Influences of Maternal Nutrition and Lifestyle Factors on Early Childhood Oral Health: A Systematic Review of Mechanisms and Intervention Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis and Analysis
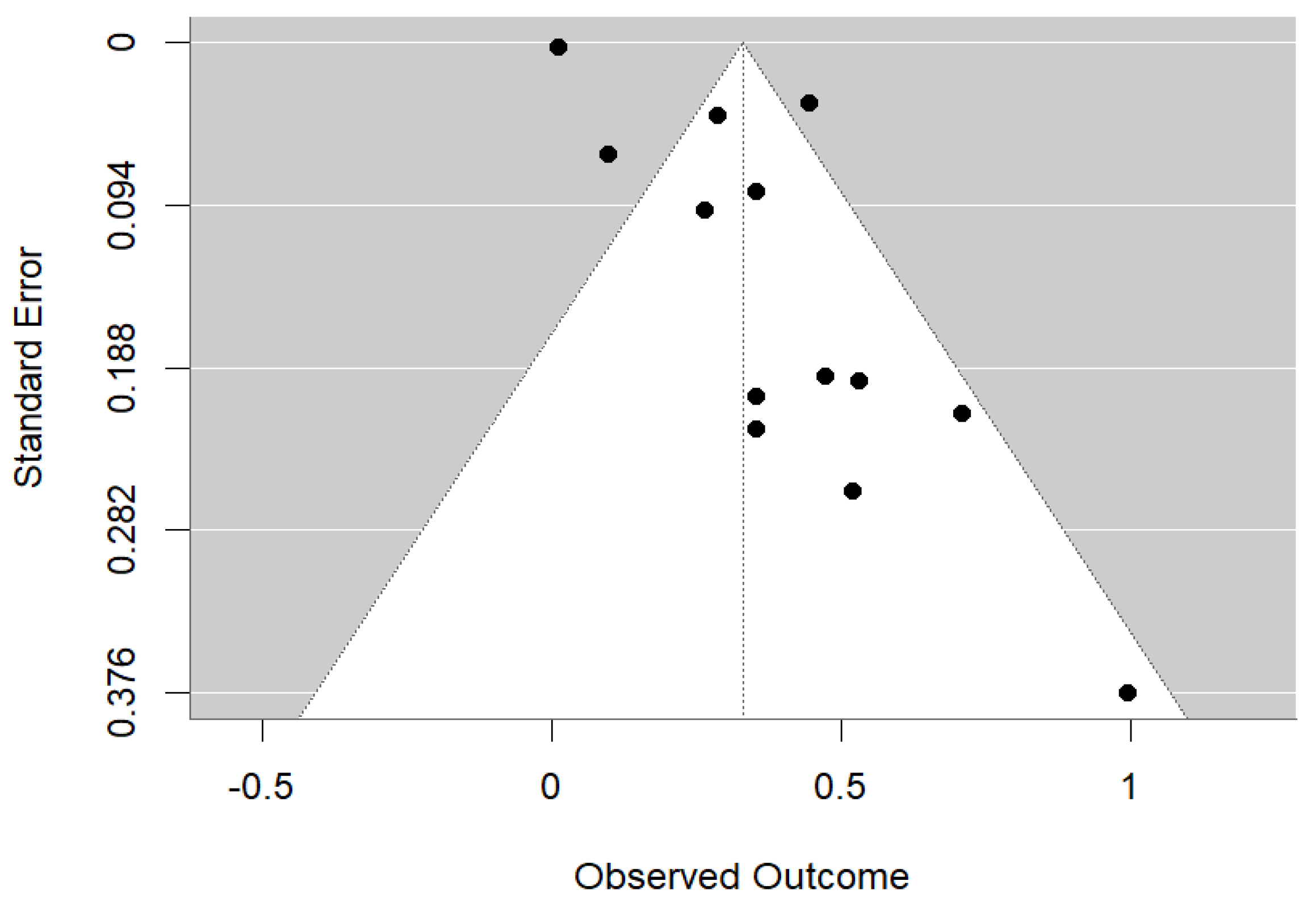
2.6. Risk of Bias Assessment
2.7. Ethical Considerations
3. Results
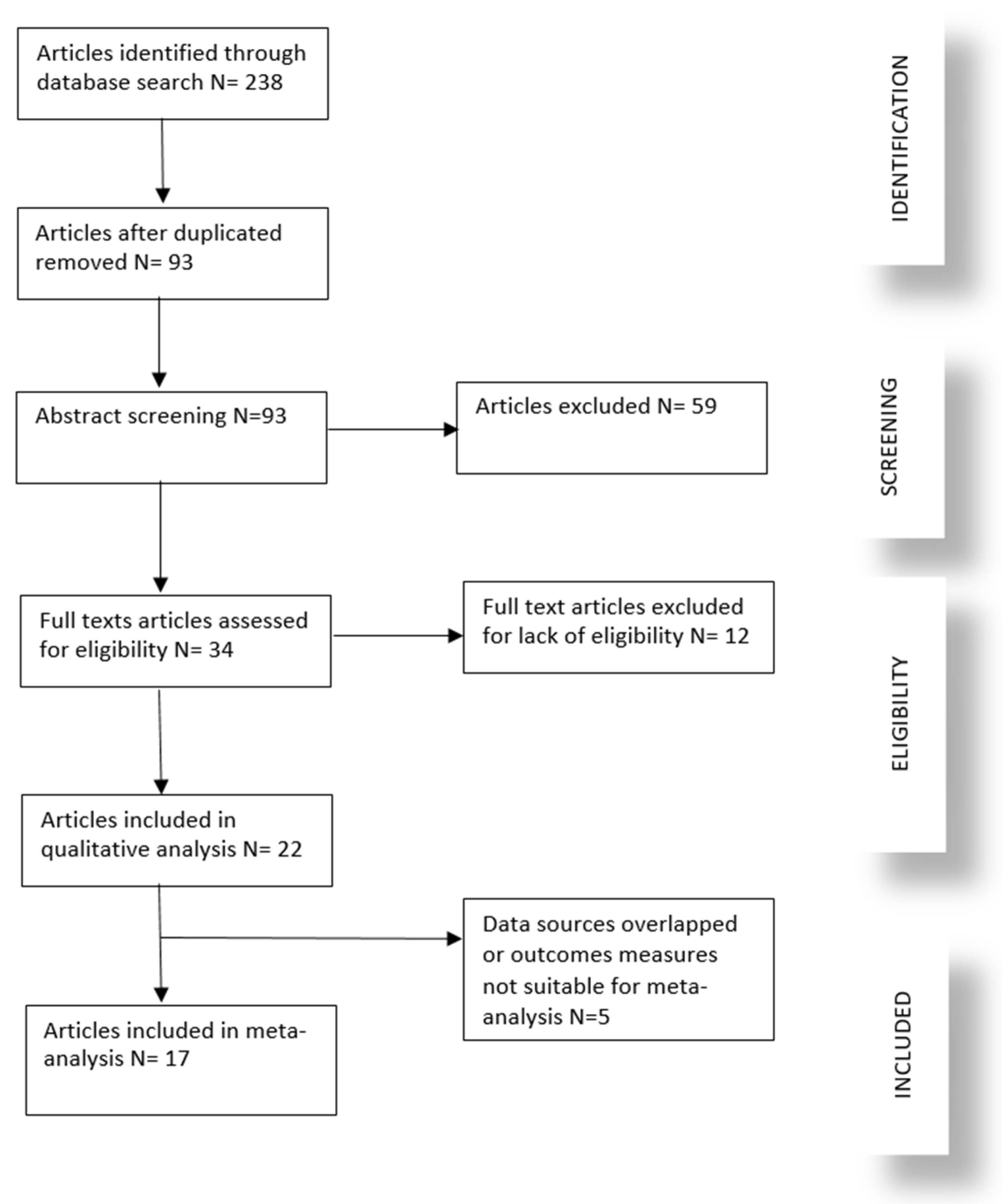
3.1. Study Selection
3.2. Study Characteristics and Outcomes
3.3. Quality of Included Studies
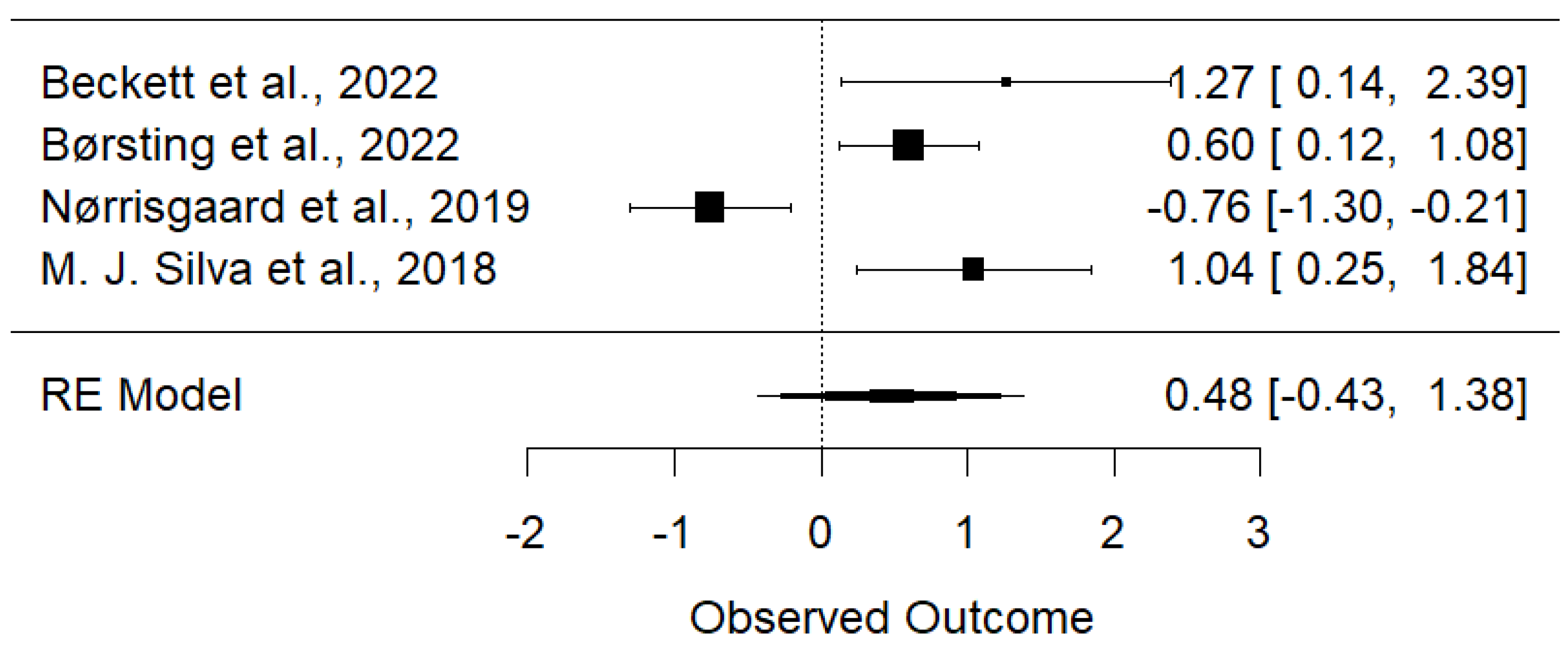
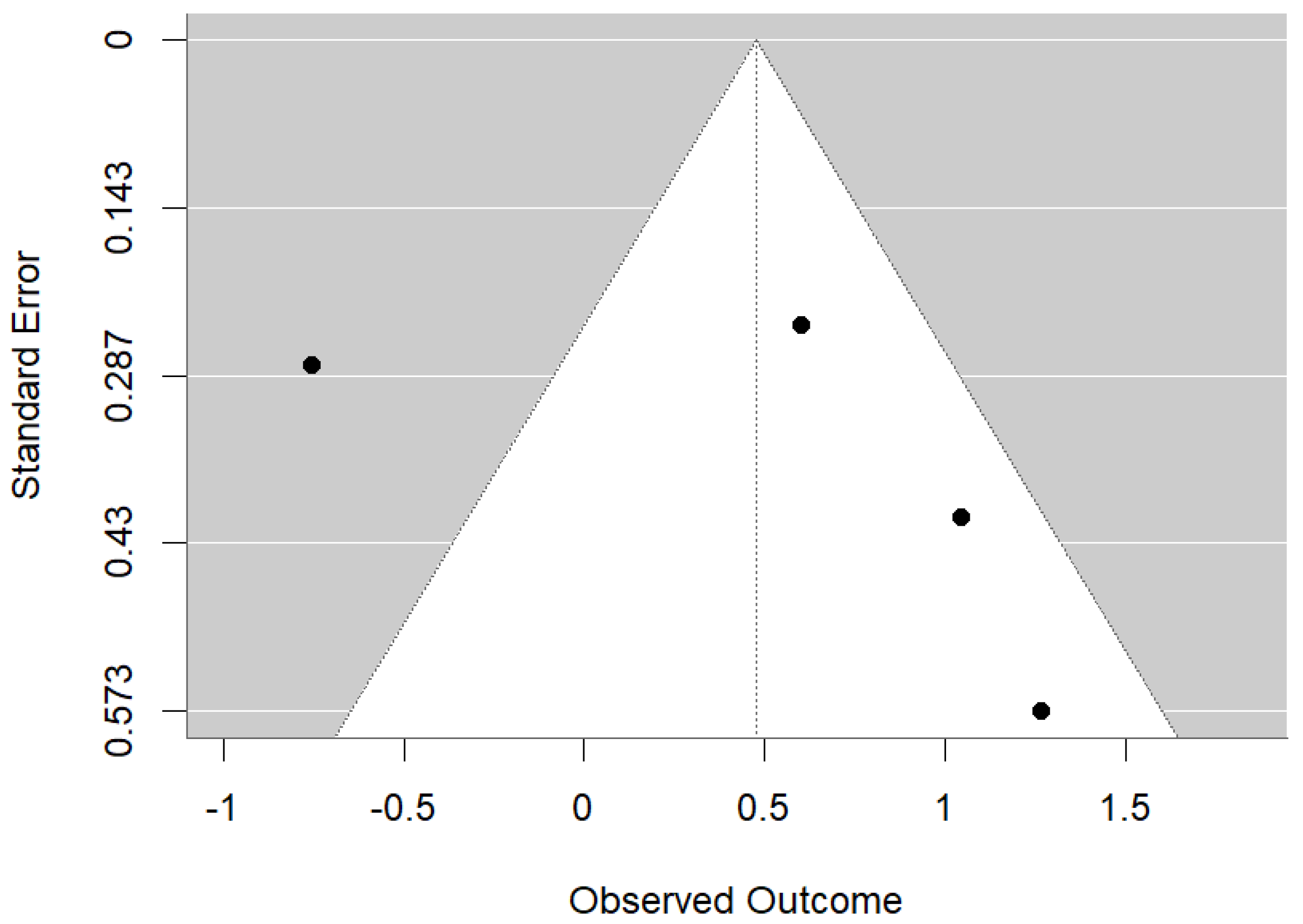
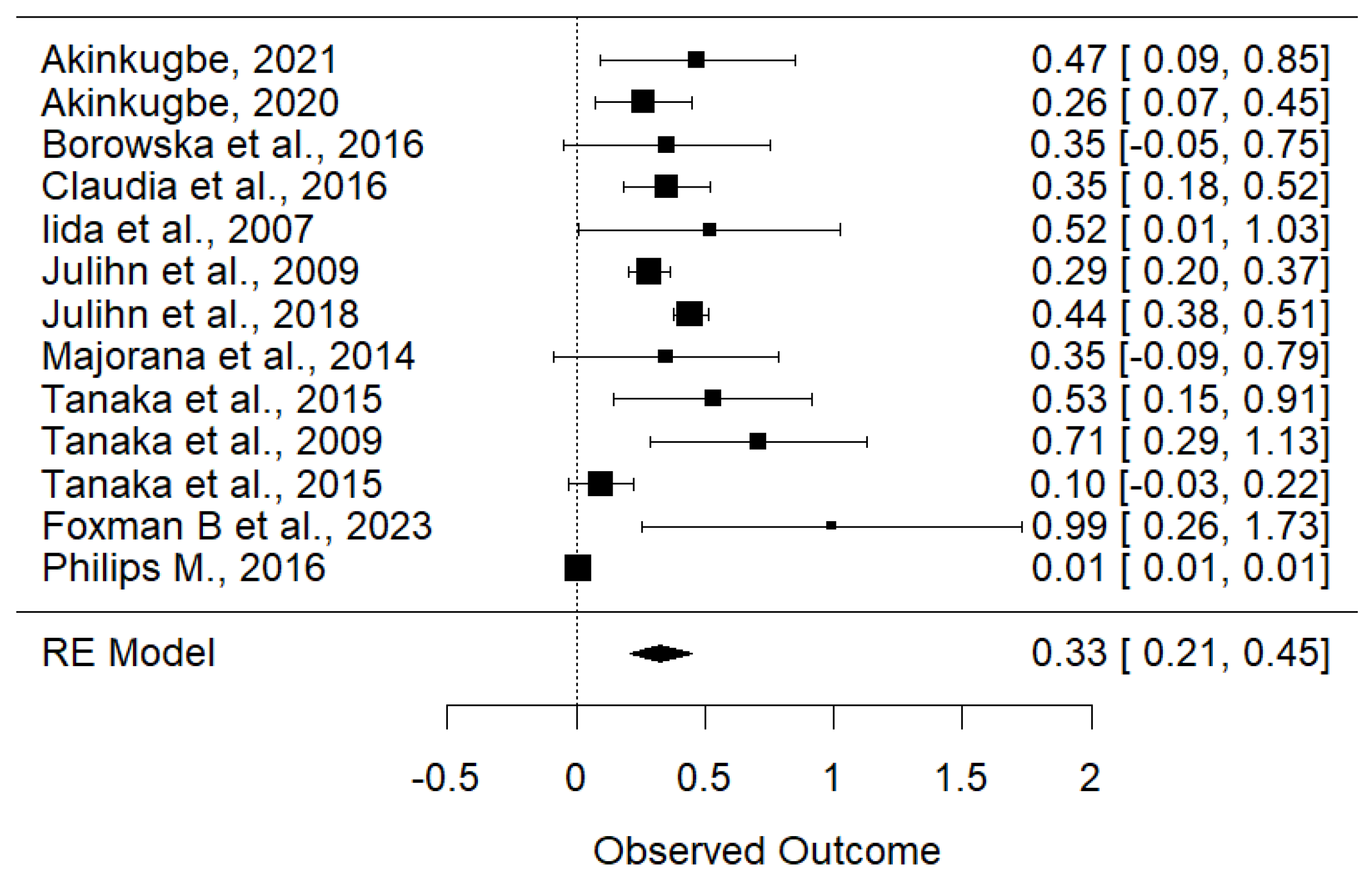
3.4. Meta-Analysis
3.4.1. Maternal Vitamin D Intake Is Associated with Reduced Early Childhood Carries and Enamel Defects
3.4.2. Maternal Smoking Is Associated with Increased Risk of Early Childhood Carries
4. Discussion
5. Limitations and Future Research
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheiham, A. Oral health, general health and quality of life. Bull. World Health Organ. 2005, 83, 644. [Google Scholar] [PubMed]
- Rodrigues, J.A.; Olegario, I.; Assunção, C.M.; Bönecker, M. Future Perspectives in Pediatric Dentistry: Where are We Now and where are We Heading? Int. J. Clin. Pediatr. Dent. 2022, 15, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Kakuma, R. Energy and protein intake in pregnancy. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2003; p. CD000032. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyake, Y.; Sasaki, S. The Effect of Maternal Smoking during Pregnancy and Postnatal Household Smoking on Dental Caries in Young Children. J. Pediatr. 