Clinical–Ultrasound Model to Predict the Clinical Course in Bronchiolitis
Abstract
1. Introduction
2. Materials and Methods
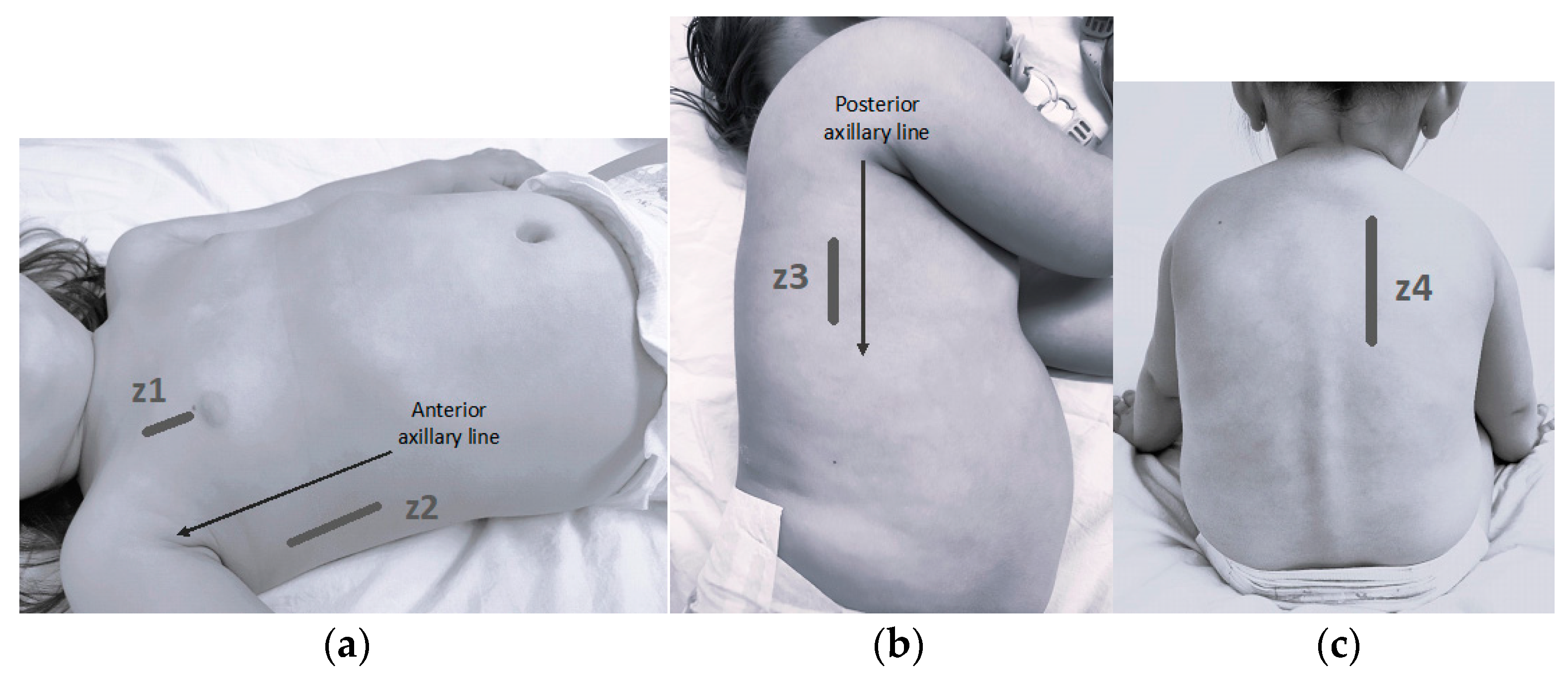
2.1. Lung Ultrasound
2.2. Statistical Analysis
3. Results
3.1. Sample Description
3.2. Ultrasound Findings
3.2.1. LUCS Relation with Outcome Variables
3.2.2. Accuracy of LUCS
3.2.3. Logistic Regression Models
- Hospital Admission: LUCS reached significant values within the 8Z and 6Z models (p = 0.029 and p = 0.045, respectively). The AUCs of the models were 0.899 and 0.889, and the explanatory power (R2) was 47% (Table S6). A score > 3 in the LUCS 8Z would increase the risk of requiring hospital admission by more than 5 times. The 4Z option did not yield significant results (Table 2).
