Frontal Plane Knee Kinematics and Kinetics During Gait in Children and Youth with Achondroplasia—Correspondence with Static X-Ray Images and Relevance to Symptoms
Abstract
:1. Introduction
- (1)
- Quantify the agreement between X-rays and standing motion capture in ACH.
- (2)
- Test whether children with ACH have abnormal knee kinematics and kinetics in the frontal plane during gait. Herein, investigate how well radiographic knee malalignments actually predict KAM while walking and whether this relationship is modified by gait compensations.
- (3)
- Investigate whether knee kinematics and KAM alterations are associated with symptoms.
2. Materials and Methods
2.1. Participants
2.2. X-Rays
2.3. Three-Dimensional Gait Analysis (3DGA)
2.4. Data Analysis
2.5. Statistics
3. Results
3.1. Patient Characteristics
3.2. X-Rays
3.3. Knee Joint Kinematics
3.4. Relationship Between X-Ray and Knee Kinematics
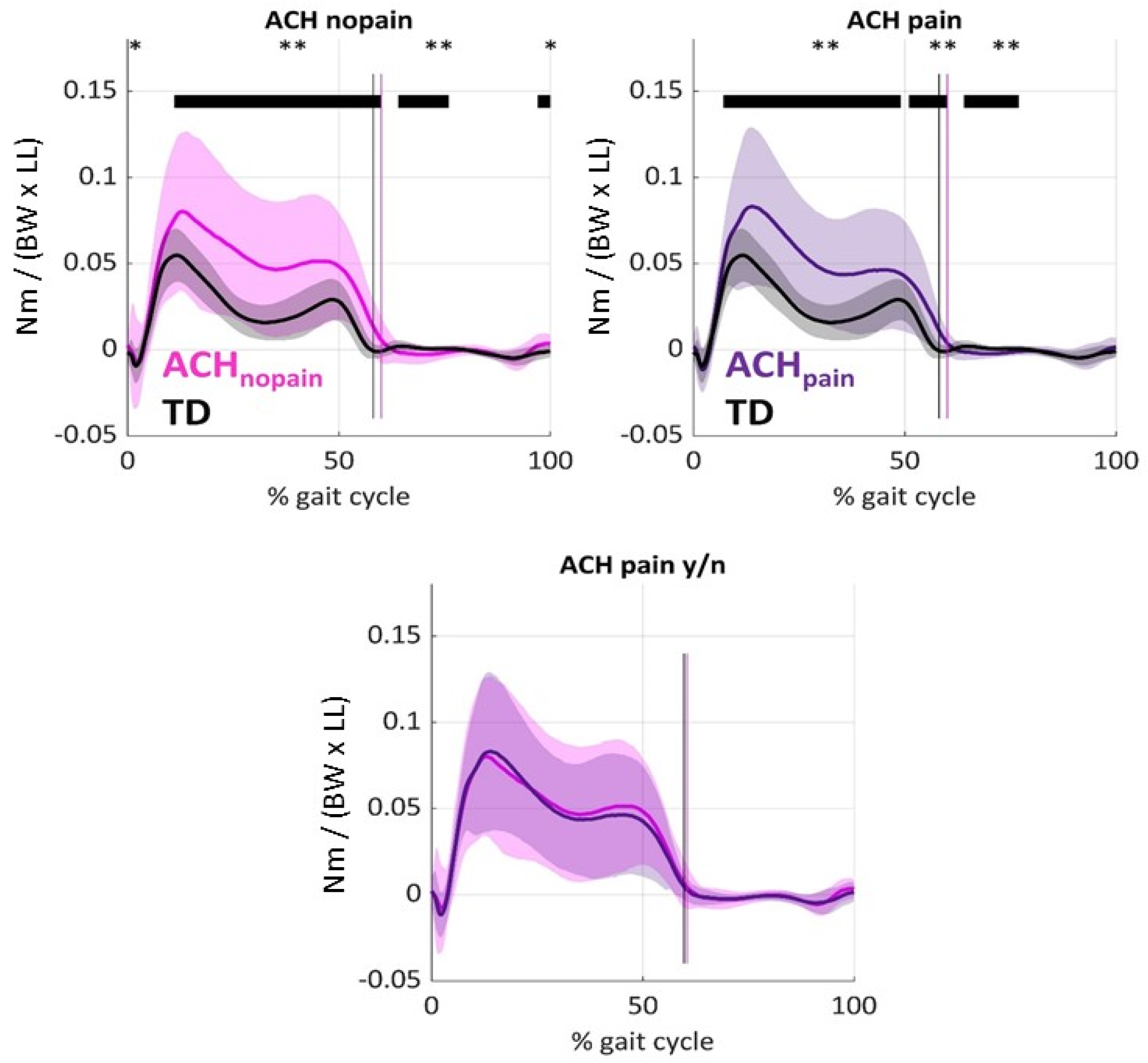
3.5. Knee Adduction Joint Moments (KAMs)
3.6. Relationship Between X-Ray, KAM, and Gait Compensations
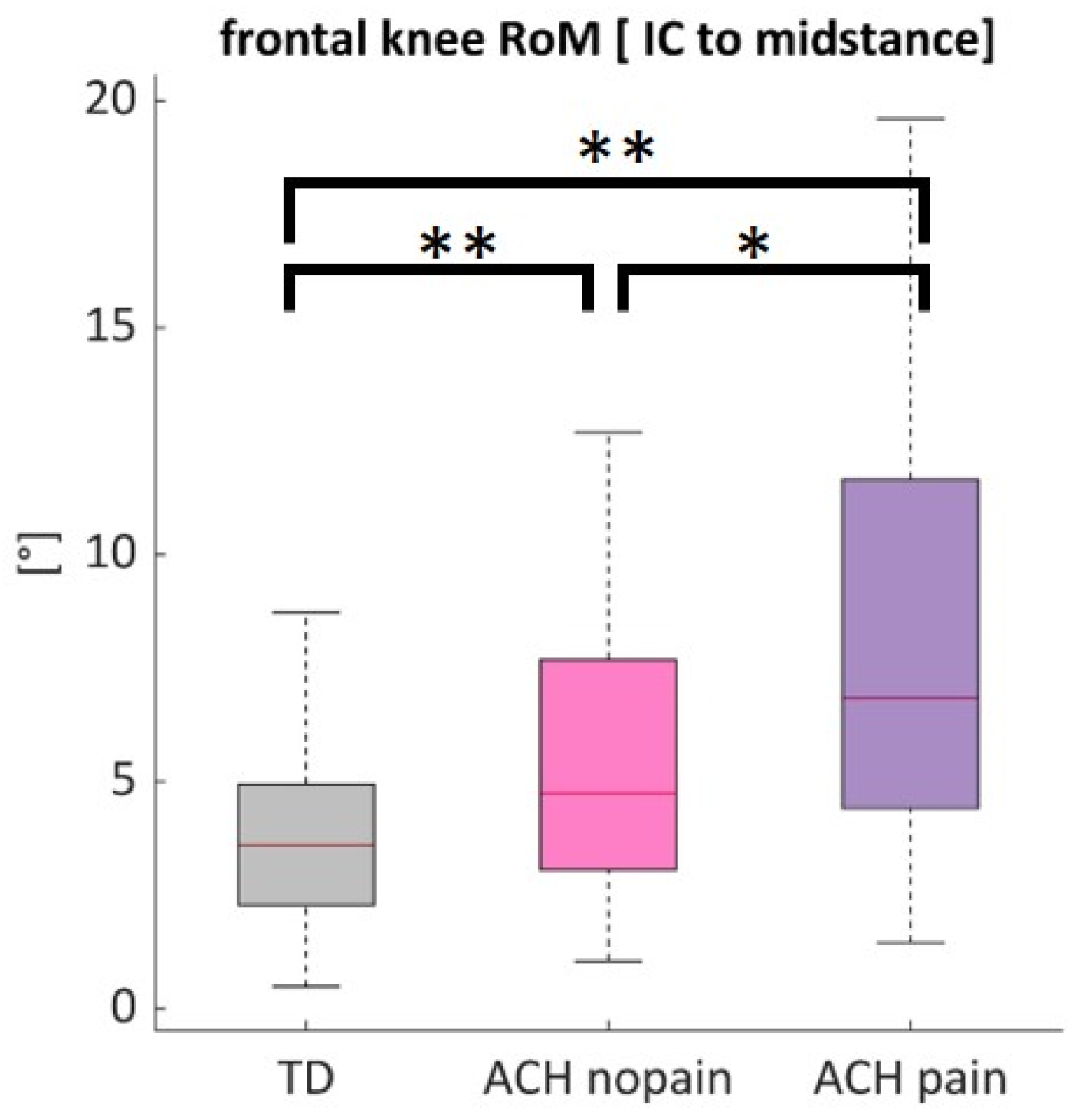
3.7. Comparisons of Knees with and Without Pain in Achondroplasia
3.7.1. X-Rays
3.7.2. Tempo-Spatial Parameters
3.7.3. Knee Joint Kinematics
3.7.4. Knee Adduction Moments [KAMs] and Kinematic Gait Compensations
4. Discussion
4.1. Relationship Between X-Ray and 3DGA in Achondroplasia
4.2. Gait Abnormalities in Knee Kinematics and Kinetics in Achondroplasia
4.3. Factors Related to Knee Pain in Achondroplasia
4.4. Limitations and Recommendations
- First, all patients were from a pediatric orthopedic department sent for clinical evaluations. High proportions of symptoms and malalignments may not be generalizable to all children with ACH.
- Second, from a methodological perspective, the gait model could be further improved. Knee anatomy in ACH is special, and the PiG model might be a simplification of this. Also, imaging technology might be used in ACH to locate the hip joint centers relative to pelvis geometry, which might be altered in ACH in comparison to TD and assist in establishing disease-specific regression equations. As foot and ankle biomechanics can also have an impact on knee biomechanics, e.g., increased rearfoot inversion, which is typical in ACH and generally increases both KAM [63] and varus thrust [64], this argues for a more specific foot model for a detailed assessment.
