Insights into Hospitalized Children with Urinary Tract Infections: Epidemiology and Antimicrobial Resistance Patterns in Israel—A Single Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Ethical Approval and Informed Consent
2.3. Statistical Methods
3. Results
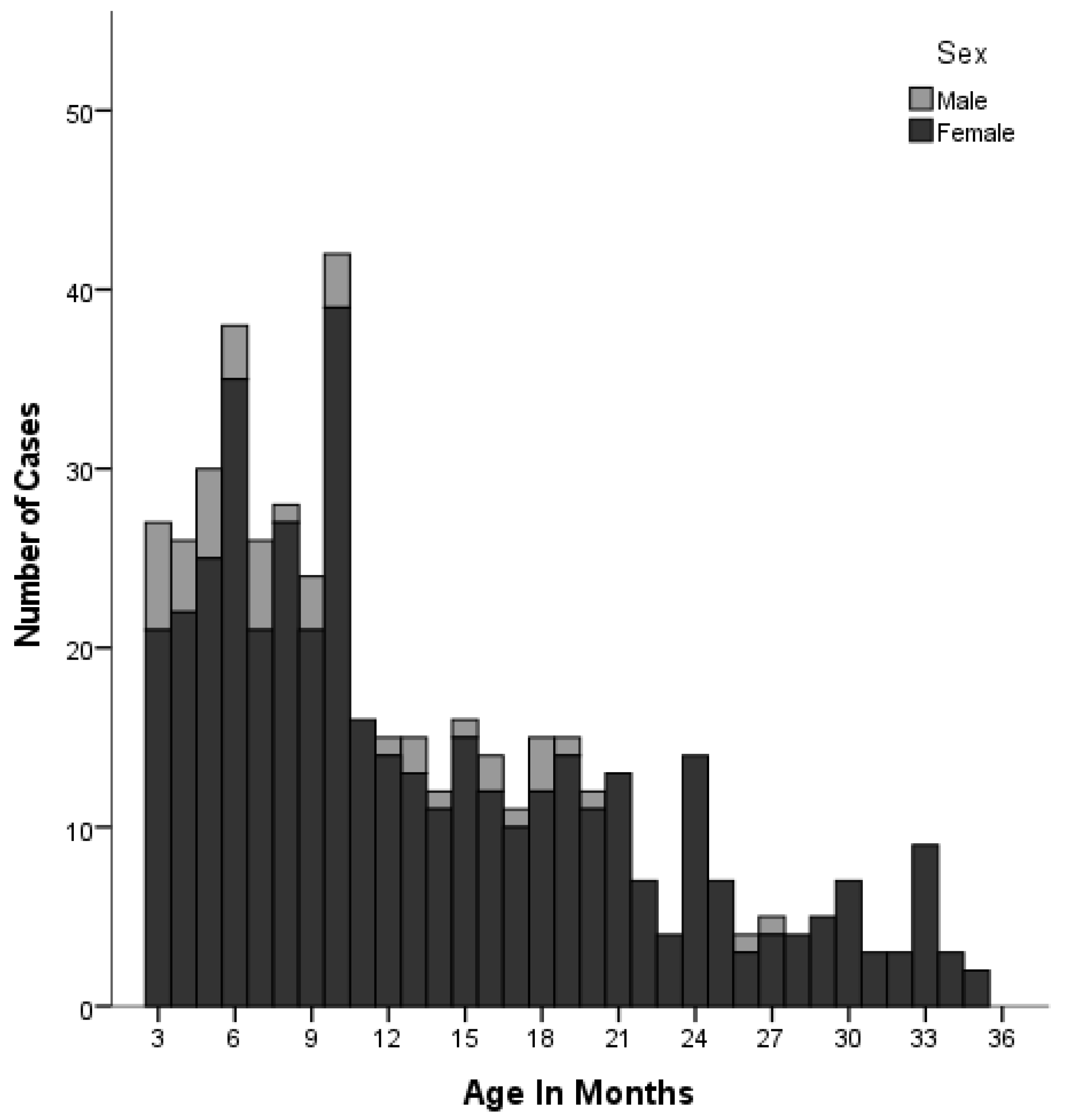
3.1. Patient and Clinical Characteristics
3.2. Pathogens
3.3. Antibiotic Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantell, R.H.; Roberts, K.B.; Adams, W.G.; Dreyer, B.P.; Kuppermann, N.; O’Leary, S.T.; Okechukwu, K.; Woods, C.R., Jr.; SUBCOMMITTEE ON FEBRILE INFANTS. Evaluation and Management of Well-Appearing Febrile Infants 8 to 60 Days Old. Pediatrics 2021, 148, e2021054063. [Google Scholar] [CrossRef] [PubMed]
- Simões e Silva, A.C.; Oliveira, E.A. Update on the Approach of Urinary Tract Infection in Childhood. J. Pediatr. 2015, 91 (Suppl. S1), S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.; Romao, R.L.P.; MacLellan, D. Urinary Tract Infection in Children. Can. Med. Assoc. J. 2017, 189, E608. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Wong, A.H.C.; Leung, A.A.M.; Hon, K.L. Urinary Tract Infection in Children. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 2–18. [Google Scholar] [CrossRef]
- Shaikh, N.; Craig, J.C.; Rovers, M.M.; Dalt, L.D.; Gardikis, S.; Hoberman, A.; Montini, G.; Rodrigo, C.; Taskinen, S.; Tuerlinckx, D.; et al. Identification of Children and Adolescents at Risk for Renal Scarring after a First Urinary Tract Infection: A Meta-Analysis with Individual Patient Data. JAMA Pediatr. 2014, 168, 893–900. [Google Scholar] [CrossRef]
- Garin, E.H.; Orta-Sibu, N.; Campos, A. Primary Vesicoureteral Reflux in Childhood. Adv. Pediatr. 2002, 49, 341–357. [Google Scholar] [CrossRef]
- Kantamalee, W.; Santanirand, P.; Saisawat, P.; Boonsathorn, S.; Techasaensiri, C.; Apiwattanakul, N. Outcomes of Empirical Antimicrobial Therapy for Pediatric Community-Onset Febrile Urinary Tract Infection in the Era of Increasing Antimicrobial Resistance. Pediatr. Infect. Dis. J. 2020, 39, 121–126. [Google Scholar] [CrossRef]
- Yakubov, R.; Van Den Akker, M.; MacHamad, K.; Hochberg, A.; Nadir, E.; Klein, A. Antimicrobial Resistance among Uropathogens That Cause Childhood Community-Acquired Urinary Tract Infections in Central Israel. Pediatr. Infect. Dis. J. 2017, 36, 113–115. [Google Scholar] [CrossRef]
