Evolutive Study of Dietary Aspects and Intestinal Microbiota of Pediatric Cohort with Cow’s Milk Protein Allergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Nutritional and Anthropometric Assessment
2.3. Fecal DNA Extraction and Quantification
2.4. High-Throughput Sequencing of 16S rRNA Gene Amplicons
2.5. Fecal Short-Chain Fatty Acids (SCFAs) Determination
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Nutritional Assessment
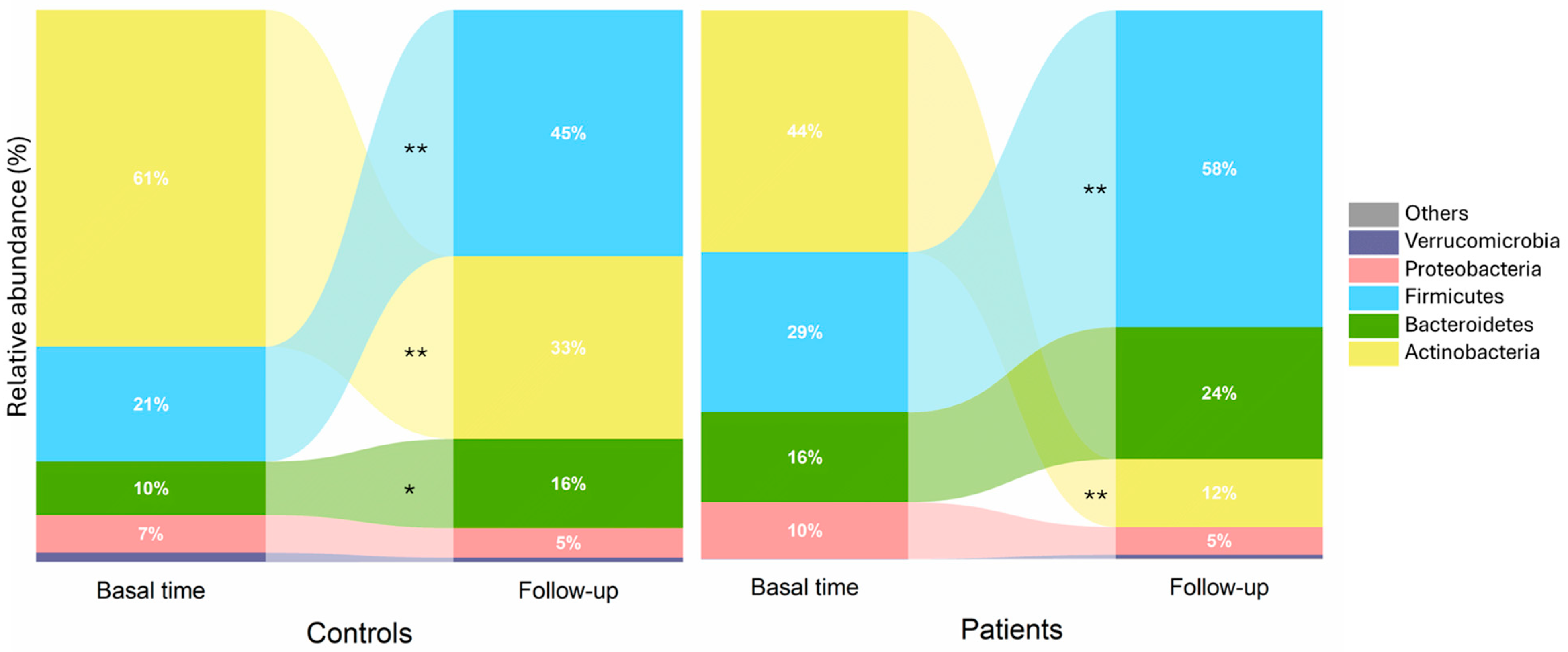
3.3. Fecal Microbiota and Compositional Profile
3.4. Fecal Metabolic Concentration of Short-Chain Fatty Acids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Yu, X.; Cong, Y.; Li, L. Cow milk αs1-casein induces allergic responses in a mouse model of atopy. Food Sci. Hum. Wellness 2022, 11, 1282–1289. [Google Scholar] [CrossRef]
- D’Auria, E.; Mameli, C.; Piras, C.; Cococcioni, L.; Urbani, A.; Zuccotti, G.V.; Roncada, P. Precision medicine in cow’s milk allergy: Proteomics perspectives from allergens to patients. J. Proteom. 2018, 188, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lajnaf, R.; Feki, S.; Ben Ameur, S.; Attia, H.; Kammoun, T.; Ayadi, M.A.; Masmoudi, H. Cow’s milk alternatives for children with cow’s milk protein allergy—Review of health benefits and risks of allergic reaction. Int. Dairy J. 2023, 141, 105624. [Google Scholar] [CrossRef]
- Mousan, G.; Kamat, D. Cow’s milk protein allergy. Clin. Pediatr. 2016, 55, 1054–1063. [Google Scholar] [CrossRef]
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-Mediated gastrointestinal food allergies in children: An update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI Guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Dupont, C.; Chouraqui, J.-P.; Linglart, A.; Bocquet, A.; Darmaun, D.; Feillet, F.; Frelut, M.-L.; Girardet, J.-P.; Hankard, R.; Rozé, J.-C.; et al. Nutritional management of cow’s milk allergy in children: An update. Arch. Pédiatrie 2018, 25, 236–243. [Google Scholar] [CrossRef]
- Kansu, A.; Yüce, A.; Dalgıç, B.; Şekerel, B.E.; Çullu-Çokuğraş, F.; Çokuğraş, H. Consensus statement on diagnosis, treatment and follow-up of cow’s milk protein allergy among infants and children in Turkey. Turk. J. Pediatr. 2016, 58, 1. [Google Scholar] [CrossRef] [PubMed]