- Third, concerning the X-rays, we focused on the mTFA only. The assessment of skeletal abnormalities and indications for possible intervention in ACH in relation to their axial abnormalities (e.g., 8-plates) includes significantly more X-ray parameters [13] than reported in the current study. To accurately guide orthopedic decision-making, the radiographic parameters that predispose to laxity need to be determined, as several joint orientation angles are usually taken into consideration when deciding on treatment; in particular, the interaction between these joint orientation angles and dynamic instability during gait should be investigated. In the future, a better understanding of the development of symptoms will likely be achieved by assessing the relationship between traditional imaging data, detailed clinical examinations, motion capturing as part of functional instrumented tests, and, most importantly, patient-reported outcomes in longitudinal follow-up studies of ACH patients throughout their maturation.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauli, R.M. Achondroplasia: A Comprehensive Clinical Review. Orphanet J. Rare Dis. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.A.; Hall, J.G.; Hecht, J.T. Achondroplasia. Lancet 2007, 370, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Coi, A.; Santoro, M.; Garne, E.; Pierini, A.; Addor, M.C.; Alessandri, J.L.; Bergman, J.E.H.; Bianchi, F.; Boban, L.; Braz, P.; et al. Epidemiology of Achondroplasia: A Population-Based Study in Europe. Am. J. Med. Genet. A 2019, 179, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Foreman, P.K.; van Kessel, F.; van Hoorn, R.; van den Bosch, J.; Shediac, R.; Landis, S. Birth prevalence of Achondroplasia: A Systematic Literature Review and Meta-Analysis. Am. J. Med. Genet. A 2020, 182, 2297–2316. [Google Scholar] [CrossRef] [PubMed]
- Karol, L.A. Spinal Deformity in Children with Achondroplasia. Spine Deform. 2012, 1, 107–113. [Google Scholar] [CrossRef]