2009, 155, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Duijster, D.; de Jong-Lenters, M.; Verrips, E.; van Loveren, C. Establishing oral health promoting behaviours in children—Parents’ views on barriers, facilitators and professional support: A qualitative study. BMC Oral Health 2015, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.; Marshall, T.A.; Milgrom, P.; Coldwell, S.E. The contribution of dietary factors to dental caries and disparities in caries. Acad. Pediatr. 2009, 9, 410–414. [Google Scholar] [CrossRef]
- Nørrisgaard, P.E.; Haubek, D.; Kühnisch, J.; Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Association of High-Dose Vitamin D Supplementation during Pregnancy with the Risk of Enamel Defects in Offspring: A 6-Year Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 924–930. [Google Scholar] [CrossRef]
- Beckett, D.M.; Broadbent, J.M.; Loch, C.; Mahoney, E.K.; Drummond, B.K.; Wheeler, B.J. Dental Consequences of Vitamin D Deficiency during Pregnancy and Early Infancy-An Observational Study. Int. J. Env. Res. Public. Health 2022, 19, 1932. [Google Scholar] [CrossRef]
- Van Der Tas, J.T.; Elfrink, M.E.; Heijboer, A.C.; Rivadeneira, F.; Jaddoe, V.W.; Tiemeier, H.; Schoufour, J.D.; Moll, H.A.; Ongkosuwito, E.M.; Wolvius, E.B.; et al. Foetal, neonatal and child vitamin D status and enamel hypomineralization. Community Dent. Oral Epidemiol. 2018, 46, 343–351. [Google Scholar] [CrossRef]
- Mortensen, N.B.; Haubek, D.; Dalgård, C.; Nørgaard, S.M.; Christoffersen, L.; Cantio, E.; Rasmussen, A.; Möller, S.; Christesen, H.T. Vitamin D status and tooth enamel hypomineralization are not associated in 4-y-old children: An Odense Child Cohort study. J. Steroid Biochem. Mol. Biol. 2022, 221, 106130. [Google Scholar] [CrossRef]
