, N.; Sutton, L.N.; Sun, P.P. In Utero Surgery for Hydrocephalus. Childs Nerv. Syst. 2003, 19, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Rault, E.; Lacalm, A.; Massoud, M.; Massardier, J.; Di Rocco, F.; Gaucherand, P.; Guibaud, L. The Many Faces of Prenatal Imaging Diagnosis of Primitive Aqueduct Obstruction. Eur. J. Paediatr. Neurol. 2018, 22, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Bar-Yosef, O.; Jacobson, J.M.; Gilboa, Y.; Derazne, E.; Achiron, R.; Katorza, E. Natural History of Fetal Isolated Ventriculomegaly: Comparison between Pre- and Post-Natal Imaging. J. Matern. Fetal Neonatal Med. 2018, 31, 1762–1767. [Google Scholar] [CrossRef]
- Kline-Fath, B.M.; Arroyo, M.S.; Calvo-Garcia, M.A.; Horn, P.S.; Thomas, C. Congenital Aqueduct Stenosis: Progressive Brain Findings in Utero to Birth in the Presence of Severe Hydrocephalus. Prenat. Diagn. 2018, 38, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.J.; Polan, R.M.; Baranano, K.W.; Burd, I.; Baschat, A.A.; Blakemore, K.J.; Ahn, E.S.; Jelin, E.B.; Jelin, A.C. Acceleration and Plateau: Two Patterns and Outcomes of Isolated Severe Fetal Cerebral Ventricular Dilation. J. Matern. Fetal Neonatal Med. 2021, 34, 3014–3020. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.S. The First Thousand Days Define a Fetal/Neonatal Neurology Program. Front. Pediatr. 2021, 9, 683138. [Google Scholar] [CrossRef]
- Agarwal, S.; Scher, M.S. Fetal-Neonatal Neurology Program Development: Continuum of Care During the First 1000 Days. J. Perinatol. 2022, 42, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Vasung, L.; Rollins, C.K.; Zhang, J.; Velasco-Annis, C.; Yang, E.; Lin, P.Y.; Sutin, J.; Warfield, S.K.; Soul, J.; Estroff, J.; et al. Abnormal Development of Transient Fetal Zones in Mild Isolated Fetal Ventriculomegaly. Cereb. Cortex 2023, 33, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Benkarim, O.M.; Hahner, N.; Piella, G.; Gratacos, E.; Ballester, M.A.G.; Eixarch, E.; Sanroma, G. Cortical Folding Alterations in Fetuses with Isolated Non-Severe Ventriculomegaly. Neuroimage Clin. 2018, 18, 103–114. [Google Scholar] [CrossRef]
- Hahner, N.; Benkarim, O.M.; Aertsen, M.; Perez-Cruz, M.; Piella, G.; Sanroma, G.; Bargallo, N.; Deprest, J.; Ballester, M.A.G.; Gratacos, E.; et al. Global and Regional Changes in Cortical Development Assessed by Mri in Fetuses with Isolated Nonsevere Ventriculomegaly Correlate with Neonatal Neurobehavior. AJNR Am. J. Neuroradiol. 2019, 40, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.J.; Palaniyappan, L.; Scerif, G.; Groom, M.J.; Liddle, E.B.; Liddle, P.F.; Hollis, C. Morphological Abnormalities in Prefrontal Surface Area and Thalamic Volume in Attention Deficit/Hyperactivity Disorder. Psychiatry Res. 2015, 233, 225–232. [Google Scholar] [CrossRef]
- Kyriakopoulou, V.; Davidson, A.; Chew, A.; Gupta, N.; Arichi, T.; Nosarti, C.; Rutherford, M.A. Characterisation of Asd Traits among a Cohort of Children with Isolated Fetal Ventriculomegaly. Nat. Commun. 2023, 14, 1550. [Google Scholar] [CrossRef]
- Evans, L.L.; Harrison, M.R. Modern Fetal Surgery-a Historical Review of the Happenings That Shaped Modern Fetal Surgery and Its Practices. Transl. Pediatr. 2021, 10, 1401–1417. [Google Scholar] [CrossRef]
- Birnholz, J.C.; Frigoletto, F.D. Antenatal Treatment of Hydrocephalus. New Engl. J. Med. 1981, 304, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.R. The University of California at San Francisco Fetal Treatment Center: A Personal Perspective. Fetal Diagn. Ther. 2004, 19, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.R.; Filly, R.A.; Golbus, M.S.; Berkowitz, R.L.; Callen, P.W.; Canty, T.G.; Catz, C.; Clewell, W.H.; Depp, R.; Edwards, M.S.; et al. Fetal Treatment 1982. New Engl. J. Med. 1982, 307, 1651–1652. [Google Scholar] [CrossRef] [PubMed]