- Ruiz-Garcia, M.; Tovar-Baudin, A.; Del Castillo-Ruiz, V.; Rodriguez, H.P.; Collado, M.A.; Mora, T.M.; Rueda-Franco, F.; Gonzalez-Astiazaran, A. Early Detection of Neurological Manifestations in Achondroplasia. Childs Nerv. Syst. 1997, 13, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Takken, T.; van Bergen, M.W.; Sakkers, R.J.; Helders, P.J.; Engelbert, R.H. Cardiopulmonary Exercise Capacity, Muscle Strength, and Physical Activity in Children and Adolescents with Achondroplasia. J. Pediatr. 2007, 150, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Fredwall, S.O.; Linge, J.; de Vries, O.; Leinhard, O.D.; Eggesbø, H.B.; Weedon-Fekjær, H.; Petersson, M.; Widholm, P.; Månum, G.; Savarirayan, R. Fat Infiltration in the Thigh Muscles is Associated with Symptomatic Spinal Stenosis and Reduced Physical Functioning in Adults with Achondroplasia. Orphanet J. Rare Dis. 2023, 18, 35. [Google Scholar] [CrossRef]
- Sims, D.T.; Onambélé-Pearson, G.L.; Burden, A.; Payton, C.; Morse, C.I. Specific Force of the Vastus Lateralis in Adults with Achondroplasia. J. Appl. Physiol. 2018, 124, 696–703. [Google Scholar] [CrossRef]
- Hecht, J.T.; Bodensteiner, J.B.; Butler, I.J. Neurologic Manifestations of Achondroplasia. Handb. Clin. Neurol. 2014, 119, 551–563. [Google Scholar] [CrossRef]
- Hunter, A.G.; Bankier, A.; Rogers, J.G.; Sillence, D.; Scott, C.I. Medical Complications of Achondroplasia: A Multicentre Patient Review. J. Med. Genet. 1998, 35, 705–712. [Google Scholar] [CrossRef]
- Llerena, J.; Kim, C.A.; Fano, V.; Rosselli, P.; Collett-Solberg, P.F.; Frassinetti Vasconcelos de Medeiros, P.; Del Pino, M.; Bertola, D.; Lourenço, C.M.; Cavalcanti, D.P.; et al. Achondroplasia in Latin America: Practical Recommendations for the Multidisciplinary Care of Pediatric Patients. BMC Pediatr. 2022, 22, 492. [Google Scholar] [CrossRef]
- Hösl, M.; Afifi, F.K.; Thamm, A.; Göttling, L.; Holzapfel, B.M.; Wagner, F.; Mohnike, K.; Nader, S. The Effectiveness of Growth Modulation Using Tension Band Plates in Children with Achondroplasia in Comparison to Children with Idiopathic Frontal Axial Deformities of the Knee. J. Pediatr. Orthop. 2025, 45, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Makarewich, C.A.; Zhang, E.; Stevens, P.M. Hemiepiphysiodesis for Lower Extremity Coronal Plane Angular Correction in theDistal Femur and Proximal Tibia in Children with Achondroplasia. J. Pediatr. Orthop. 2023, 43, 639–642. [Google Scholar] [CrossRef]