- Børsting, T.; Schuller, A.; van Dommelen, P.; Stafne, S.N.; Skeie, M.S.; Skaare, A.B.; Mørkved, S.; Salvesen, K.Å.; Stunes, A.K.; Mosti, M.P.; et al. Maternal vitamin D status in pregnancy and molar incisor hypomineralisation and hypomineralised second primary molars in the offspring at 7–9 years of age: A longitudinal study. Eur. Arch. Paediatr. Dent. 2022, 23, 557–566. [Google Scholar] [CrossRef]
- Reed, S.G.; Miller, C.S.; Wagner, C.L.; Hollis, B.W.; Lawson, A.B. Toward Preventing Enamel Hypoplasia: Modeling Maternal and Neonatal Biomarkers of Human Calcium Homeostasis. Caries Res. 2020, 54, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Halchuk, S.; Star, L. Prevalence and risk factors of caregiver reported Severe Early Childhood Caries in Manitoba First Nations children: Results from the RHS Phase 2 (2008–2010). Int. J. Circumpolar Health 2013, 72 (Suppl. S1), 21167. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Kilpatrick, N.M.; Craig, J.M.; Manton, D.J.; Leong, P.; Burgner, D.P.; Scurrah, K.J. Genetic and early-life environmental influences on dental caries risk: A twin study. Pediatrics 2019, 143, e20183499. [Google Scholar] [CrossRef]
- Neto, M.B.C.; da Silva-Souza, K.P.; Maranhão, V.F.; Botelho, K.V.G.; Heimer, M.V.; Santos-Junior, V.E.D. Enamel Defects in Deciduous Dentition and Their Association with the Occurrence of Adverse Effects from Pregnancy to Early Childhood. Oral Health Prev. Dent. 2020, 18, 741–746. [Google Scholar] [PubMed]
