Association of Right Ventricular Dysfunction with Risk of Neurodevelopmental Impairment in Infants with Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
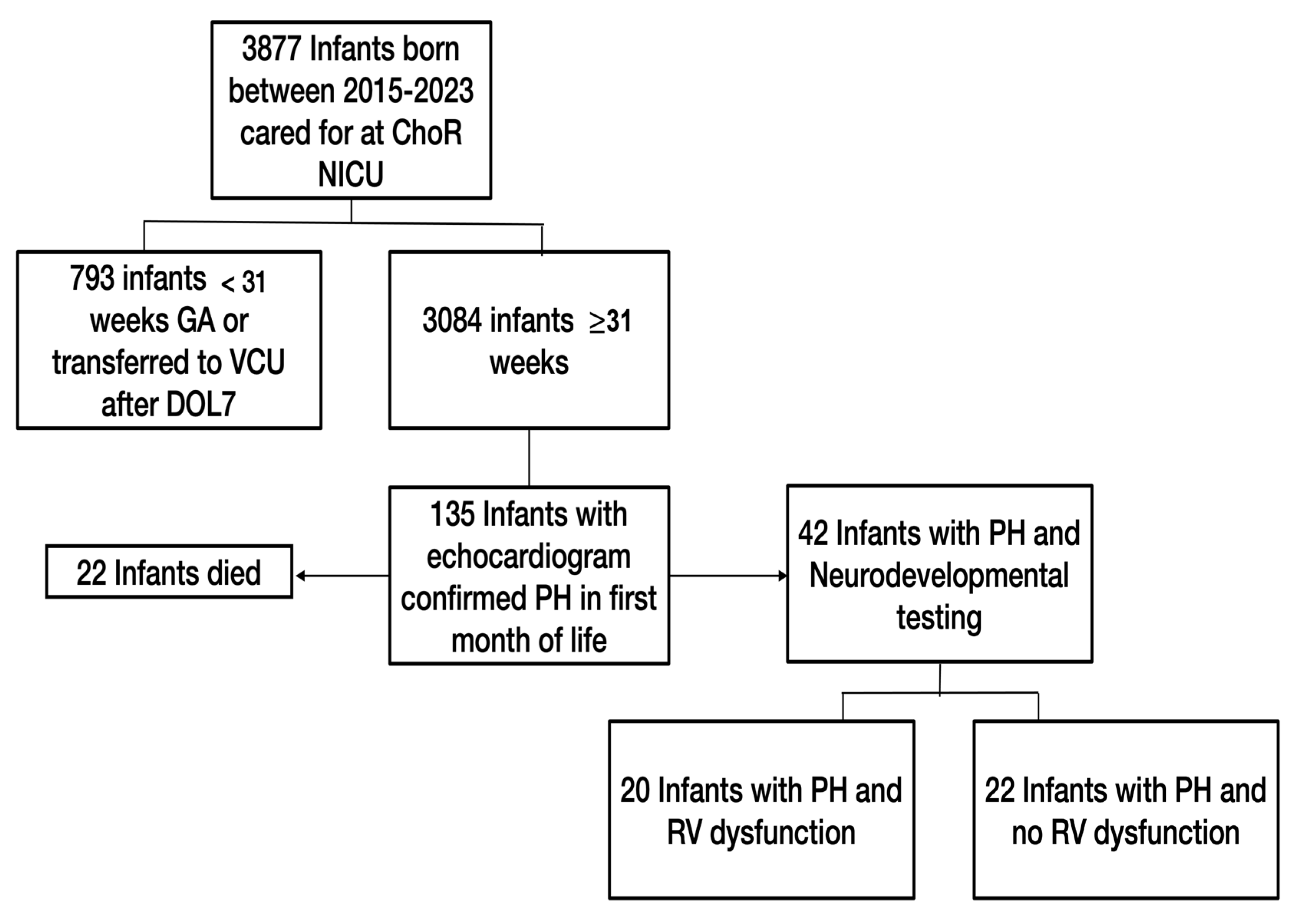
2.2. Study Population
2.3. Data Inclusion and Analysis
2.4. Data Analysis
3. Results
3.1. Population Characteristics
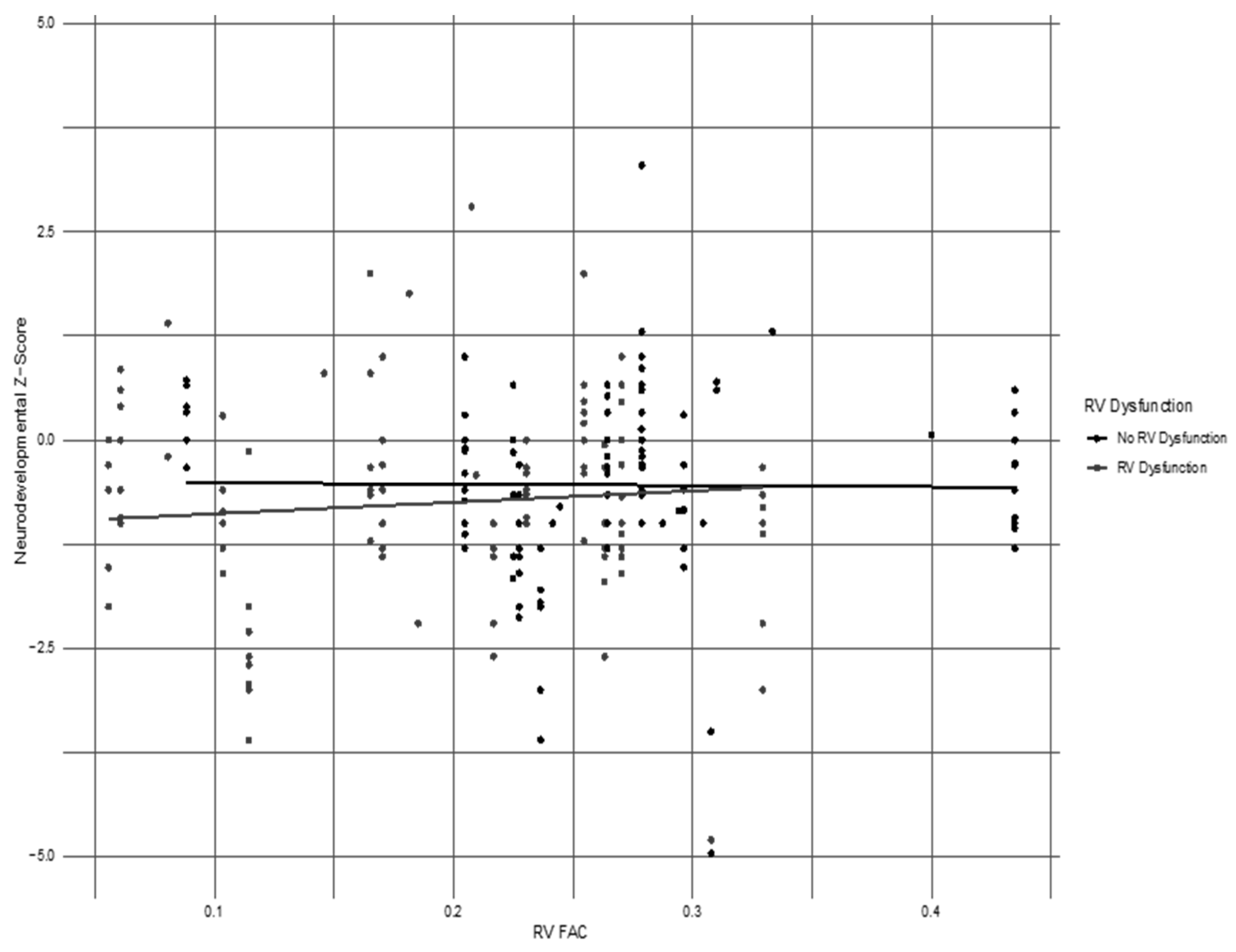
3.2. Assessment of the RV FAC as a Marker of RV Dysfunction
3.3. Neurodevelopmental Impairment in Cohort
3.4. Morbidities and Hospital Characteristics in Population Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sankaran, D.; Lakshminrusimha, S. Pulmonary hypertension in the newborn- etiology and pathogenesis. Semin. Fetal Neonatal Med. 2022, 27, 101381. [Google Scholar] [CrossRef] [PubMed]
- Mandell, E.; Kinsella, J.P.; Abman, S.H. Persistent pulmonary hypertension of the newborn. Pediatr. Pulmonol. 2021, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Tissot, C. Echocardiographic Evaluation of Transitional Circulation for the Neonatologists. Front. Pediatr. 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, P.H.; Davidson, D.; Spivak, L.; Straube, R.; Rhines, J.; Chang, C.T. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with inhaled nitric oxide. J. Pediatr. 2002, 140, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Fraisse, A.; Geva, T.; Gaudart, J.; Wessel, D.L. Doppler echocardiographic predictors of outcome in newborns with persistent pulmonary hypertension. Cardiol. Young 2004, 14, 277–283. [Google Scholar] [CrossRef]
- Ruoss, J.L.; Moronta, S.C.; Bazacliu, C.; Giesinger, R.E.; McNamara, P.J. Management of cardiac dysfunction in neonates with pulmonary hypertension and the role of the ductus arteriosus. Semin. Fetal Neonatal Med. 2022, 27, 101368. [Google Scholar] [CrossRef]
- Hoette, S.; Creuzé, N.; Günther, S.; Montani, D.; Savale, L.; Jais, X.; Parent, F.; Sitbon, O.; Rochitte, C.E.; Simonneau, G.; et al. RV Fractional Area Change and TAPSE as Predictors of Severe Right Ventricular Dysfunction in Pulmonary Hypertension: A CMR Study. Lung 2018, 196, 157–164. [Google Scholar] [CrossRef]