- Oxygen Therapy: Significant values were obtained for the score in all its versions (p = 0.001 for 8Z and 6Z, and p = 0.021 for 4Z) with AUC > 0.85 and R2 up to 55% exploring 6 zones (Table S6). In the LUCS 8Z, a score >6 would increase the risk of requiring oxygen by almost 7 times, and more than 2 points in the LUCS 4Z would increase it by 4 times (Table 2).
- PICU Admission: The LUCS score did not reach a significant value in any extension (8Z, 6Z, or 4Z) (Table 2).
3.2.4. Linear Regression Models
- Hospital Stay: LUCS was significant within the 8 and 6 zone models (p = 0.001). A LUCS score > 3 or 2 (depending on whether it is 8Z or 6Z) would increase the hospital stay by almost 2 days, with an R2 for both models around 45%. The 4Z model did not reach statistical significance (p = 0.082).
- Oxygen Therapy Duration: Significant results were obtained only with the 8Z option (p < 0.001), although the results with 6 zones were close to reaching significance (p = 0.051).
- PICU Stay: No statistical significance was reached for the models in 8Z, 6Z, or 4Z.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [PubMed]
- Bronchiolitis in Children: Diagnosis and Management|Guidance|NICE [Internet]. NICE. Available online: https://www.nice.org.uk/guidance/ng9 (accessed on 1 February 2024).
- Gil-Prieto, R.; Gonzalez-Escalada, A.; Marín-García, P.; Gallardo-Pino, C.; Gil-De-Miguel, A. Respiratory Syncytial Virus Bronchiolitis in Children up to 5 Years of Age in Spain: Epidemiology and Comorbidities: An Observational Study. Medicine 2015, 94, e831. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Di Mauro, A.; Scalini, E.; Comes, P.; Lofù, I.; Mostert, M.; Tafuri, S.; Manzionna, M.M. Lung ultrasound: A useful tool in diagnosis and management of bronchiolitis. BMC Pediatr. 2015, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Caiulo, V.A.; Gargani, L.; Caiulo, S.; Fisicaro, A.; Moramarco, F.; Latini, G.; Picano, E. Lung ultrasound in bronchiolitis: Comparison with chest X-ray. Eur. J. Pediatr. 2011, 170, 1427–1433. [Google Scholar] [CrossRef]
- Chong, S.; Teoh, O.H.; Nadkarni, N.; Yeo, J.G.; Lwin, Z.; Ong, Y.G.; Lee, J.H. The modified respiratory index score (RIS) guides resource allocation in acute bronchiolitis. Pediatr. Pulmonol. 2017, 52, 954–961. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Plint, A.C.; Terwee, C.B.; Sampaio, C.; Klassen, T.P.; Offringa, M.; van der Lee, J.H. Validity of bronchiolitis outcome measures. Pediatrics 2015, 135, e1399–e1408. [Google Scholar] [CrossRef]
- Destino, L.; Weisgerber, M.C.; Soung, P.; Bakalarski, D.; Yan, K.; Rehborg, R.; Wagner, D.R.; Gorelick, M.H.; Simpson, P. Validity of respiratory scores in bronchiolitis. Hosp. Pediatr. 2012, 2, 202–209. [Google Scholar] [CrossRef]
- Bekhof, J.; Reimink, R.; Brand, P.L.P. Systematic review: Insufficient validation of clinical scores for the assessment of acute dyspnoea in wheezing children. Paediatr. Respir. Rev. 2014, 15, 98–112. [Google Scholar] [CrossRef]
- Granda, E.; Urbano, M.; Andrés, P.; Corchete, M.; Cano, A.; Velasco, R. Comparison of severity scales for acute bronchiolitis in real clinical practice. Eur. J. Pediatr. 2023, 182, 1619–1626. [Google Scholar] [CrossRef]
- Balaguer, M.; Alejandre, C.; Vila, D.; Esteban, E.; Carrasco, J.L.; Cambra, F.J.; Jordan, I. Bronchiolitis Score of Sant Joan de Déu: BROSJOD Score, validation and usefulness. Pediatr. Pulmonol. 2017, 52, 533–539. [Google Scholar] [CrossRef]
- Bueno-Campaña, M.; Sainz, T.; Alba, M.; del Rosal, T.; Mendez-Echevarría, A.; Echevarria, R.; Tagarro, A.; Ruperez-Lucas, M.; Herrreros, M.L.; Latorre, L.; et al. Lung ultrasound for prediction of respiratory support in infants with acute bronchiolitis: A cohort study. Pediatr. Pulmonol. 2019, 54, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Supino, M.C.; Buonsenso, D.; Scateni, S.; Scialanga, B.; Mesturino, M.A.; Bock, C.; Chiaretti, A.; Giglioni, E.; Reale, A.; Musolino, A.M. Point-of-care lung ultrasound in infants with bronchiolitis in the pediatric emergency department: A prospective study. Eur. J. Pediatr. 2019, 178, 623–632. [Google Scholar] [CrossRef]