- Clewell, W.H.; Johnson, M.L.; Meier, P.R.; Newkirk, J.B.; Zide, S.L.; Hendee, R.W.; Bowes, W.A., Jr.; Hecht, F.; O’Keeffe, D.; Henry, G.P.; et al. A Surgical Approach to the Treatment of Fetal Hydrocephalus. New Engl. J. Med. 1982, 306, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Deprest, J.A.; Flake, A.W.; Gratacos, E.; Ville, Y.; Hecher, K.; Nicolaides, K.; Johnson, M.P.; Luks, F.I.; Adzick, N.S.; Harrison, M.R. The Making of Fetal Surgery. Prenat. Diagn. 2010, 30, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Manning, F.A.; Harrison, M.R.; Rodeck, C. Catheter Shunts for Fetal Hydronephrosis and Hydrocephalus. Report of the International Fetal Surgery Registry. New Engl. J. Med. 1986, 315, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Peiro, J.L.; Duru, S.; Fernandez-Tome, B.; Peiro, L.; Encinas, J.L.; Sanchez-Margallo, F.M.; Oria, M. Fetal Endoscopic Third Ventriculostomy Is Technically Feasible in Prenatally Induced Hydrocephalus Ovine Model. Neurosurgery 2023, 92, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Winkler, S.M.; Harrison, M.R.; Messersmith, P.B. Biomaterials in Fetal Surgery. Biomater. Sci. 2019, 7, 3092–3109. [Google Scholar] [CrossRef]
- Szaflik, K.; Czaj, M.; Polis, L.; Wojtera, J.; Szmanski, W.; Krzeszowski, W.; Polis, B.; Litwinska, M.; Mikolajczyk, W.; Janiak, K.; et al. Fetal Therap—Evaluation of Ventriculo-Amniotic Shunts in the Treatment of Hydrocephalus. Ginekol. Pol. 2014, 85, 16–22. [Google Scholar] [CrossRef]
- Chen, Y.; Emery, S.P.; Maxey, A.P.; Gu, X.; Wagner, W.R.; Chun, Y. A Novel Low-Profile Ventriculoamniotic Shunt for Foetal Aqueductal Stenosis. J. Med. Eng. Technol. 2016, 40, 186–198. [Google Scholar] [CrossRef]
- Emery, S.P.; Greene, S.; Elsisy, M.; Chung, K.; Ye, S.H.; Kim, S.; Wagner, W.R.; Hazen, N.; Chun, Y. In Vitro and in Vivo Assessment of a Novel Ultra-Flexible Ventriculoamniotic Shunt for Treating Fetal Hydrocephalus. J. Biomater. Appl. 2023, 37, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, S.; Moron, A.F.; Zymberg, S.T.; Dastoli, P. Fetal Hydrocephalus—Prenatal Treatment. Childs Nerv. Syst. 2003, 19, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, S.; da Costa, M.D.S.; Mendonca, J.N.; Dastoli, P.A.; Suriano, I.C.; Barbosa, M.M.; Moron, A.F. Antenatal Management of Fetal Neurosurgical Diseases. Childs Nerv. Syst. 2017, 33, 1125–1141. [Google Scholar] [CrossRef] [PubMed]
- Litwinska, M.; Litwinska, E.; Czaj, M.; Polis, B.; Polis, L.; Szaflik, K. Ventriculo-Amniotic Shunting for Severe Fetal Ventriculomegaly. Acta Obstet. Gynecol. Scand. 2019, 98, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Bruner, J.P.; Davis, G.; Tulipan, N. Intrauterine Shunt for Obstructive Hydrocephalus--Still Not Ready. Fetal Diagn. Ther. 2006, 21, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.; Al-Mejhim, F.; Al-Qahtani, N. In Uteroventriculo-Amniotic Shunt for Hydrocephalus. Childs Nerv. Syst. 2008, 24, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.R. In Utero Ventriculo-Uterine Shunt Treatment for Fetal Hydrocephalus: Preliminary Study of Al-Anazi Ventriculo-Uterine Shunt. Neurosurg. Q. 2010, 20, 1–4. [Google Scholar] [CrossRef]
- Peralta, C.F.A.; Medrado, A.P.; Botelho, R.D.; da Costa, K.J.R.; Imada, V.; Lamis, F. Percutaneous Fetal Endoscopic Third Ventriculostomy for Severe Isolated Cerebral Ventriculomegaly. Prenat. Diagn. 2023, 43, 1614–1621. [Google Scholar] [CrossRef]
- Zamlynski, M.; Olejek, A.; Koszutski, T.; Bohosiewicz, J.; Mandera, M.; Zamlynski, J.; Maruniak-Chudek, I.; Herman-Sucharska, I.; Pastuszka, A. Open Fetal Surgery for Ventricular-Amniotic Valve Implantation in Aqueductal Stenosis-Dependent Severe Fetal Hydrocephalus—A Case Report with 7-Year Follow-Up. Fetal Diagn. Ther. 2024, 51, 278–284. [Google Scholar] [CrossRef]