- Malowitz, J.R.; Forsha, D.E.; Smith, P.B.; Cotten, C.M.; Barker, P.C.; Tatum, G.H. Right ventricular echocardiographic indices predict poor outcomes in infants with persistent pulmonary hypertension of the newborn. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Boode, W.P.; Singh, Y.; Molnar, Z.; Schubert, U.; Savoia, M.; Sehgal, A.; Levy, P.T.; McNamara, P.J.; El-Khuffash, A. Application of Neonatologist Performed Echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr. Res. 2018, 84 (Suppl. 1), 68–77. [Google Scholar] [CrossRef]
- Jain, A.; Mohamed, A.; El-Khuffash, A.; Connelly, K.A.; Dallaire, F.; Jankov, R.P.; McNamara, P.J.; Mertens, L. A comprehensive echocardiographic protocol for assessing neonatal right ventricular dimensions and function in the transitional period: Normative data and z scores. J. Am. Soc. Echocardiogr. 2014, 27, 1293–1304. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Lee, N.R.; Vaver, K.N.; Werner, D.; Fashaw, L.; Hale, K.; Waas, N. School-age outcomes of newborns treated for persistent pulmonary hypertension. J. Perinatol. 2010, 30, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, V.; Nielsen, L.H.; Klokker, M.; Greisen, G. Follow-up of 5- to 11-year-old children treated for persistent pulmonary hypertension of the newborn. Acta Paediatr. 2009, 98, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Berti, A.; Janes, A.; Furlan, R.; Macagno, F. High prevalence of minor neurologic deficits in a long-term neurodevelopmental follow-up of children with severe persistent pulmonary hypertension of the newborn: A cohort study. Ital. J. Pediatr. 2010, 13, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campbell, S.K.; Hedeker, D. Validity of the Test of Infant Motor Performance for discriminating among infants with varying risk for poor motor outcome. J. Pediatr. 2001, 139, 546–551. [Google Scholar] [CrossRef] [PubMed]
- van Haastert, I.C.; de Vries, L.S.; Helders, P.J.; Jongmans, M.J. Early gross motor development of preterm infants according to the Alberta Infant Motor Scale. J. Pediatr. 2006, 149, 617–622. [Google Scholar] [CrossRef]
- Bayley, N. Test Review: Bayley Scales of Infant and Toddler Development, 3rd ed.; Harcourt Assessment: San Antonio, TX, USA, 2006. [Google Scholar]