- Akinkugbe, A.A. Does the Trimester of Smoking Matter in the Association between Prenatal Smoking and the Risk of Early Childhood Caries? Caries Res. 2021, 55, 114–121. [Google Scholar] [CrossRef]
- Akinkugbe, A.A.; Brickhouse, T.H.; Nascimento, M.M.; Slade, G.D. Prenatal smoking and the risk of early childhood caries: A prospective cohort study. Prev. Med. Rep. 2020, 20, 101201. [Google Scholar] [CrossRef]
- Borowska-Struginska, B.; Zadzinska, E.; Bruzda-Zwiech, A.; Filipinska, R.; Lubowiecka-Gontarek, B.; Szydlowska-Walendowska, B.; Wochna-Sobańska, M. Prenatal and familial factors of caries in first permanent molars in schoolchildren living in an urban area of Lodz, Poland. Homo J. Comp. Hum. Biol. 2016, 67, 226–234. [Google Scholar] [CrossRef]
- Claudia, C.; Ju, X.; Mejia, G.; Jamieson, L.M. The relationship between maternal smoking during pregnancy and parental-reported experience of dental caries in Indigenous Australian children. Community Dent. Health 2016, 33, 297–302. [Google Scholar] [CrossRef]
- Iida, H.; Auinger, P.; Billings, R.J.; Weitzman, M. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics 2007, 120, e944–e952. [Google Scholar] [CrossRef]
- Julihn, A.; Ekbom, A.; Modéer, T. Maternal overweight and smoking: Prenatal risk factors for caries development in offspring during the teenage period. Eur. J. Epidemiol. 2009, 24, 753–762. [Google Scholar] [CrossRef]
- Julihn, A.; Soares, F.C.; Hjern, A.; Dahllöf, G. Socioeconomic Determinants, Maternal Health, and Caries in Young Children. JDR Clin. Transl. Res. 2018, 3, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Majorana, A.; Cagetti, M.G.; Bardellini, E.; Amadori, F.; Conti, G.; Strohmenger, L.; Campus, G. Feeding