- Waller, T.A.; Pantin, S.A.L.; Yenior, A.L.; Pujalte, G.G.A. Urinary Tract Infection Antibiotic Resistance in the United States. Prim. Care 2018, 45, 455–466. [Google Scholar] [CrossRef]
- Kutasy, B.; Coyle, D.; Fossum, M. Urinary Tract Infection in Children: Management in the Era of Antibiotic Resistance—A Pediatric Urologist’s View. Eur. Urol. Focus 2017, 3, 207–211. [Google Scholar] [CrossRef]
- Mahony, M.; McMullan, B.; Brown, J.; Kennedy, S.E. Multidrug-Resistant Organisms in Urinary Tract Infections in Children. Pediatr. Nephrol. 2020, 35, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Al Mana, H.; Sundararaju, S.; Tsui, C.K.M.; Perez-Lopez, A.; Yassine, H.; Al Thani, A.; Al-Ansari, K.; Eltai, N.O. Whole-Genome Sequencing for Molecular Characterization of Carbapenem-Resistant Enterobacteriaceae Causing Lower Urinary Tract Infection among Pediatric Patients. Antibiotics 2021, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Megged, O. Extended-Spectrum β-Lactamase-Producing Bacteria Causing Community-Acquired Urinary Tract Infections in Children. Pediatr. Nephrol. 2014, 29, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N.; Dabbah, H.; Weissman, I.; Aga, I.; Even, L.; Glikman, D. Urinary Tract Infections Caused by Community-Acquired Extended-Spectrum β-Lactamase-Producing and Non-Producing Bacteria: A Comparative Study. J. Pediatr. 2013, 163, 1417–1421. [Google Scholar] [CrossRef]
- Tamas, V.; Shah, S.; Hollenbach, K.A.; Kanegaye, J.T. Emergence of Extended-Spectrum β-Lactamase-Producing Pathogens in Community-Acquired Urinary Tract Infections among Infants at a Pediatric Emergency Department. Pediatr. Emerg. Care 2022, 38, E1053–E1057. [Google Scholar] [CrossRef]
- Esposito, S.; Biasucci, G.; Pasini, A.; Predieri, B.; Vergine, G.; Crisafi, A.; Malaventura, C.; Casadio, L.; Sella, M.; Pierantoni, L.; et al. Antibiotic Resistance in Paediatric Febrile Urinary Tract Infections. J. Glob. Antimicrob. Resist. 2022, 29, 499–506. [Google Scholar] [CrossRef]
- Roberts, K.B.; Subcommittee on Urinary Tract Infection; Steering Committee on Quality Improvement and Management. Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef]
- Renko, M.; Salo, J.; Ekstrand, M.; Pokka, T.; Pieviläinen, O.; Uhari, M.; Tapiainen, T. Meta-Analysis of the Risk Factors for Urinary Tract Infection in Children. Pediatr. Infect. Dis. J. 2022, 41, 787–792. [Google Scholar] [CrossRef]