- Nandula, P.S.; Shah, S.D. Persistent Pulmonary Hypertension of the Newborn. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Giesinger, R.E.; El Shahed, A.I.; Castaldo, M.P.; Breatnach, C.R.; Chau, V.; Whyte, H.; El-Khuffash, A.F.; Mertens, L.; McNamara, P.J. Impaired Right Ventricular Performance Is Associated with Adverse Outcome after Hypoxic Ischemic Encephalopathy. Am. J. Respir. Crit. Care Med. 2019, 200, 1294–1305. [Google Scholar] [CrossRef]
- Levy, P.T.; Dioneda, B.; Holland, M.R.; Sekarski, T.J.; Lee, C.K.; Mathur, A.; Cade, W.T.; Cahill, A.G.; Hamvas, A.; Singh, G.K. Right ventricular function in preterm and term neonates: Reference values for right ventricle areas and fractional area of change. J. Am. Soc. Echocardiogr. 2015, 28, 559–569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giesinger, R.E.; El Shahed, A.I.; Castaldo, M.P.; Bischhoff, A.R.; Chau, V.; Whyte, H.E.A.; El-Khuffash, A.F.; Mertens, L.; McNamara, P. J Neurodevelopmental outcome following hypoxic ischaemic encephalopathy and therapeutic hypothermia is related to right ventricular performance at 24-hour postnatal age. Arch. Dis. Child Fetal Neonatal Ed. 2022, 107, 70–75. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Kennaugh, J.M.; Moreland, S.G.; Fashaw, L.M.; Hale, K.A.; Torielli, F.M.; Abman, S.H.; Kinsella, J.P. Longitudinal followup of a cohort of newborn infants treated with inhaled nitric oxide for persistent pulmonary hypertension. J. Pediatr. 1997, 131, 70–75. [Google Scholar] [CrossRef]
- Konduri, G.G.; Vohr, B.; Robertson, C.; Sokol, G.M.; Solimano, A.; Singer, J.; Ehrenkranz, R.A.; Sihghal, N.; Wright, L.; van Meurs, K.; et al. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: Neurodevelopmental follow-up. J. Pediatr. 2007, 150, 235–240.e1. [Google Scholar] [CrossRef]
- Jain, A.; El-Khuffash, A.F.; van Herpen, C.H.; Resende, M.H.F.; Giesinger, R.E.; Weisz, D.; Mertens, L.; Jankov, R.; McNamara, J.P. Cardiac Function and Ventricular Interactions in Persistent Pulmonary Hypertension of the Newborn. Pediatr. Crit. Care Med. 2021, 22, e145–e157. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.U.; Jabri, A.; Hamade, H.; Al Abdouh, A.; Mahanna, M.; Haddadin, F.; Nasser, F.; Hammad, N.; Abu Jazar, D.; Toumar, A.J.; et al. Predicting the Severity and Outcome of Persistent Pulmonary Hypertension of the Newborn Using New Echocardiography Parameters. Curr. Probl. Cardiol. 2023, 48, 101181. [Google Scholar] [CrossRef] [PubMed]