and smoking habits as cumulative risk factors for early childhood caries in toddlers, after adjustment for several behavioral determinants: A retrospective study. BMC Pediatr. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Shinzawa, M.; Tokumasu, H.; Seto, K.; Tanaka, S.; Kawakami, K. Secondhand smoke and incidence of dental caries in deciduous teeth among children in Japan: Population based retrospective cohort study. BMJ 2015, 351, h5397. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Davis, E.; Neiswanger, K.; McNeil, D.; Shaffer, J.; Marazita, M.L. Maternal factors and risk of early childhood caries: A prospective cohort study. Community Dent. Oral Epidemiol. 2023, 51, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Masterson, E.; Sabbah, W. Association between child caries and maternal health-related behaviours. Community Dent. Health 2016, 33, 133–137. [Google Scholar] [CrossRef]
- Tapalaga, G.; Bumbu, B.A.; Reddy, S.R.; Vutukuru, S.D.; Nalla, A.; Bratosin, F.; Fericean, R.M.; Dumitru, C.; Crisan, D.C.; Nicolae, N.; et al. The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review. Nutrients 2023, 15, 3863. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Huang, R. The effect of smoking on caries-related microorganisms. Tob. Induc. Dis. 2019, 17, 32. [Google Scholar] [CrossRef]
- Yanagita, M.; Kashiwagi, Y.; Kobayashi, R.; Tomoeda, M.; Shimabukuro, Y.; Murakami, S. Nicotine inhibits mineralization of human dental pulp cells. J. Endod. 2008, 34, 1061–1065. [Google Scholar] [CrossRef]