- Mattoo, T.K.; Shaikh, N.; Nelson, C.P. Contemporary Management of Urinary Tract Infection in Children. Pediatrics 2021, 147, e2020012138. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 23 April 2024).
- Cag, Y.; Haciseyitoglu, D.; Ozdemir, A.A.; Cag, Y. Antibiotic Resistance and Bacteria in Urinary Tract Infections in Pediatric Patients. Medeni Med. J. 2021, 36, 217–224. [Google Scholar] [CrossRef] [PubMed]
- El, M.; Al, N.; Hospital, Q.; Rabah, F.; Idrees, A.B.; Al Muharrmi, Z.; El Nour, I. Urinary Tract Infection in Omani Children: Etiology and Antimicrobial Resistance. A Comparison between First Episode and Recurrent Infection. J. Adv. Microbiol. 2021, 21, 51–62. [Google Scholar] [CrossRef]
- Eremenko, R.; Barmatz, S.; Lumelsky, N.; Colodner, R.; Strauss, M.; Alkan, Y. Urinary Tract Infection in Outpatient Children and Adolescents: Risk Analysis of Antimicrobial Resistance. Isr. Med. Assoc. J. 2020, 22, 236–240. Available online: https://europepmc.org/article/med/32286027 (accessed on 23 April 2024). [PubMed]
- Gunduz, S.; Uludağ Altun, H. Antibiotic Resistance Patterns of Urinary Tract Pathogens in Turkish Children. Glob. Health Res. Policy 2018, 3, 10. [Google Scholar] [CrossRef]
- Kalaitzidou, I.; Ladomenou, F.; Athanasopoulos, E.; Anatoliotaki, M.; Vlachaki, G. Susceptibility Patterns of Uropathogens Identified in Hospitalized Children. Pediatr. Int. 2019, 61, 246–251. [Google Scholar] [CrossRef]
- Hanna-Wakim, R.H.; Ghanem, S.T.; El Helou, M.W.; Khafaja, S.A.; Shaker, R.A.; Hassan, S.A.; Saad, R.K.; Hedari, C.P.; Khinkarly, R.W.; Hajar, F.M.; et al. Epidemiology and Characteristics of Urinary Tract Infections in Children and Adolescents. Front. Cell. Infect. Microbiol. 2015, 5, 00045. [Google Scholar] [CrossRef]
- Haque, R.; Akter, M.L.; Salam, M.A. Prevalence and Susceptibility of Uropathogens: A Recent Report from a Teaching Hospital in Bangladesh. BMC Res. Notes 2015, 8, 416. [Google Scholar] [CrossRef]
- Swerkersson, S.; Jodal, U.; Åhrén, C.; Hansson, S. Urinary Tract Infection in Small Outpatient Children: The Influence of Age and Gender on Resistance to Oral Antimicrobials. Eur. J. Pediatr. 2014, 173, 1075–1081. [Google Scholar] [CrossRef]