- Sackesen, C.; Altintas, D.U.; Bingol, A.; Bingol, G.; Buyuktiryaki, B.; Demir, E.; Kansu, A.; Kuloglu, Z.; Tamay, Z.; Sekerel, B.E. Current trends in tolerance induction in cow’s milk allergy: From passive to proactive strategies. Front. Pediatr. 2019, 7, 372. [Google Scholar] [CrossRef]
- Martín-Masot, R.; Díaz-Martín, J.J.; Santamaría-Orleans, A.; Navas-López, V.M. Spanish pediatricians’ positions regarding prevention, diagnosis, nutritional management, and challenges in cow’s milk protein Allergy. Nutrients 2023, 15, 3586. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Broekaert, I.; Domellöf, M.; Indrio, F.; Lapillonne, A.; Pienar, C.; Ribes-Koninckx, C.; Shamir, R.; Szajewska, H.; Thapar, N.; et al. An ESPGHAN Position Paper on the Diagnosis, Management, and Prevention of Cow’s Milk Allergy. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 386–413. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of human milk bioactives on infants’ gut and immune health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, D.M.; An, R.; Boeren, S.; Mutte, S.K.; Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; et al. Assessment of infant outgrowth of cow’s milk allergy in relation to the faecal microbiome and metaproteome. Sci. Rep. 2023, 13, 12029. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Yang, Y.; Shoaie, S.; Zhang, C.; Ji, B.; Wei, Y. Advances in the relationships between cow’s milk protein allergy and gut microbiota in infants. Front. Microbiol. 2021, 12, 716667. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Gutiérrez-Díaz, I.; Saiz, M.L.; Navarro, S.; Suárez, M.; Carbajal, I.; García, Á.; Sariego, L.; Toyos, P.; Rodríguez, S.; et al. Gut microbiota and inflammatory mediators differentiate IgE mediated and non-IgE mediated cases of cow’s milk protein at diagnosis. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 836–845. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Organizador Dietético Metabólico ODIMET. Available online: http://www.odimet.es/ (accessed on 20 February 2024).