- Manavi, K.R.; Alston-Mills, B.P.; Thompson, M.P. History of tobacco, vitamin D and women. Int. J. Vitam. Nutr. Res. 2020, 90, 389–394. [Google Scholar] [CrossRef]
- Kellesarian, S.; Malignaggi, V.; De Freitas, P.; Ahmed, H.; Javed, F. Association between prenatal maternal cigarette smoking and early childhood caries. A systematic review. J. Clin. Exp. Dent. 2017, 9, e1141–e1146. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, Q.; Tan, B.; Huang, R. Correlation Between Maternal Smoking during Pregnancy and Dental Caries in Children: A Systematic Review and Meta-Analysis. Front. Oral. Health 2021, 2, 673449. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Country | Study Design | Sample Size | Outcomes |
|---|---|---|---|---|
| Mortensen et al., 2022 [10] | Denmark | Retrospective Cohort | 1241 | Prevalence of HSPM: 54.7%. No significant association between 25(OH) D and HSPM. |
| Beckett et al., 2022 [8] | New Zealand | Retrospective Cohort | 81 | Vitamin D insufficiency (<50 nmol/L) in the third trimester was associated with an increased risk of caries by age 6 (IRR: 3.55, CI: 1.15–10.92). |
| Børsting et al., 2022 [11] | Norway | Randomized Trial | 176 pairs | Prevalence: 32% MIH, 22% HSPM. Insufficient maternal vitamin D during gestational weeks 18–22 was significantly associated with MIH (adjusted RR: 1.82, 95% CI: 1.13–2.93). |
| Nørrisgaard et al., 2019 [7] | Denmark | Randomized Trial | 623 mothers; 588 of their children | High-dose vitamin D (2400 IU/day) supplementation during pregnancy was associated with a lower risk of enamel defects in permanent OR: 0.47 (95% CI: 0.27–0.81) and deciduous dentitions OR: 0.50 (95% CI: 0.28–0.87) of the offspring. |