- Ahmed, M.; Long, W.N.W.; Javed, S.; Reynolds, T. Rising Resistance of Urinary Tract Pathogens in Children: A Cause for Concern. Ir. J. Med. Sci. 2022, 191, 279–282. [Google Scholar] [CrossRef]
- Zorc, J.J.; Levine, D.A.; Platt, S.L.; Dayan, P.S.; Macias, C.G.; Krief, W.; Schor, J.; Bank, D.; Shaw, K.N.; Kuppermann, N.; et al. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics 2005, 116, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Morone, N.E.; Bost, J.E.; Farrell, M.H. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatr. Infect. Dis. J. 2008, 27, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Keren, R.; Shaikh, N.; Pohl, H.; Gravens-Mueller, L.; Ivanova, A.; Zaoutis, L.; Patel, M.; de Berardinis, R.; Parker, A.; Bhatnagar, S.; et al. Risk Factors for Recurrent Urinary Tract Infection and Renal Scarring. Pediatrics 2015, 136, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Duicu, C.; Cozea, I.; Delean, D.; Aldea, A.A.; Aldea, C. Antibiotic resistance patterns of urinary tract pathogens in children from Central Romania. Exp. Ther. Med. 2021, 22, 748. [Google Scholar] [CrossRef] [PubMed]
- Bitsori, M.; Maraki, S.; Koukouraki, S.; Galanakis, E. Pseudomonas aeruginosa urinary tract infection in children: Risk factors and outcomes. J. Urol. 2012, 187, 260–264. [Google Scholar] [CrossRef]
- Khan, A.; Jhaveri, R.; Seed, P.C.; Arshad, M. Update on Associated Risk Factors, Diagnosis, and Management of Recurrent Urinary Tract Infections in Children. J. Pediatr. Infect. Dis. Soc. 2019, 8, 152–159. [Google Scholar] [CrossRef]
- Larcombe, J. Urinary tract infection in children: Recurrent infections. BMJ Clin. Evid. 2015, 2015, 306. [Google Scholar]
- Silva, A.; Costa, E.; Freitas, A.; Almeida, A. Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics 2022, 11, 768. [Google Scholar] [CrossRef]
- Shaikh, N.; Hoberman, A.; Keren, R.; Ivanova, A.; Gotman, N.; Chesney, R.W.; Carpenter, M.A.; Moxey-Mims, M.; Wald, E.R. Predictors of Antimicrobial Resistance among Pathogens Causing Urinary Tract Infection in Children. J. Pediatr. 2016, 171, 116–121. [Google Scholar] [CrossRef]
- Israel Medical Association. Diagnosis and Treatment of Urinary Tract Infection in Children—Clinical Guidelines. 2014. Available online: https://www.ima.org.il/userfiles/image/clinical_45_sheten.pdf (accessed on 20 April 2024).