- World Health Organization (WHO). Exclusive Breastfeeding for Six Months Best for Babies Everywhere. Available online: https://www.who.int/mediacentre/news/statements/2011/breastfeeding_20110115/en/ (accessed on 1 March 2024).
- World Health Organization (WHO). Child Growth Standards OMS Anthro; [Version 3.2.2]; World Health Organization (WHO): Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization (WHO). UNICEF WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children; World Health Organization (WHO): Geneva, Switzerland, 2009; pp. 1–12. [Google Scholar]
- Costea, P.I.; Zeller, G.; Sunagawa, S.; Pelletier, E.; Alberti, A.; Levenez, F.; Tramontano, M.; Driessen, M.; Hercog, R.; Jung, F.E.; et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 2017, 35, 1069–1076. [Google Scholar] [CrossRef]
- Milani, C.; Hevia, A.; Foroni, E.; Duranti, S.; Turroni, F.; Lugli, G.A.; Sanchez, B.; Martín, R.; Gueimonde, M.; van Sinderen, D.; et al. Assessing the fecal microbiota: An Optimized Ion Torrent 16S RRNA gene-based analysis protocol. PLoS ONE 2013, 8, e68739. [Google Scholar] [CrossRef]
- Mozota, M.; Castro, I.; Gómez-Torres, N.; Arroyo, R.; Gutiérrez-Díaz, I.; Delgado, S.; Rodríguez, J.M.; Alba, C. Administration of Ligilactobacillus salivarius CECT 30632 to elderly during the COVID-19 pandemic: Nasal and fecal metataxonomic analysis and fatty acid profiling. Front. Microbiol. 2022, 13, 1052675. [Google Scholar] [CrossRef]
- Abrams, E.M.; Sicherer, S.H. Cow’s milk allergy prevention. Ann. Allergy Asthma Immunol. 2021, 127, 36–41. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, C.; Guo, C.; Wang, J.; Chen, I.; Wen, S.W.; Krewski, D.; Yue, L.; Xie, R. The prevalence of food allergy in cesarean-born children aged 0–3 years: A systematic review and meta-analysis of cohort studies. Front. Pediatr. 2023, 10, 1044954. [Google Scholar] [CrossRef]
- Giannetti, A.; Toschi Vespasiani, G.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s milk protein allergy as a model of food allergies. Nutrients 2021, 13, 1525. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.B.; Johnston, W.H.; Deulofeut, H.; Leader, J.; Rhodes, R.; Yeiser, M.; Harris, C.L.; Wampler, J.L.; Hill, R.J.; Cooper, T. Growth and tolerance of healthy, term infants fed lower protein extensively hydrolyzed or amino acid-based formula: Double-blind, randomized, controlled trial. BMC Pediatr. 