- Zoido Garrote, E.; García Aparicio, C.; Camila Torrez Villarroel, C.; Pedro Vega García, A.; Muniz Fontán, M.; Oulego Erroz, I. Usefulness of early lung ultrasound in acute mild-moderate acute bronchiolitis. A pilot study. An. Pediatr. 2019, 90, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, A.K.; Yilmaz, H.L.; Kendir, Ö.T.; Gökay, S.S.; Eyüboglu, I. Lung Ultrasound Findings and Bronchiolitis Ultrasound Score for Predicting Hospital Admission in Children With Acute Bronchiolitis. Pediatr. Emerg. Care 2020, 36, e135–e142. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Cappiello, A.R.; Ammirabile, A.; Abbondanza, N.; Bianchi, F.P.; Tafuri, S.; Manzionna, M.M. Lung Ultrasound and Clinical Progression of Acute Bronchiolitis: A Prospective Observational Single-Center Study. Medicina 2020, 56, 314. [Google Scholar] [CrossRef] [PubMed]
- Bobillo-Perez, S.; Sorribes, C.; Gebellí, P.; Lledó, N.; Castilla, M.; Ramon, M.; Rodriguez-Fanjul, J. Lung ultrasound to predict pediatric intensive care admission in infants with bronchiolitis (LUSBRO study). Eur. J. Pediatr. 2021, 180, 2065–2072. [Google Scholar] [CrossRef]
- Ruiz, N.S.S.; Albarrán, I.R.; Gorostiza, I.; Laka, I.G.; Lejonagoitia, C.D.; Samson, F. Point-of-care lung ultrasound in children with bronchiolitis in a pediatric emergency department. Arch. Pédiatr. 2021, 28, 64–68. [Google Scholar] [CrossRef]
- Gori, L.; Amendolea, A.; Buonsenso, D.; Salvadori, S.; Supino, M.C.; Musolino, A.M.; Adamoli, P.; Coco, A.D.; Trobia, G.L.; Biagi, C.; et al. Prognostic Role of Lung Ultrasound in Children with Bronchiolitis: Multicentric Prospective Study. JCM 2022, 11, 4233. [Google Scholar] [CrossRef]
- Smith, J.A.; Stone, B.S.; Shin, J.; Yen, K.; Reisch, J.; Fernandes, N.; Cooper, M.C. Association of outcomes in point-of-care lung ultrasound for bronchiolitis in the pediatric emergency department. Am. J. Emerg. Med. 2024, 75, 22–28. [Google Scholar] [CrossRef]
- Hernández-Villarroel, A.C.; Ruiz-García, A.; Manzanaro, C.; Echevarría-Zubero, R.; Bote-Gascón, P.; Gonzalez-Bertolin, I.; Sainz, T.; Calvo, C.; Bueno-Campaña, M. Lung Ultrasound: A Useful Prognostic Tool in the Management of Bronchiolitis in the Emergency Department. JPM 2023, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Camporesi, A.; Morello, R.; Guzzardella, A.; Pierucci, U.M.; Izzo, F.; De Rose, C.; Buonsenso, D. A combined rapid clinical and lung ultrasound score for predicting bronchiolitis severity. Intensive Care Med. Paediatr. Neonatal. 2023, 1, 14. [Google Scholar] [CrossRef]
- Pisani, L.; Vercesi, V.; van Tongeren, P.S.; Lagrand, W.K.; Leopold, S.J.; Huson, M.A.; Henwood, P.C.; Walden, A.; Smit, M.R.; Riviello, E.D.; et al. The diagnostic accuracy for ARDS of global versus regional lung ultrasound scores—A post hoc analysis of an observational study in invasively ventilated ICU patients. Intensive Care Med. Exp. 2019, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Kok, B.; Schuit, F.; Lieveld, A.; Azijli, K.; Nanayakkara, P.W.; Bosch, F. Comparing lung ultrasound: Extensive versus short in COVID-19 (CLUES): A multicentre, observational study at the emergency department. BMJ Open 2021, 11, e048795. [Google Scholar] [CrossRef] [PubMed]
- Buessler, A.; Chouihed, T.; Duarte, K.; Bassand, A.; Huot-Marchand, M.; Gottwalles, Y.; Pénine, A.; André, E.; Nace, L.; Jaeger, D.; et al. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED: A Multicenter Prospective Study. Chest 2020, 157, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Pilar Orive FJLópez Fernández, Y.M. Manejo de la Bronquiolitis Aguda en la UCIP. Protocol of the Spanish Society of Paediatric Intensive Care, 2021. [Internet]. Available online: https://www.aeped.es/sites/default/files/documentos/21_bronquiolitis_ucip.pdf (accessed on 1 February 2024).