- Koudela, K., Jr.; Koudela, K., Sr.; Koudelová, J. Total Knee Arthroplasty in Severe Valgus Deformity in a Patient with Achondroplasia. Acta Chir. Orthop. Traumatol. Cechoslov. 2011, 78, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.; Thacker, M.; Church, C.; Miller, F.; Mackenzie, W.G.; Conklin, D. Dynamic Lower Extremity Alignment in Children with Achondroplasia. J. Pediatr. Orthop. 2006, 26, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Stanley, G.; McLoughlin, S.; Beals, R.K. Observations on the Cause of Bowlegs in Achondroplasia. J. Pediatr. Orthop. 2002, 22, 112–116. [Google Scholar] [CrossRef]
- Lee, S.T.; Song, H.R.; Mahajan, R.; Makwana, V.; Suh, S.W.; Lee, S.H. Development of Genu Varum in Achondroplasia: Relation to Fibular Overgrowth. J. Bone Jt. Surg. Br. 2007, 89, 57–61. [Google Scholar] [CrossRef]
- Liang, H.; Qi, W.; Jin, C.; Pang, Q.; Liu, W.; Jiang, Y.; Wang, O.; Li, M.; Xing, X.; Pan, H.; et al. Evaluation of Volumetric Bone Mineral Density, Bone Microarchitecture, and Bone Strength in Patients with Achondroplasia Caused by FGFR3 c.1138G>A Mutation. Calcif. Tissue Int. 2023, 112, 13–23. [Google Scholar] [CrossRef]
- Pfeiffer, K.M.; Brod, M.; Smith, A.; Gianettoni, J.; Viuff, D.; Ota, S.; Charlton, R.W. Assessing Physical Symptoms, Daily Functioning, and Well-Being in Children with Achondroplasia. Am. J. Med. Genet. A 2021, 185, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kurian, B.T.; Belthur, M.V.; Jones, S.; Giles, S.N.; Fernandes, J.A. Correction of Bowleg Deformity in Achondroplasia through Combined Bony Realignment and Lateral Collateral Ligament Tightening. Strateg. Trauma Limb Reconstr. 2019, 14, 132–138. [Google Scholar] [CrossRef]
- Onesimo, R.; Sforza, E.; Bedeschi, M.F.; Leoni, C.; Giorgio, V.; Rigante, D.; De Rose, C.; Kuczynska, E.M.; Romeo, D.M.; Palmacci, O.; et al. How Pain Affect Real Life of Children and Adults with Achondroplasia: A Systematic Review. Eur. J. Med. Genet. 2023, 66, 104850. [Google Scholar] [CrossRef] [PubMed]
- Okenfuss, E.; Moghaddam, B.; Avins, A.L. Natural History of Achondroplasia: A Retrospective Review of Longitudinal Clinical Data. Am. J. Med. Genet. A 2020, 182, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Del Pilar Duque Orozco, M.; Record, N.C.; Rogers, K.J.; Bober, M.B.; Mackenzie, W.G.; Atanda, A., Jr. Arthroscopic Knee Anatomy in Young Achondroplasia Patients. J. Child. Orthop. 2017, 11, 169–174. [Google Scholar] [CrossRef]
- Sims, D.T.; Burden, A.; Payton, C.; Onambélé-Pearson, G.L.; Morse, C.I. A Spatio-Temporal and Kinematic Description of Self-Selected Walking in Adults with Achondroplasia. Gait Posture 2020, 80, 391–396. [Google Scholar] [CrossRef]