2021, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Al-Hussaini, B.; Al-Mannaei, K.; Al-Sunaid, A.; Helmi Ayesh, W.; El-Degeir, M.; El-Kabbany, N.; Haddad, J.; Hashmi, A.; Kreishan, F.; et al. Prevention of allergic sensitization and treatment of cow’s milk protein allergy in early life: The middle-east step-down consensus. Nutrients 2019, 11, 1444. [Google Scholar] [CrossRef]
- Reche, M.; Pascual, C.; Fiandor, A.; Polanco, I.; Rivero-Urgell, M.; Chifre, R.; Johnston, S.; Martín-Esteban, M. The effect of a partially hydrolysed formula based on rice protein in the treatment of infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2010, 21, 577–585. [Google Scholar] [CrossRef]
- Vieira, M.C.; Morais, M.B.; Spolidoro, J.V.; Toporovski, M.S.; Cardoso, A.L.; Araujo, G.T.; Nudelman, V.; Fonseca, M.C. A Survey on clinical presentation and nutritional status of infants with suspected cow’ milk allergy. BMC Pediatr. 2010, 10, 25. [Google Scholar] [CrossRef]
- Jardim-Botelho, A.; Martins, T.G.; Motta-Franco, J.; Meyer, R.; Fontes Vieira, S.C.; Protásio, B.F.; Santos Silva, M.L.; Pontes, R.S.; de Oliveira, M.B.B.; de Carvalho Barreto, I.D.; et al. Growth and nutritional biomarkers in brazilian infants with cow’s milk allergy at diagnosis and 18-month follow-up: A prospective cohort study. Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26, 355. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Nocerino, R.; Frediani, T.; Lucarelli, S.; Di Scala, C.; Varin, E.; Leone, L.; Muraro, A.; Agostoni, C. Amino acid–based formula in cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 632–638. [Google Scholar] [CrossRef]
- Nocerino, R.; Coppola, S.; Carucci, L.; de Giovanni di Santa Severina, A.F.; Oglio, F.; de Michele, R.; di Sessa, I.; Masino, A.; Bedogni, G.; Berni Canani, R. The step-down approach in children with cow’s milk allergy: Results of a randomized controlled trial. Allergy 2023, 78, 2477–2486. [Google Scholar] [CrossRef]
- Kotchetkoff, E.C.D.A.; Mendonça, R.B.; Barreto, T.L.N.; Boaventura, R.M.; Sarni, R.O.S. Cow’s milk allergy immunoglobulin e-mediated: Intake of proteins and amino acids. Rev. Assoc. Med. Bras. 2022, 68, 1027–1032. [Google Scholar] [CrossRef]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Kouwenhoven, S.M.P.; Muts, J.; Finken, M.J.J.; Goudoever, J.B. van Low-protein infant formula and obesity risk. Nutrients 2022, 14, 2728. [Google Scholar] [CrossRef] [PubMed]