- Rohana, J.; Boo, N.Y.; Chandran, V.; Sarvananthan, R. Neurodevelopmental outcome of newborns with persistent pulmonary hypertension. Malays. J. Med. Sci. 2011, 18, 58–62. [Google Scholar] [PubMed]

| Groups | ||||
|---|---|---|---|---|
| Maternal Characteristics | Overall, n = 42 1 | No RV Dysfunction, n = 22 1 | RV Dysfunction, n = 20 1 | p-Value 2 |
| Age | 29.7 (5.4) | 30.0 (4.8) | 29.3 (6.1) | 0.8 |
| Race | 0.2 | |||
| White | 21 (50%) | 14 (64%) | 7 (35%) | |
| Black | 18 (43%) | 7 (32%) | 11 (55%) | |
| Other | 3 (7.1%) | 1 (4.5%) | 2 (10%) | |
| Average BMI | 34 (11) | 32 (13) | 37 (9) | 0.10 |
| Tobacco use during pregnancy | 4 (9.5%) | 3 (14%) | 1 (5.0%) | 0.6 |
| SSRI use during pregnancy | 4 (9.5%) | 3 (14%) | 1 (5.0%) | 0.6 |
| Asthma history | 1 (2.4%) | 0 (0%) | 1 (5.0%) | 0.5 |
| Gestational hypertension | 6 (14%) | 2 (9.1%) | 4 (20%) | 0.4 |
| Gestational diabetes | 7 (17%) | 4 (18%) | 3 (15%) | >0.9 |
| Prenatal steroids given | 9 (21%) | 7 (32%) | 2 (10%) | 0.13 |
| Oligohydramnios | 3 (7.1%) | 1 (4.5%) | 2 (10%) | 0.6 |
| Vaginal delivery | 19 (45%) | 9 (41%) | 10 (50%) | 0.6 |
| C-section | 23 (55%) | 13 (59%) | 10 (50%) | 0.6 |
| Groups | ||||
|---|---|---|---|---|
| Infant Characteristics | Overall, n = 42 1 | No RV Dysfunction, n = 22 1 | RV Dysfunction, n = 20 1 | p-Value 2 |
| Gestational age (weeks) | 37.07 (2.64) | 37.14 (2.66) | 37.00 (2.70) | 0.9 |
| Gestational age less than or equal to 35 weeks | 13 (31%) | 7 (32%) | 6 (30%) | 0.9 |
| Birth weight (grams) | 2847 (780) | 2665 (789) | 3047 (737) | 0.2 |
| Size for gestational age | 0.3 | |||
| AGA | 28 (67%) | 15 (68%) | 13 (65%) | |
| LGA | 5 (12%) | 1 (4.5%) | 4 (20%) | |
| SGA | 9 (21%) | 6 (27%) | 3 (15%) | |
| Male gender | 27 (64%) | 12 (55%) | 15 (75%) | 0.2 |
| Apgar at 5 min (average score) | 6 (2) | 7 (3) | 6 (2) | 0.4 |
| Apgar at 5 min (number with score less than 5) | 8 (20%) | 5 (23%) | 3 (16%) | 0.7 |