- Copetti, R.; Cattarossi, L. Ultrasound diagnosis of pneumonia in children. Radiol. Med. 2008, 113, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M.; Yousef, N.; Miatello, J.; Roy, C.; Claude, C.; Boutillier, B.; Dubois, C.; Pierre, A.-F.; Tissières, P.; Durand, P. Can a simple lung ultrasound score predict length of ventilation for infants with severe acute viral bronchiolitis? Arch. Pediatr. 2018, 25, 112–117. [Google Scholar] [CrossRef]
- Buonsenso, D.; Soldati, G.; Curatola, A.; Morello, R.; De Rose, C.; Vacca, M.E.; Lazzareschi, I.; Musolino, A.M.; Valentini, P. Lung Ultrasound Pattern in Healthy Infants During the First 6 Months of Life. J. Ultrasound Med. 2020, 39, 2379–2388. [Google Scholar] [CrossRef]
- Ingelse, S.A.; Pisani, L.; Westdorp, M.H.A.; Almakdase, M.; Schultz, M.J.; van Woensel, J.B.M.; Bem, R.A. Lung ultrasound scoring in invasive mechanically ventilated children with severe bronchiolitis. Pediatr. Pulmonol. 2020, 55, 2799–2805. [Google Scholar] [CrossRef]

| Patients’ Characteristics | Patients Outcome | |||
|---|---|---|---|---|
| Age (m) | 3.68 (1.80–6.65) | Discharged home | 13 (14.4) | |
| Weight (kg) | 5.80 (4.71–7.70) | Hospital admission | 77 (85.6) | |
| Gestational age (w + d) | 39 + 1 (37 + 5–40 + 2) | Hospital stay (d) | 4 (2–6) | |
| Birth weight (g) | 3.080 (2.735–3.455) | PICU admission | 15 (16.6) | |
| Gender | Male | 49 (54.43) | PICU stay (d) | 4 (3–5.5) |
| Female | 41 (45.6) | Oxygen therapy (low flow/with NIV) | 43 (47.8) | |
| Prematurity (<37 weeks) | 8 (8.9%) | Low-flow oxygen therapy only | 30 (69.8) | |
| Breastfeeding | 58 (64.4) | Duration of oxygen therapy (d) | 3 (1.5–5) | |
| Daycare attendance | 13 (14.4) | Need of NIV | 13 (14.4) | |
| Previous episodes of respiratory distress | 22 (24.4) | Duration of NIV support (d) | 2.5 (2–3) | |
| Need of IMV | 0 (0) | |||
| Household members with respiratory symptoms | 70 (77.8) | |||
| Nasopharyngeal Swab | ||||
| Not collected | 10 (11.1) | RSV | 54 (67.5) | |
| Collected | 80 (88.9) | Adenovirus | 5 (6.25) | |
| Negative | 13 (16.25) | Influenza virus | 4 (5) | |
| Coinfection | 10 (12.5) | Others | 4 (5) | |
| Cutoff Point | OR Univariate | OR Multivariate | |||
|---|---|---|---|---|---|
| Hospitalization | Model 8Z variables | Age (m) | >3.47 | 0.07 (0.00–0.36) p = 0.011 | 0.05 (0.00–0.32) p = 0.008 |
| BROSJOD | >6 | 7.33 (1.81–49.50) p = 0.013 | 12.71 (2.60–101.04) p = 0.005 | ||
| LUCS 8Z | >3 | 6.41 (1.87–25.86) p = 0.005 | 5.47 (1.28–28.60) p = 0.029 | ||
| Model 6Z variables | Age (m) | >3.47 | 0.07 (0.00–0.36) p = 0.011 | 0.06 (0.00–0.36) p = 0.011 | |
| BROSJOD | >6 | 7.33 (1.81–49.50) p = 0.013 | 10.85 (2.27–82.31) p = 0.007 | ||
| LUCS 6Z | >2 | 9.10 (2.24–61.57) p = 0.006 | 5.90 (1.20–45.00) p = 0.045 | ||
| Model 4Z variables | Age (m) | >3.47 | 0.07 (0.00–0.36) p = 0.011 | 0.07 (0.00–0.42) p = 0.016 | |