- Trakulpark, C.; Densupsoontorn, N. Twelve-month growth and accession of tolerance in infants with cow’s milk protein allergy compared among those fed with breast milk or alternative formulae. Asian Pac. J. Allergy Immunol. 2023. [Google Scholar] [CrossRef]
- Kvammen, J.A.; Thomassen, R.A.; Eskerud, M.B.; Rugtveit, J.; Henriksen, C. Micronutrient status and nutritional intake in 0- to 2-year-old children consuming a cows’ milk exclusion diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Maleknejad, S.; Dashti, K.; Safaei-Asl, A.; Roshan, Z.A.; Salehi, S.; Hassanzadeh-Rad, A. Micronutrients in infants suffering from cow’s milk allergy fed with dietary formulas and breast milk. BMC Pediatr. 2024, 24, 115. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef]
- Molloy, J.; Allen, K.; Collier, F.; Tang, M.; Ward, A.; Vuillermin, P. The potential link between gut microbiota and ige-mediated food allergy in early life. Int. J. Envion. Res. Public Health 2013, 10, 7235–7256. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the gut microbiota by breastfeeding: The gateway to allergy prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Mennini, M.; Reddel, S.; Del Chierico, F.; Gardini, S.; Quagliariello, A.; Vernocchi, P.; Valluzzi, R.L.; Fierro, V.; Riccardi, C.; Napolitano, T.; et al. Gut microbiota profile in children with IgE-mediated cow’s milk allergy and cow’s milk sensitization and probiotic intestinal persistence evaluation. Int. J. Mol. Sci. 2021, 22, 1649. [Google Scholar] [CrossRef]
- Mera-Berriatua, L.; Zubeldia-Varela, E.; Martín-Antoniano, I.; López de Maturana, E.; Rojo, D.; Bazire, R.; Cabrera-Freitag, P.; Barker-Tejeda, T.; Ubeda, C.; Barber, C.; et al. unravelling the gut microbiota of cow’s milk–allergic infants, their mothers, and their grandmothers. J. Investig. Allergol. Clin. Immunol. 2022, 32, 395–398. [Google Scholar] [CrossRef]
- Campbell, E.; Maccio-Maretto, L.; Hesser, L.A.; Kemter, A.M.; Canani, R.B.; Nocerino, R.; Paparo, L.; Patry, R.T.; Nagler, C.R. Tlr4 Regulates proinflammatory intestinal immune responses mediated by an atopic gut microbiota. bioRxiv 2022. [Google Scholar] [CrossRef]