- Kiernan, D. Lower Limb Biomechanics during Gait in Children with Achondroplasia. J. Biomech. 2021, 119, 110313. [Google Scholar] [CrossRef]
- Broström, E.W.; Antonissen, L.; von Heideken, J.; Esbjörnsson, A.C.; Hagenäs, L.; Naili, J.E. Gait in Children with Achondroplasia—A Cross-Sectional Study on Joint Kinematics and Kinetics. BMC Musculoskelet. Disord. 2022, 23, 397. [Google Scholar] [CrossRef] [PubMed]
- Hoover-Fong, J.; Cheung, M.S.; Fano, V.; Hagenas, L.; Hecht, J.T.; Ireland, P.; Irving, M.; Mohnike, K.; Offiah, A.C.; Okenfuss, E.; et al. Lifetime Impact of Achondroplasia: Current Evidence and Perspectives on the Natural History. Bone 2021, 146, 115872. [Google Scholar] [CrossRef]
- Chang, A.; Hochberg, M.; Song, J.; Dunlop, D.; Chmiel, J.S.; Nevitt, M.; Hayes, K.; Eaton, C.; Bathon, J.; Jackson, R.; et al. Frequency of Varus and Valgus Thrust and Factors Associated with Thrust Presence in Persons with or at Higher Risk of Developing Knee Osteoarthritis. Arthritis Rheum. 2010, 62, 1403–1411. [Google Scholar] [CrossRef]
- Chang, A.; Hayes, K.; Dunlop, D.; Hurwitz, D.; Song, J.; Cahue, S.; Genge, R.; Sharma, L. Thrust During Ambulation and the Progression of Knee Osteoarthritis. Arthritis Rheum. 2004, 50, 3897–3903. [Google Scholar] [CrossRef]
- Stief, F.; Feja, Z.; Holder, J.; van Drongelen, S.; Adolf, S.; Braun, S.; Böhm, H.; Meurer, A. Non-Invasive Determination of Frontal Plane Lower Limb Alignment Using Motion Capture Technique—An Alternative for Full-Length Radiographs in Young Patients treated by a Temporary Hemiepiphysiodesis? Gait Posture 2020, 79, 26–32. [Google Scholar] [CrossRef]
- Gaebe, G.; Kruse, R.; Rogers, K.; Mackenzie, W.G.; Holmes, L., Jr. Dynamic Lower Extremity Deformity in Children with Pseudoachondroplasia. J. Pediatr. Orthop. 2018, 38, 157–162. [Google Scholar] [CrossRef]
- Farr, S.; Kranzl, A.; Pablik, E.; Kaipel, M.; Ganger, R. Functional and Radiographic Consideration of Lower Limb Malalignment in Children and Adolescents with Idiopathic Genu Valgum. J. Orthop. Res. 2014, 32, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.; Stief, F.; Sander, K.; Hösl, M.; Döderlein, L. Correction of Static Axial Alignment in Children with Knee Varus or Valgus Deformities through Guided Growth: Does it also Correct Dynamic Frontal Plane Moments during Walking? Gait Posture 2015, 42, 394–397. [Google Scholar] [CrossRef]
