Lactoferrin in Pediatric Chronic Kidney Disease and Its Relationship with Cardiovascular Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Cardiovascular Assessment
2.3. Determination of Lactoferrin Concentration
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
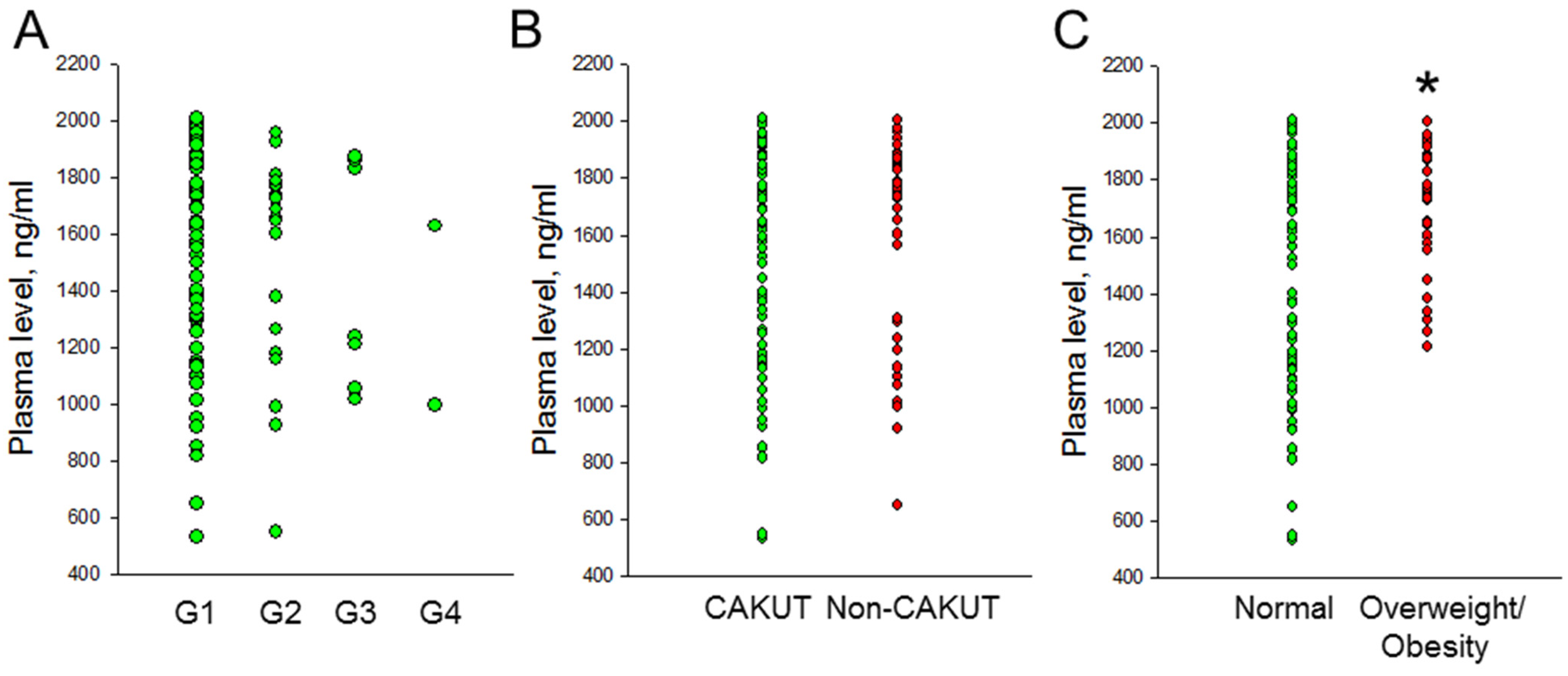
3.2. Plasma Lactoferrin Concentration
3.3. Cardiovascular Assessment
3.4. Plasma LF Concentration vs. ABPM Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, J.R.; Kalantar-Zadeh, K.; Schaefer, F.; World Kidney Day Steering Committee. World Kidney Day 2016: Averting the legacy of kidney disease-focus on childhood. Pediatr. Nephrol. 2016, 31, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. The First Thousand Days: Kidney Health and Beyond. Healthcare 2021, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.J.; Mitsnefes, M. Cardiovascular Disease in Children and Adolescents with Chronic Kidney Disease. Semin. Nephrol. 2018, 38, 559–569. [Google Scholar] [CrossRef]
- Lousa, I.; Reis, F.; Beirão, I.; Alves, R.; Belo, L.; Santos-Silva, A. New Potential Biomarkers for Chronic Kidney Disease Management—A Review of the Literature. Int. J. Mol. Sci. 2020, 22, 43. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Cardiovascular Risks of Hypertension: Lessons from Children with Chronic Kidney Disease. Children 2022, 9, 1650. [Google Scholar] [CrossRef]
- Cummins, T.D.; Korte, E.A.; Bhayana, S.; Merchant, M.L.; Barati, M.T.; Smoyer, W.E.; Klein, J.B. Advances in proteomic profiling of pediatric kidney diseases. Pediatr. Nephrol. 2022, 37, 2255–2265. [Google Scholar] [CrossRef]
- Chen, W.L.; Tain, Y.L.; Chen, H.E.; Hsu, C.N. Cardiovascular Disease Risk in Children with Chronic Kidney Disease: Impact of Apolipoprotein C-II and Apolipoprotein C-III. Front. Pediatr. 2021, 9, 706323. [Google Scholar] [CrossRef]
- Hsu, C.N.; Liao, W.T.; Chen, W.L.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Plasma and Urinary Platelet Factor 4 as Biomarkers for Cardiovascular Risk in Children with Chronic Kidney Disease. Biomedicines 2023, 11, 3318. [Google Scholar] [CrossRef] [PubMed]