- Aitoro, R.; Paparo, L.; Amoroso, A.; Di Costanzo, M.; Cosenza, L.; Granata, V.; Di Scala, C.; Nocerino, R.; Trinchese, G.; Montella, M.; et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 2017, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial signature in IgE-mediated food allergies. Genome Med. 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Guadamuro, L.; Espinosa-Martos, I.; Mancabelli, L.; Jiménez, S.; Molinos-Norniella, C.; Pérez-Solis, D.; Milani, C.; Rodríguez, J.M.; Ventura, M.; et al. Microbiota and derived parameters in fecal samples of infants with non-ige cow’s milk protein allergy under a restricted diet. Nutrients 2018, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy 2021, 76, 1398–1415. [Google Scholar] [CrossRef]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Sekerel, B.E.; Bingol, G.; Cullu Cokugras, F.; Cokugras, H.; Kansu, A.; Ozen, H.; Tamay, Z. An expert panel statement on the beneficial effects of human milk oligosaccharides (HMOs) in early life and potential utility of HMO-supplemented infant formula in cow’s milk protein allergy. J. Asthma Allergy 2021, 14, 1147–1164. [Google Scholar] [CrossRef]
- Ståhlberg, M.R. Breast feeding, cow milk feeding, and allergy. Allergy 1985, 40, 612–615. [Google Scholar] [CrossRef]



| Controls (n = 24) | Patients (n = 21) | p Value | ||
|---|---|---|---|---|
| Gender | Male | 12 (50.00%) | 13 (61.90%) | |
| Female | 12 (50.00%) | 8 (38.10%) | 0.423 | |
| Born at term | 24 (100.00%) | 20 (95.20%) | 0.365 | |
| Type of birth | Vaginal | 17 (70.80%) | 18 (85.70%) | |
| C-section | 7 (29.20%) | 3 (14.30%) | 0.231 | |
| Weight at birth (kg) | 3.30 ± 0.54 | 3.34 ± 0.57 | 0.665 | |
| Height at birth (cm) | 49.90 ± 2.80 | 49.30 ± 1.90 | 0.204 | |
| First allergic reaction age (m) | - | 4.46 ± 2.75 | - | |
| Weight at diagnosis (kg) | 7.40 ± 0.89 | 6.62 ± 2.04 | 0.044 | |
| Height at diagnosis (cm) | 66.10 ± 2.50 | 63.80 ± 7.50 | 0.094 | |
| CMPA type | M-CMPA | - | 12 (57.10%) | |
| NIM-CMPA | - | 9 (42.90%) | ||
| Special infant dietary replacement | EHF | - | 13 (62.00%) | |
| RF | - | 4 (19.00%) | ||
| BF | 7 (29.17%) | 4 (19.00%) | ||
| Others | 17 (70.83%) | - | ||
| Age at follow-up (m) | 11.79 ± 0.57 | 12.24 ± 3.90 | 0.973 | |
| Weight at follow-up (kg) | 9.41 ± 0.89 | 9.58 ± 1.53 | 0.982 | |
| Height at follow-up (cm) | 75.50 ± 2.60 | 74.80 ± 4.30 | 0.192 | |
| Weight-for-age at follow-up (z-score) | 0.11 ± 0.74 | 0.14 ± 1.28 | 0.927 | |
| Height-for age at follow-up (z-score) | 0.35 ± 1.09 | −0.10 ± 1.16 | 0.585 | |