| Van der Tas et al., 2018 [9] | The Netherland | Prospective Cohort | 4750 mothers; 3983 children | No significant association between 25(OH) D concentrations in prenatal, early postnatal, and later postnatal life and the presence of HSPMs or MIH at the age of six. |
| Reed et al., 2017 [12] | USA | Randomized Trial | 29 Pairs | EH prevalence: 45%. Maternal vitamin D insufficiency might be associated with EH in children’s teeth. |
| Schroth et al., 2014 [13] | USA | Prospective Cohort | 207 | Mothers of children with ECC had significantly lower 25(OH) D levels compared to those whose children were caries-free (41 ± 20 nmol/L vs. 52 ± 27 nmol/L; p = 0.05). |
| M. J. Silva et al., 2018 [14] | Australia | Prospective Cohort | 250 twin pregnancies; 344 twins | Prevalence of HSPM was 19.8%. No significant evidence (p = 0.172) was found for genetic influence on HSPM. Maternal smoking in the second OR: 2.84, 95% CI (1.28–6.30), p = 0.01 and third OR: 3.23, 95% CI (1.42–7.33), p = 0.05 trimester was associated with HSPM. |
| Neto et al., 2020 [15] | Brazil | Cross-section | 152 | Children with vitamin D deficiency during gestation were 6.40 times more likely to have DDE. |
| Author, Year | Country | Study Design | Sample Size | Outcomes |
|---|---|---|---|---|
| Akinkugbe, 2021 [16] | England | Retrospective Cohort | 1429 | RR: 1.60 (1.09–2.32) |
| Akinkugbe, 2020 [17] | England | Prospective Cohort | 1429 | RR: 1.30 (1.08–1.58) |
| Borowska-Struginska et al., 2016 [18] | Poland | Cross-section | 1131 | OR: 1.42 (0.95–2.12) |