- Shini, V.S.; Udayarajan, C.T.; Nisha, P. A comprehensive review on lactoferrin: A natural multifunctional glycoprotein. Food Funct. 2022, 13, 11954–11972. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C.; Zhang, J. Lactoferrin and Its Detection Methods: A Review. Nutrients 2021, 13, 2492. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chiu, I.J.; Lin, Y.F.; Chen, Y.J.; Lee, Y.H.; Chiu, H.W. Lactoferrin Contributes a Renoprotective Effect in Acute Kidney Injury and Early Renal Fibrosis. Pharmaceutics 2020, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Abrink, M.; Larsson, E.; Gobl, A.; Hellman, L. Expression of lactoferrin in the kidney: Implications for innate immunity and iron metabolism. Kidney Int. 2000, 57, 2004–2010. [Google Scholar] [CrossRef]
- Urbina, E.M.; Williams, R.V.; Alpert, B.S.; Collins, R.T.; Daniels, S.R.; Hayman, L.; Jacobson, M.; Mahoney, L.; Mietus-Snyder, M.; Rocchini, A.; et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: Recommendations for standard assessment for clinical research: A scientific statement from the American Heart Association. Hypertension 2009, 54, 919–950. [Google Scholar] [CrossRef]
- Taal, M.W. Arterial stiffness in chronic kidney disease: An update. Curr. Opin. Nephrol. Hypertens. 2014, 23, 169–173. [Google Scholar] [CrossRef]
- Kupferman, J.C.; Aronson Friedman, L.; Cox, C.; Flynn, J.; Furth, S.; Warady, B.; Mitsnefes, M.; CKiD Study Group. BP control and left ventricular hypertrophy regression in children with CKD. J. Am. Soc. Nephrol. 2014, 25, 167–174. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Nicolaou, N.; Renkema, K.Y.; Bongers, E.M.; Giles, R.H.; Knoers, N.V. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat. Rev. Nephrol. 2015, 11, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Kubota, M.; Nagai, A.; Mamemoto, K.; Tokuda, M. Hyperuricemia in obese children and adolescents: The relationship with metabolic syndrome. Pediatr. Rep. 2010, 2, e12. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.M. Dyslipidemia in children and adolescents: When and how to diagnose and treat? Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Administration. Ministry of Health and Welfare. Definition of Underweight, Normal Weight, Overweight, and Obesity in Children and Adolescents. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=542&pid=705 (accessed on 4 August 2024).