| BMI-for-age at follow-up (z-score) | Underweight (−5.99 to <−1) | 2 (8.33%) | 4 (19.00%) | |
| Normal weight (−0.99 to 0.99) | 19 (73.17%) | 11 (52.40%) | ||
| Overweight risk (1 to 1.99) | 3 (12.50%) | 6 (28.6%) |
| Controls (n = 24) | Patients (n = 21) | |
|---|---|---|
| Total energy (kcal/day) | 1083.46 ± 169.26 (987.79–1208.71) | 1088.87 ± 210.65 (923.81–1265.64) |
| Protein (g/day) | 38.70 ± 11.38 (31.17–45.80) | 37.88 ± 10.80 (29.96–43.02) |
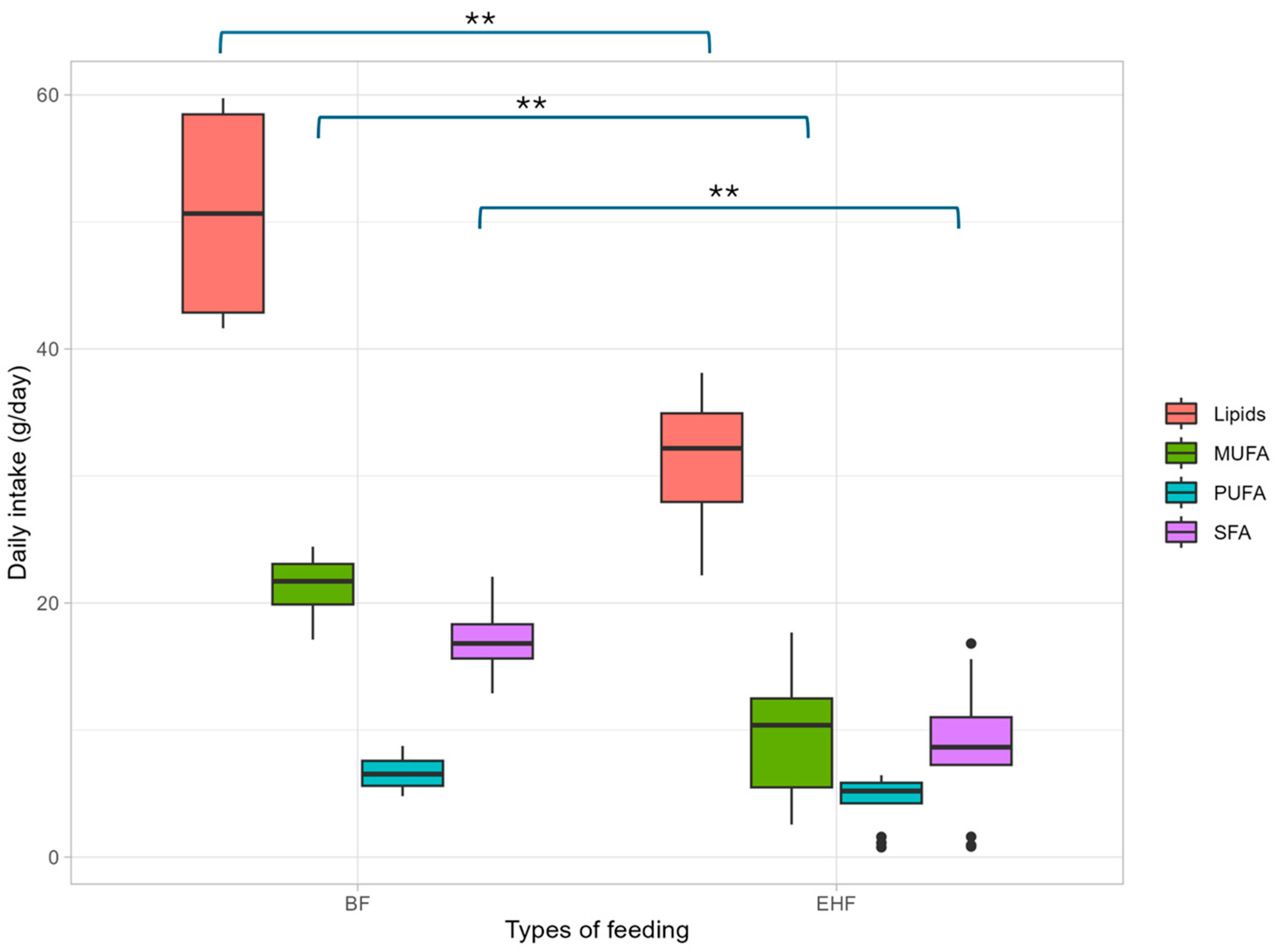
| Lípids (g/day) | 39.65 ± 8.65 (34.08–44.40) * | 35.78 ± 9.39 (29.30–38.12) |
| SFA (g/day) | 14.65 ± 5.15 (11.29–18.16) * | 10.92 ± 5.54 (7.77 ± 15.58) |
| 16:0 (Palmitic) | 4.60 ± 2.95 2.29–6.56) | 3.69 ± 3.04 (1.03 ± 6.18) |
| 18:0 (Estearic) | 1.24 ± 0.83 (0.68–1.81) | 0.95 ± 0.96 (0.22–1.33) |
| MUFA (g/day) | 14.84 ± 5.21 (10.90–19.14) | 12.41 ± 6.42 (8.85–17.12) |
| 16:1 (Palmitoleic) | 0.49 ± 0.38 (0.20–0.68) | 0.41 ± 0.47 (0.11–0.45) |
| 18:1 (Oleic) | 11.30 ± 5.59 (6.95–16.80) | 10.40 ± 6.31 (4.69–15.56) |
| PUFA (g/day) | 4.48 ± 1.84 (3.06–5.59) | 4.69 ± 2.10 (4.23–5.88) |
| 18:2 (Linoleic) | 3.27 ± 1.75 (1.76–4.42) | 3.38 ± 1.62 (2.38–4.69) |
| 18:3 (Linolenic) | 0.41 ± 0.24 (0.20–0.57) | 0.45 ± 0.24 (0.24–0.59) |
| Carbohydrates (g/day) | 142.94 ± 29.04 (118.96–163.21) | 153.83 ± 37.56 (129.46–187.63) |
| Total sugars | 69.28 ± 15.86 (59.91–77.22) | 57.12 ± 26.14 (36.97–76.10) |
| Dietary fiber (g/day) | 9.63 ± 4.62 (6.81–12.83) * | 12.89 ± 5.39 (9.15–16.05) |
| Controls (n = 24) | Patients (n = 21) | |
|---|---|---|
| Antioxidants | ||
| Vitamin C (mg/d) | 110.33 ± 50.90 (68.85–158.95) * | 145.62 ± 56.71 (102.91–187.02) |
| Vitamin E (mg/d) | 6.39 ± 4.23 (3.26–8.78) * | 9.04 ± 3.65 (6.65–11.87) |
| B Vitamins | ||
| Vitamin B-1 (mg/d) | 0.88 ± 0.46 (0.52–1.31) | 0.91 ± 0.30 (0.66–1.14) |
| Vitamin B-2 (mg/d) | 1.21 ± 0.62 (0.85–1.37) | 1.09 ± 0.36 (0.84–1.29) |
| Vitamin B-3 (mg/d) | 10.16 ± 4.1 (7.82–11.69) ** | 14.34 ± 4.58 (10.88–18.01) |
| Vitamin B-6 (mg/d) | 1.40 ± 0.52 (1.02 ± 1.72) | 1.49 ± 0.41 (1.07–1.73) |
| Folic acid (µg/d) | 185.92 ± 83.57 (118.40–247.77) | 218.11 ± 120.28 (130.04–259.84) |
| Vitamin B-12 (µg/d) | 2.02 ± 1.03 (1.32–2.62) | 1.83 ± 0.75 (1.41–2.28) |
| Bone-related nutrients | ||
| Calcium (mg/d) | 683.57 ± 276.18 (467.39–909.95) | 562.02 ± 183.62 (430.83–654.23) |
| Phosphorus (mg/d) | 623.14 ± 195.43 (494.72–732.93) | 542.34 ± 183.62 (494.27–607.00) |