| Claudia et al., 2016 [19] | Australia | Retrospective Cohort | 1687 | RR: 1.42 (1.20–1.68) |
| Iida et al., 2007 [20] | USA | Cross-section | 1576 | OR: 1.68 (1.01–2.79) |
| Julihn et al., 2009 [21] | Sweden | Cross-section | 15,538 | OR: 1.33 (1.22–1.44) |
| Julihn et al., 2018 [22] | Sweden | Cross-section | 65,259 | OR: 1.56 (1.42–1.63) |
| Majorana et al., 2014 [23] | Italy | Cross-section | 2395 | OR: 1.42 (0.92–2.21) |
| Tanaka et al., 2015 [24] | Japan | Cross-section | 6412 | OR: 1.70 (1.15–2.48) |
| Tanaka et al., 2009 [4] | Japan | Cross-section | 2015 | OR: 2.03 (1.33–3.09) |
| Tanaka et al., 2015 [24] | Japan | Retrospective Cohort | 76,920 | HR: 1.10 (0.97–1.25) |
| Foxman B et al., 2023 [25] | USA | Prospective Cohort | 1015 | OR: 2.7 (1.29–5.64) |
| Philips M., 2016 [26] | USA | Retrospective Cohort | 917 | OR: 1.01 (1.01–1.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashdi, M. Influences of Maternal Nutrition and Lifestyle Factors on Early Childhood Oral Health: A Systematic Review of Mechanisms and Intervention Strategies. Children 2024, 11, 1107. https://doi.org/10.3390/children11091107
Alrashdi M. Influences of Maternal Nutrition and Lifestyle Factors on Early Childhood Oral Health: A Systematic Review of Mechanisms and Intervention Strategies. Children. 2024; 11(9):1107. https://doi.org/10.3390/children11091107
Chicago/Turabian StyleAlrashdi, Murad. 2024. "Influences of Maternal Nutrition and Lifestyle Factors on Early Childhood Oral Health: A Systematic Review of Mechanisms and Intervention Strategies" Children 11, no. 9: 1107. https://doi.org/10.3390/children11091107