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; Falkner, B.; Flinn, S.K.; Gidding, S.S.; Goodwin, C. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.; Stergiou, W.E.; Witte, K.; Soergelm, M.; Mehls, O.; Schaefer, F. German Working Group on Pediatric Hypertension. Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. J. Hypertens. 2002, 20, 1995–2007. [Google Scholar]
- Raina, R.; Polaconda, S.; Nair, N.; Chakraborty, R.; Sethi, S.; Krishnappa, V.; Kapur, G.; Mhanna, M.; Kusumi, K. Association of pulse pressure, pulse pressure index, and ambulatory arterial stiffness index with kidney function in a cross-sectional pediatric chronic kidney disease cohort from the CKiD study. J. Clin. Hypertens. 2020, 22, 1059–1069. [Google Scholar] [CrossRef]
- Kollias, A.; Stergiou, G.S.; Dolan, E.; O’Brien, E. Ambulatory arterial stiffness index: A systematic review and meta-analysis. Atherosclerosis 2012, 224, 291–301. [Google Scholar] [CrossRef]
- Daniels, S.R.; Kimball, T.R.; Morrison, J.A.; Khoury, P.; Meyer, R.A. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am. J. Cardiol. 1995, 76, 699–701. [Google Scholar] [CrossRef]
- Halbach, S. Practical application of ABPM in the pediatric nephrology clinic. Pediatr. Nephrol. 2020, 35, 2067–2076. [Google Scholar] [CrossRef]
- Zahan, M.S.; Ahmed, K.A.; Moni, A.; Sinopoli, A.; Ha, H.; Uddin, M.J. Kidney protective potential of lactoferrin: Pharmacological insights and therapeutic advances. Korean J. Physiol. Pharmacol. 2022, 26, 1–13. [Google Scholar] [CrossRef]
- Kim, J.Y.; Campbell, L.E.; Shaibi, G.Q.; Coletta, D.K. Gene expression profiling and association of circulating lactoferrin level with obesity-related phenotypes in Latino youth. Pediatr. Obes. 2015, 10, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Veilleux, A.; Pouliot, Y.; Lamarche, B.; Beaulieu, J.F.; Hould, F.S.; Richard, D.; Tchernof, A.; Levy, E. Plasma Lactoferrin Levels Positively Correlate with Insulin Resistance despite an Inverse Association with Total Adiposity in Lean and Severely Obese Patients. PLoS ONE 2016, 11, e0166138. [Google Scholar] [CrossRef] [PubMed]
- Jamka, M.; Krzyżanowska-Jankowska, P.; Mądry, E.; Lisowska, A.; Bogdański, P.; Walkowiak, J. No Difference in Lactoferrin Levels between Metabolically Healthy and Unhealthy Obese Women. Nutrients 2019, 11, 1976. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; An, Q.; Huang, K.; Dai, Y.; Meng, Q.; Zhang, Y. Unlocking the power of Lactoferrin: Exploring its role in early life and its preventive potential for adult chronic diseases. Food Res. Int. 2024, 182, 114143. [Google Scholar] [CrossRef] [PubMed]
- Brady, T.M.; Schneider, M.F.; Flynn, J.T.; Cox, C.; Samuels, J.; Saland, J.; White, C.T.; Furth, S.; Warady, B.A.; Mitsnefes, M. Carotid intima-media thickness in children with CKD: Results from the CKiD study. Clin. J. Am. Soc. Nephrol. 2012, 7, 1930–1937. [Google Scholar] [CrossRef]
- Day, T.G.; Park, M.; Kinra, S. The association between blood pressure and carotid intima-media thickness in children: A systematic review. Cardiol. Young 2017, 27, 1295–1305. [Google Scholar] [CrossRef]
- Conkar, S.; Mir, S.; Dogan, E.; Ülger Tutar, Z. Association of Vitamin D Deficiency with Increased Pulse Wave Velocity and Augmentation Index in Children with Chronic Kidney Disease. Iran. J. Kidney Dis. 2018, 12, 275–280. [Google Scholar]
- Hsu, C.N.; Hou, C.Y.; Lu, P.C.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Association between Acrylamide Metabolites and Cardiovascular Risk in Children with Early Stages of Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 5855. [Google Scholar] [CrossRef]