| Magnesium (mg/d) | 144.92 ± 37.58 (118.72–175.72) | 139.98 ± 43.99 (111.80–163.73) |
| Vitamin D (µg/d) | 5.72 ± 4.06 (1.55–8.92) * | 8.9. ± 5.38 (6.66–10.88) |
| Other micronutrients | ||
| Sodium (mg/d) | 467.70 ± 265.02 (280.88–550.44) | 369.89 ± 163.48 (246.30–507.50) |
| Potassium (mg/d) | 1880.49 ± 479.35 (1605.71–2126.69) | 1799.43 ± 512.76 (1412.60–2211.90) |
| Iron (mg/d) | 7.32 ± 3.42 (4.63–9.12) | 9.78 ± 4.51 (6.13–11.29) |
| Selenium (µg/d) | 35.84 ± 24.29 (24.42–41.98) | 29.80–16.31 (16.84–38.39) |
| Manganese (µg/d) | 2.71 ± 5.94 (0.74–1.56) | 1.06–0.46 (0.71–1.37) |
| Iodine (mg/d) | 67.26 ± 42.55 (36.77–94.28) | 67.57 ± 27.95 (56.95–90.07) |
| Zinc (mg/d) | 4.97 ± 2.39 (3.39–6.61) | 5.12 ± 1.75 (4.14–6.30) |
| Vitamin A/ (µg/d) | 741.72 ± 264.11 (584.58–912.13) | 841.16 ± 426.23 (553.55–1119.80) |
| Vitamin K (µg/d) | 49.57 ± 34.00 (27.13–60.29) * | 125.42 ± 207.17 (44.48–90.09) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, A.M.; Navarro, S.; Carvajal, I.; García, A.; Suárez, M.; Toyos, P.; Rodríguez, S.; Jimenez, S.; González, D.; Molinos, C.; et al. Evolutive Study of Dietary Aspects and Intestinal Microbiota of Pediatric Cohort with Cow’s Milk Protein Allergy. Children 2024, 11, 1113. https://doi.org/10.3390/children11091113
Castro AM, Navarro S, Carvajal I, García A, Suárez M, Toyos P, Rodríguez S, Jimenez S, González D, Molinos C, et al. Evolutive Study of Dietary Aspects and Intestinal Microbiota of Pediatric Cohort with Cow’s Milk Protein Allergy. Children. 2024; 11(9):1113. https://doi.org/10.3390/children11091113
Chicago/Turabian StyleCastro, Ana M., Sandra Navarro, Ignacio Carvajal, Agueda García, Marta Suárez, Paula Toyos, Silvia Rodríguez, Santiago Jimenez, David González, Cristina Molinos, and et al. 2024. "Evolutive Study of Dietary Aspects and Intestinal Microbiota of Pediatric Cohort with Cow’s Milk Protein Allergy" Children 11, no. 9: 1113. https://doi.org/10.3390/children11091113
APA StyleCastro, A. M., Navarro, S., Carvajal, I., García, A., Suárez, M., Toyos, P., Rodríguez, S., Jimenez, S., González, D., Molinos, C., Pérez-Solís, D., Fernández, P., Margolles, A., Díaz-Martín, J. J., Gutiérrez-Díaz, I., & Delgado, S. (2024). Evolutive Study of Dietary Aspects and Intestinal Microbiota of Pediatric Cohort with Cow’s Milk Protein Allergy. Children, 11(9), 1113. https://doi.org/10.3390/children11091113