- Bradley, J.S. Nelson’s Pediatric Antimicrobial Therapy, 29th ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2023. [Google Scholar]
- Gilbert, D.N.; Chambers, H.F.; Saag, M.S.; Pavia, A.T.; Boucher, H.W. The Sanford Guide to Antimicrobial Therapy, 53rd ed.; Antimicrobial Therapy: Sperryville, VA, USA, 2023. [Google Scholar]
- Zappitelli, M.; Moffett, B.S.; Hyder, A.; Goldstein, S.L. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: A retrospective cohort study. Nephrol. Dial. Transplant. 2011, 26, 144–150. [Google Scholar] [CrossRef]
- McWilliam, S.J.; Antoine, D.J.; Smyth, R.L.; Pirmohamed, M. Aminoglycoside-induced nephrotoxicity in children. Pediatr. Nephrol. 2017, 32, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Pittet, L.F.; Hikmat, S.; Silvester, E.J.; Clifford, V.; Hunt, R.; Gwee, A. Short-Course Intravenous Antibiotics for Young Infants with Urinary Tract Infection. Arch. Dis. Child. 2022, 107, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Zaoutis, T.; Shaikh, N.; Fisher, B.T.; Coffin, S.E.; Bhatnagar, S.; Downes, K.J.; Gerber, J.S.; Shope, T.R.; Martin, J.M.; Muniz, G.B.; et al. Short-Course Therapy for Urinary Tract Infections in Children: The SCOUT Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Montini, G.; Tessitore, A.; Console, K.; Ronfani, L.; Barbi, E.; Pennesi, M.; STOP Trial Group. Short Oral Antibiotic Therapy for Pediatric Febrile Urinary Tract Infections: A Randomized Trial. Pediatrics 2024, 153, e2023062598. [Google Scholar] [CrossRef]
- Bryce, A.; Hay, A.D.; Lane, I.F.; Thornton, H.V.; Wootton, M.; Costelloe, C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: Systematic review and meta-analysis. BMJ 2016, 352, i939. [Google Scholar] [CrossRef]

| Characteristic | Complicated UTI (N = 453) | Non-Complicated UTI (N = 308) | p |
|---|---|---|---|
| Male sex | 59 (13.0) | 0 (0.0) | --- |
| Median patient age (mo) | 17.5 [9.0–48.25] | 21 [9–54.5] | 0.18 |
| Urine tract pathology | 266/443 * (60.0%) | N/A | |
| Hydronephrosis | 191 (43.1) | ||
| Duplex collecting system | 14 (3.1) | ||
| Vesico-ureteric reflux | 11 (2.5) | ||
| Stenosis | 2 (0.5) | ||
| Other | 48 (10.8) | ||
| CRP (mg/dL) | 86.75 [39.75–153.25] | 84.50 [34.00–155.00] | 0.46 |
| Max CRP (mg/dL) | 103.50 [43.75–170.00] | 95.30 [47.78–169.80] | 0.32 |
| Urea (mg/dL) | 20.0 [16–25] | 20.0 [16–25] | 0.93 |
| Creatinine (mg/dL) | 0.30 [0.30–0.40] | 0.30 [0.30–0.50] | 0.52 |
| Pathogen | |||
| Escherichia coli | 368 (81.2) | 286 (92.9) | <0.001 |
| Klebsiella pneumoniae | 21 (4.6) | 5 (1.6) | 0.03 |
| Pseudomonas aeruginosa | 24 (5.3) | 3 (1.0) | 0.002 |
| Proteus mirabilis | 14 (3.1) | 7 (2.3) | 0.65 |
| Enterococcus faecalis | 12 (2.6) | 3 (1.0) | 0.10 |
| Enterobacter spp. | 5 (1.1) | 1 (0.3) | 0.41 |
| Staphylococcus aureus | 5 (1.1) | 1 (0.3) | 0.41 |
| Citrobacter spp. | 1 (0.2) | 2 (0.6) | 0.57 |
| Other | 3 (0.7) | 1 (0.3) | 0.65 |
| All (N = 761) | 3–11 mo (N = 230) | 1–2 yr (N = 192) | 3–4 yr (N = 136) | 5–11 yr (N = 131) | 12–17 yr (N = 72) | p | |
|---|---|---|---|---|---|---|---|