- Michejda, M.; Queenan, J.T.; McCullough, D. Present Status of Intrauterine Treatment of Hydrocephalus and Its Future. Am. J. Obstet. Gynecol. 1986, 155, 873–882. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W., 3rd; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A Randomized Trial of Prenatal Versus Postnatal Repair of Myelomeningocele. New Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A.; Blackwell, S.B.; Chatterjee, D.; Cummings, J.J.; Emery, S.P.; Hirose, S.; Hollier, L.M.; Johnson, A.; Kilpatrick, S.J.; Luks, F.I.; et al. Care Levels for Fetal Therapy Centers. Obstet. Gynecol. 2022, 139, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, J.S.; Johnson, A.; Van Mieghem, T. International Society for Prenatal Diagnosis 2022 Debate: There Should Be Formal Accreditation and Ongoing Quality Assurance/Review for Units Offering Fetal Therapy That Includes Public Reporting of Outcomes. Prenat. Diagn. 2023, 43, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Who: Recommended Definitions, Terminology and Format for Statistical Tables Related to the Perinatal Period and Use of a New Certificate for Cause of Perinatal Deaths. Modifications Recommended by Figo as Amended October 14, 1976. Acta Obstet. Gynecol. Scand. 1977, 56, 247–253.
- Carta, S.; Agten, A.K.; Belcaro, C.; Bhide, A. Outcome of Fetuses with Prenatal Diagnosis of Isolated Severe Bilateral Ventriculomegaly: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2018, 52, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Michejda, M.; Hodgen, G.D. In Utero Diagnosis and Treatment of Non-Human Primate Fetal Skeletal Anomalies. I. Hydrocephalus. JAMA 1981, 246, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, D.K.; Harrison, M.R.; Berger, M.S.; Chinn, D.H.; Halks-Miller, M.; Edwards, M.S. Correction of Congenital Hydrocephalus in Utero I. The Model: Intracisternal Kaolin Produces Hydrocephalus in Fetal Lambs and Rhesus Monkeys. J. Pediatr. Surg. 1983, 18, 331–338. [Google Scholar] [CrossRef]
- Clark, R.G.; Milhorat, T.H. Experimental Hydrocephalus. 3. Light Microscopic Findings in Acute and Subacute Obstructive Hydrocephalus in the Monkey. J. Neurosurg. 1970, 32, 400–413. [Google Scholar] [CrossRef]
- Sutton, L.N.; Sun, P.; Adzick, N.S. Fetal Neurosurgery. Neurosurgery 2001, 48, 124–142; discussion 42–44. [Google Scholar]
- Donoho, D.A.; Syed, H.R. Fetal Neurosurgical Interventions for Spinal Malformations, Cerebral Malformations, and Hydrocephalus: Past, Present, and Future. Semin. Pediatr. Neurol. 2022, 42, 100964. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, S.; da Costa, M.D.S.; Barbosa, M.M.; Suriano, I.C.; Ottaiano, A.C.; de Andrade Lourencao Freddi, T.; Ferreira, N.; Kusano, C.U.; Dastoli, P.A.; Nicacio, J.M.; et al. Fetal Neurosurgery. Childs Nerv. Syst. 2023, 39, 2899–2927. [Google Scholar] [CrossRef] [PubMed]
- Minta, K.J.; Kannan, S.; Kaliaperumal, C. Outcomes of Endoscopic Third Ventriculostomy (Etv) and Ventriculoperitoneal Shunt (Vps) in the Treatment of Paediatric Hydrocephalus: Systematic Review and Meta-Analysis. Childs Nerv. Syst. 2023, 40, 1045–1052. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamłyński, M.; Zhemela, O.; Olejek, A. Isolated Fetal Ventriculomegaly: Diagnosis and Treatment in the Prenatal Period. Children 2024, 11, 957. https://doi.org/10.3390/children11080957
Zamłyński M, Zhemela O, Olejek A. Isolated Fetal Ventriculomegaly: Diagnosis and Treatment in the Prenatal Period. Children. 2024; 11(8):957. https://doi.org/10.3390/children11080957
Chicago/Turabian StyleZamłyński, Mateusz, Olena Zhemela, and Anita Olejek. 2024. "Isolated Fetal Ventriculomegaly: Diagnosis and Treatment in the Prenatal Period" Children 11, no. 8: 957. https://doi.org/10.3390/children11080957