- Safaeian, L.; Zabolian, H. Antioxidant effects of bovine lactoferrin on dexamethasone-induced hypertension in rat. ISRN Pharmacol. 2014, 2014, 943523. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chen, W.L.; Liao, W.T.; Hsu, C.N. Lactoferrin Supplementation during Pregnancy and Lactation Protects Adult Male Rat Offspring from Hypertension Induced by Maternal Adenine Diet. Nutrients 2024, 16, 2607. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | CKD Stage G1 | CKD Stage G2–G4 |
|---|---|---|
| n = 73 | n = 29 | |
| Age, years | 9.7 (6.1–12.8) | 10.9 (6.8–15.8) |
| Male | 35 (46.6%) | 22 (75.9%) * |
| CAKUT | 45 (61.6%) | 22 (75.9%) |
| Body height (percentile) | 50 (25–85) | 25 (15–75) |
| Body weight (percentile) | 50 (25–85) | 50 (15–85) |
| Body mass index (kg·m−2) | 17.5 (15.4–20.5) | 19 (15.4–21) |
| Systolic BP (percentile) | 50 (50–95) | 90 (50–99) * |
| Diastolic BP (percentile) | 50 (50–95) | 90 (50–95) |
| Blood urea nitrogen (mg/dL) | 12 (10–14) | 16 (13–24.5) * |
| Creatinine (mg/dL) | 0.51 (0.43–0.6) | 0.8 (0.65–1.16) * |
| eGFR (mL/min/1.73 m2) | 107.3 (98.3–119.4) | 72.7 (52–84.9) * |
| UTCR (mg/g) | 65.7 (38.4–113.9) | 152.2 (37.5–336.4) |
| Hemoglobin (g/dL) | 13.5 (13–14.1) | 13.5 (12.7–14.4) |
| Hematocrit (%) | 40 (38.5–41.4) | 40.3 (38.9–42.8) |
| Fasting glucose (mg/dL) | 87 (84–92) | 90 (82–92) |
| Total cholesterol (mg/dL) | 172 (150–207) | 185 (144–208) |
| Low-density lipoprotein (mg/dL) | 94.5 (78–117.5) | 100 (74–122) |
| Triglyceride (mg/dL) | 67 (45–92) | 66.5 (51–108.8) |
| Sodium (mmol/L) | 140 (139–141) | 140 (139–141) |
| Potassium (mmol/L) | 4.3 (4.1–4.5) | 4.5 (4.2–4.8) |
| Uric acid (mg/dL) | 4.7 (4.2–5.5) | 6.7 (5.6–7.5) * |
| Calcium (mg/dL) | 9.9 (9.7–10.1) | 10.1 (9.7–10.3) |
| Phosphate (mg/dL) | 4.9 (4.6–5.2) | 4.8 (4.5–5.2) |
| Hypertension (by office BP) | 26 (35.6%) | 13 (44.8%) |
| Overweight/obesity | 24 (32.9%) | 11 (37.9%) |
| Hyperlipidemia | 23 (31.5%) | 11 (37.9%) |
| Hyperuricemia | 5 (6.8%) | 15 (51.7%) * |
| Proteinuria | 13 (17.8%) | 12 (41.4) |
| CV Assessment | Normal | Overweight/Obesity |
|---|---|---|
| n = 43 | n = 19 | |
| Left ventricular mass (g) | 73.8 (56.6–82.2) | 101 (86.9–140) * |
| LVMI (g/m2.7) | 25 (22–29.4) | 32 (24.9–44.4) * |
| cIMT | 0.35 (0.32–0.42) | 0.38 (0.31–0.42) |
| Augmentation index | 1.1 (−10.5–8.6) | −1.3 (−7–0.9) |
| PWV | 3.9 (3.4–4.2) | 4.3 (3.7–4.7) * |
| AASI | 0.3 (0.15–0.44) | 0.27 (0.2–0.49) |
| Cardiovascular Markers | Total (n = 62) | |
|---|---|---|
| r | p | |
| Left ventricular mass | 0.185 | 0.15 |
| LVMI | 0.164 | 0.203 |
| cIMT | −0.262 | 0.04 * |
| Augmentation index | −0.257 | 0.043 * |
| PWV | −0.008 | 0.954 |
| AASI | −0.357 | 0.004 * |
| ABPM Profile | n | Normal | n | Abnormal | |
|---|---|---|---|---|---|
| Lactoferrin, ng/mL | Lactoferrin, ng/mL | p Value | |||
| 24-h BP | 54 | 1754 (1363–1874) | 8 | 1312 (1025–1803) | 0.11 |
| Awake BP | 53 | 1743 (1334–1873) | 9 | 1306 (1013–1736) | 0.062 |
| Asleep BP | 40 | 1798 (1586–1885) | 22 | 1473 (1138–1767) | 0.015 |
| BP load | 36 | 1777 (1392–1882) | 26 | 1550 (1099–1861) | 0.084 |
| Night dipping | 29 | 1845 (1730–1901) | 33 | 1525 (1119–1791) | 0.004 |
| Total ABPM profile | 23 | 1845 (1731–1914) | 39 | 1566 (1138–1858) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-Y.; Lu, P.-C.; Chen, W.-L.; Liao, W.-T.; Hsu, C.-N.; Tain, Y.-L. Lactoferrin in Pediatric Chronic Kidney Disease and Its Relationship with Cardiovascular Risk. Children 2024, 11, 1124. https://doi.org/10.3390/children11091124
Ho C-Y, Lu P-C, Chen W-L, Liao W-T, Hsu C-N, Tain Y-L. Lactoferrin in Pediatric Chronic Kidney Disease and Its Relationship with Cardiovascular Risk. Children. 2024; 11(9):1124. https://doi.org/10.3390/children11091124
Chicago/Turabian StyleHo, Chun-Yi, Pei-Chen Lu, Wei-Ling Chen, Wei-Ting Liao, Chien-Ning Hsu, and You-Lin Tain. 2024. "Lactoferrin in Pediatric Chronic Kidney Disease and Its Relationship with Cardiovascular Risk" Children 11, no. 9: 1124. https://doi.org/10.3390/children11091124