| Intraventricular hemorrhage | 9 (21%) | 6 (27%) | 3 (15%) | 0.5 |
| Seizures | 8 (19%) | 5 (23%) | 3 (15%) | 0.7 |
| Respiratory distress syndrome | 16 (38%) | 10 (45%) | 6 (30%) | 0.3 |
| Congenital heart disease | 5 (12%) | 1 (4.5%) | 4 (20%) | 0.2 |
| Meconium aspiration syndrome | 12 (29%) | 7 (32%) | 5 (25%) | 0.6 |
| Hypoxic-ischemic encephalopathy | 10 (24%) | 6 (27%) | 4 (20%) | 0.7 |
| Pulmonary hypoplasia | 4 (9.5%) | 1 (4.5%) | 3 (15%) | 0.3 |
| RV Dysfunction | No RV Dysfunction | |||||
|---|---|---|---|---|---|---|
| TIMP/AIMS 3–6 mo n = 18/20 | Bayley 1 year n = 11/20 | Bayley 2 years n = 4/20 | TIMP/AIMS 3–6 mo n = 21/22 | Bayley 1 year n = 9/22 | Bayley 2 years n = 4/22 | |
| Gross motor delay % (n) | 22 (4) | 36 (4) | 25 (1) | 19 (4) | 55 (5) | 25 (1) |
| Fine motor delay % (n) | x | 18 (2) | 25 (1) | x | 11 (1) | 0 |
| Expressive language delay % (n) | x | 27 (3) | 50 (2) | x | 44 (4) | 0 |
| Receptive language delay % (n) | x | 72 (8) | 25 (1) | x | 44 (4) | 25 (1) |
| Cognitive delay % (n) | x | 18 (2) | 25 (1) | x | 11 (1) | 25 (1) |
| Lost to follow-up % (n) | 10 (2) | 45 (9) | 80 (16) | 0.5 (1) | 59 (13) | 81 (18) |
| Neurodevelopment Test | RV FAC Coefficient | 95% CI Lower | 95% CI Upper | Standard Error | Neurodevelopment Z-Score | p-Value |
|---|---|---|---|---|---|---|
| TIMP | −0.50 | −7.12 | 6.12 | 3.38 | −0.15 | 0.88 |
| AIMS | −39.73 | −136.21 | 56.75 | 49.22 | −0.81 | 0.45 |
| Bayley cognitive | −0.50 | −9.60 | 8.60 | 4.64 | −0.11 | 0.91 |
| Bayley receptive language | 2.29 | −2.31 | 6.89 | 2.35 | 0.98 | 0.34 |
| Bayley expressive language | −0.24 | −5.13 | 4.65 | 2.50 | −0.10 | 0.92 |
| Bayley comprehensive language | 1.13 | −3.88 | 6.14 | 2.56 | 0.44 | 0.66 |
| Bayley fine motor | −1.02 | −8.93 | 6.89 | 4.04 | −0.25 | 0.80 |
| Bayley gross motor | −5.48 | −13.17 | 2.21 | 3.92 | −1.40 | 0.18 |
| Bayley comprehensive motor | −3.73 | −12.33 | 4.87 | 4.39 | −0.85 | 0.40 |
| Groups | ||||
|---|---|---|---|---|
| Secondary Outcomes | Overall, n = 42 1 | No RV Dysfunction, n = 22 1 | RV Dysfunction, n = 20 1 | p-Value 2 |
| Average pH level | 7.22 (0.15) | 7.24 (0.18) | 7.20 (0.12) | 0.2 |
| Resuscitated at birth | 29 (69%) | 15 (68%) | 14 (70%) | 0.9 |