| Escherichia coli | 654 (85.9) | 201 (87.4) | 171 (89.1) | 106 (77.9) | 113 (86.3) | 63 (87.5) | 0.072 |
| Klebsiella pneumoniae | 26 (3.4) | 9 (3.5) | 5 (2.6) | 5 (3.7) | 5 (3.8) | 2 (2.8) | 0.96 |
| Pseudomonas aeruginosa | 27 (3.5) | 5 (2.2) | 7 (3.6) | 10 (7.4) | 2 (1.5) | 3 (4.2) | 0.086 |
| Proteus mirabilis | 21 (2.8) | 3 (1.3) | 5 (2.6) | 9 (6.6) | 4 (3.1) | 0 (0.0) | 0.033 |
| Enterococcus fecalis | 15 (2.0) | 5(2.2) | 3 (1.6) | 3 (2.2) | 3 (2.3) | 1 (1.4) | 0.98 |
| Enterobacter spp. | 6 (0.8) | 2 (0.9) | 1 (0.5) | 1 (0.7) | 1 (0.8) | 1 (1.4) | 0.94 |
| Staphylococcus aureus | 6 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 3 (2.3) | 2 (2.8) | 0.008 |
| Citrobacter spp. | 2(0.3) | 2 (0.7) | 0 (0.0) | 0(0.0) | 0 (0.0) | 0 (0.0) | 0.73 |
| Other | 4 (0.6) | 3 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0.16 |
| All Patients with Cultured Gram-Negative Bacteria (N = 739) Resistant/Number Tested (%) | Complicated UTI Resistant/Number Tested (%) | Non-Complicated UTI Resistant/Number Tested (%) | p Value | |
|---|---|---|---|---|
| Ampicillin | 352/579 (60.9) | 216/347 (62.3) | 136/231 (58.3) | 0.42 |
| TMP-SMX | 107/436 (24.5) | 71/275 (25.8) | 36/161 (22.4) | 0.42 |
| Cephalexin | 76/426 (17.8) | 41/212 (19.3) | 35/213 (16.4) | 0.03 |
| Amoxicillin–clavulanic | 104/600 (17.3) | 58/346 (16.8) | 46/243 (18.2) | 0.50 |
| Cefuroxime | 34/397 (8.6) | 24/237 (10.1) | 10/160 (6.2) | 0.18 |
| Ciprofloxacin | 31/472 (6.6) | 21/307 (6.8) | 10/165 (6.1) | 0.75 |
| Nitrofurantion | 26/472 (5.2) | 17/267 (6.4) | 9/236 (3.8) | 0.20 |
| Ceftriaxone | 26/597 (4.4) | 17/344 (4.9) | 9/252 (3.6) | 0.42 |
| Gentamicin | 38/632 (6.0) | 25/375 (6.7) | 13/256 (5.1) | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitoon, H.; Garkaby, J.; Nassrallah, B.; Sharkansky, L.; Shnaider, M.; Chistyakov, I.; Genizi, J.; Nathan, K. Insights into Hospitalized Children with Urinary Tract Infections: Epidemiology and Antimicrobial Resistance Patterns in Israel—A Single Center Study. Children 2024, 11, 1142. https://doi.org/10.3390/children11091142
Zaitoon H, Garkaby J, Nassrallah B, Sharkansky L, Shnaider M, Chistyakov I, Genizi J, Nathan K. Insights into Hospitalized Children with Urinary Tract Infections: Epidemiology and Antimicrobial Resistance Patterns in Israel—A Single Center Study. Children. 2024; 11(9):1142. https://doi.org/10.3390/children11091142
Chicago/Turabian StyleZaitoon, Hussein, Jenny Garkaby, Basheer Nassrallah, Livnat Sharkansky, Morya Shnaider, Irina Chistyakov, Jacob Genizi, and Keren Nathan. 2024. "Insights into Hospitalized Children with Urinary Tract Infections: Epidemiology and Antimicrobial Resistance Patterns in Israel—A Single Center Study" Children 11, no. 9: 1142. https://doi.org/10.3390/children11091142
APA StyleZaitoon, H., Garkaby, J., Nassrallah, B., Sharkansky, L., Shnaider, M., Chistyakov, I., Genizi, J., & Nathan, K. (2024). Insights into Hospitalized Children with Urinary Tract Infections: Epidemiology and Antimicrobial Resistance Patterns in Israel—A Single Center Study. Children, 11(9), 1142. https://doi.org/10.3390/children11091142