| BROSJOD | >6 | 7.33 (1.81–49.50) p = 0.013 | 11.07 (2.40–81.80) p = 0.005 | ||
| LUCS 4Z | >2 | 11.10 (2.04–207.16) p = 0.024 | 6.01 (0.88–121.47) p = 0.116 | ||
| Oxygen Therapy | Model 8Z variables | Age (m) | >4.07 | 0.44 (0.19–1.01) p = 0.057 | 0.23 (0.06–0.73) p = 0.018 |
| BROSJOD | >6 | 11.02 (4.28–31.04) p < 0.001 | 15.77 (5.01–60.80) p < 0.001 | ||
| LUCS 8Z | >6 | 6.39 (2.60–16.86) p < 0.001 | 6.88 (2.28–23.61) p = 0.001 | ||
| Model 6Z variables | Age (m) | >4.07 | 0.44 (0.19–1.01) p = 0.057 | 0.35 (0.09–1.14) p = 0.093 | |
| BROSJOD | >6 | 11.02 (4.28–31.04) p < 0.001 | 16.98 (5.29–67.49) p < 0.001 | ||
| LUCS 6Z | >1 | 16.56 (4.37–109.05) p < 0.001 | 17.45 (3.67–134.55) p = 0.001 | ||
| Model 4Z variables | Age (m) | >4.07 | 0.44 (0.19–1.01) p = 0.057 | 0.36 (0.10–1.11) p = 0.086 | |
| BROSJOD | >6 | 11.02 (4.28–31.04) p < 0.001 | 17.22 (5.66–64.96) p < 0.001 | ||
| LUCS 4Z | >2 | 3.63 (1.53–8.98) p = 0.004 | 3.97 (1.29–13.91) p = 0.021 | ||
| Picu Admission | Model 8Z variables | Age (m) | >3.37 | 0.10 (0.02–0.41) p = 0.004 | 0.03 (0.00–0.22) p = 0.005 |
| BROSJOD | >8 | 38.86 (9.83–205.02) p < 0.001 | 94.99 (13.22–2086.29) p < 0.001 | ||
| LUCS 8Z | >8 | 5.84 (1.80–22.86) p = 0.005 | 3.23 (0.52–26.56) p = 0.220 | ||
| Model 6Z variables | Age (m) | >3.37 | 0.10 (0.02–0.41) p = 0.004 | 0.03 (0.00–0.28) p = 0.008 | |
| BROSJOD | >8 | 38.86 (9.83–205.02) p < 0.001 | 101.08 (14.33–2197.85) p < 0.001 | ||
| LUCS 6Z | >3 | 6.00 (1.73–27.98) p = 0.009 | 2.95 (0.46–26.36) p = 0.273 | ||
| Model 4Z variables | Age (m) | >3.37 | 0.10 (0.02–0.41) p = 0.004 | 0.03 (0.00–0.26) p = 0.007 | |
| BROSJOD | >8 | 38.86 (9.83–205.02) p < 0.001 | 114.50 (16.64–2453.94) p < 0.001 | ||
| LUCS 4Z | >2 | 2.38 (0.78–7.77) p = 0.134 | 1.27 (0.19–9.29) p = 0.803 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez García, L.; Hierro Delgado, E.; Oulego Erroz, I.; Rey Galán, C.; Mayordomo Colunga, J. Clinical–Ultrasound Model to Predict the Clinical Course in Bronchiolitis. Children 2024, 11, 987. https://doi.org/10.3390/children11080987
Rodríguez García L, Hierro Delgado E, Oulego Erroz I, Rey Galán C, Mayordomo Colunga J. Clinical–Ultrasound Model to Predict the Clinical Course in Bronchiolitis. Children. 2024; 11(8):987. https://doi.org/10.3390/children11080987
Chicago/Turabian StyleRodríguez García, Lucía, Elena Hierro Delgado, Ignacio Oulego Erroz, Corsino Rey Galán, and Juan Mayordomo Colunga. 2024. "Clinical–Ultrasound Model to Predict the Clinical Course in Bronchiolitis" Children 11, no. 8: 987. https://doi.org/10.3390/children11080987
APA StyleRodríguez García, L., Hierro Delgado, E., Oulego Erroz, I., Rey Galán, C., & Mayordomo Colunga, J. (2024). Clinical–Ultrasound Model to Predict the Clinical Course in Bronchiolitis. Children, 11(8), 987. https://doi.org/10.3390/children11080987