| Therapeutic hypothermia performed | 9 (21%) | 5 (23%) | 4 (20%) | >0.9 |
| Inhaled nitric oxide | 33 (79%) | 17 (77%) | 16 (80%) | >0.9 |
| Duration of iNO (days) | 5 (5) | 5 (4) | 6 (5) | 0.3 |
| ECMO performed | 13 (32%) | 5 (24%) | 8 (40%) | 0.3 |
| Duration of ECMO (days) | 3 (5) | 2 (3) | 4 (6) | 0.2 |
| Milrinone | 17 (40%) | 4 (18%) | 13 (65%) | 0.002 |
| Vasopressors | 20 (48%) | 8 (36%) | 12 (60%) | 0.13 |
| Diuretics | 25 (60%) | 11 (50%) | 14 (70%) | 0.2 |
| Sedation | 23 (55%) | 11 (50%) | 12 (60%) | 0.5 |
| Days of sedation | 15 (23) | 12 (16) | 18 (29) | 0.6 |
| Mechanical ventilation required | 38 (90%) | 18 (82%) | 20 (100%) | 0.11 |
| Days on mechanical ventilation | 14 (14) | 12 (12) | 16 (16) | 0.4 |
| Oxygen required at discharge | 8 (19%) | 4 (18%) | 4 (20%) | >0.9 |
| Tracheostomy required | 1 (2.4%) | 1 (4.5%) | 0 (0%) | >0.9 |
| Gastrostomy tube required at discharge | 6 (14%) | 3 (14%) | 3 (15%) | >0.9 |
| Nasogastric tube required at discharge | 8 (19%) | 6 (27%) | 2 (10%) | 0.2 |
| Length of stay (days) | 50 (38) | 45 (36) | 56 (40) | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero Orozco, R.; Mohammed, T.A.; Carter, K.; Brown, S.; Miller, S.; Sabo, R.T.; Joseph, M.C.; Truong, U.; Nair, M.; Anderson, V.; et al. Association of Right Ventricular Dysfunction with Risk of Neurodevelopmental Impairment in Infants with Pulmonary Hypertension. Children 2024, 11, 1121. https://doi.org/10.3390/children11091121
Romero Orozco R, Mohammed TA, Carter K, Brown S, Miller S, Sabo RT, Joseph MC, Truong U, Nair M, Anderson V, et al. Association of Right Ventricular Dysfunction with Risk of Neurodevelopmental Impairment in Infants with Pulmonary Hypertension. Children. 2024; 11(9):1121. https://doi.org/10.3390/children11091121
Chicago/Turabian StyleRomero Orozco, Rossana, Tazuddin A. Mohammed, Kerri Carter, Shaaron Brown, Stephen Miller, Roy T. Sabo, Meredith Campbell Joseph, Uyen Truong, Megha Nair, Victoria Anderson, and et al. 2024. "Association of Right Ventricular Dysfunction with Risk of Neurodevelopmental Impairment in Infants with Pulmonary Hypertension" Children 11, no. 9: 1121. https://doi.org/10.3390/children11091121