- Holder, J.; van Drongelen, S.; Uhlrich, S.D.; Herrmann, E.; Meurer, A.; Stief, F. Peak Knee Joint Moments Accurately Predict Medial and Lateral Knee Contact Forces in Patients with Valgus Malalignment. Sci. Rep. 2023, 13, 2870. [Google Scholar] [CrossRef]
- Simic, M.; Hinman, R.S.; Wrigley, T.V.; Bennell, K.L.; Hunt, M.A. Gait Modification Strategies for Altering Medial Knee Joint Load: A Systematic Review. Arthritis Care Res. 2011, 63, 405–526. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.A.; Birmingham, T.B.; Bryant, D.; Jones, I.; Giffin, J.R.; Jenkyn, T.R.; Vandervoort, A.A. Lateral Trunk Lean Explains Variation in Dynamic Knee Joint Load in Patients with Medial Compartment Knee Osteoarthritis. Osteoarthr. Cartil. 2008, 16, 591–599. [Google Scholar] [CrossRef] [PubMed]
- van Drongelen, S.; Kaldowski, H.; Tarhan, T.; Assi, A.; Meurer, A.; Stief, F. Are Changes in Radiological Leg Alignment and Femoral Parameters After Total Hip Replacement Responsible for Joint Loading During Gait? BMC Musculoskelet. Disord. 2019, 20, 526. [Google Scholar] [CrossRef] [PubMed]
- Foxen-Craft, E.; Scott, E.L.; Kullgren, K.A.; Philliben, R.; Hyman, C.; Dorta, M.; Murphy, A.; Voepel-Lewis, T. Pain Location and Widespread Pain in Youth with Orthopaedic Conditions: Exploration of the Reliability and Validity of a Body Map. Eur. J. Pain 2019, 23, 57–65. [Google Scholar] [CrossRef]
- Sabharwal, S.; Zhao, C. The Hip-Knee-Ankle Angle in Children: Reference Values Based on a Full-Length Standing Radiograph. J. Bone Jt. Surg. Am. 2009, 91, 2461–2468. [Google Scholar] [CrossRef]
- Pinzone, O.; Schwartz, M.H.; Baker, R. Comprehensive Non-Dimensional Normalization of Gait Data. Gait Posture 2016, 44, 68–73. [Google Scholar] [CrossRef]
- Chang, A.H.; Chmiel, J.S.; Moisio, K.C.; Almagor, O.; Zhang, Y.; Cahue, S.; Sharma, L. Varus Thrust and Knee Frontal Plane Dynamic Motion in Persons with Knee Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1668–1673. [Google Scholar] [CrossRef]
- Hof, L. Scaling Gait Data to Body Size. Gait Posture 1996, 4, 222–223. [Google Scholar] [CrossRef]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Vector Field Statistical Analysis of Kinematic and Force Trajectories. J. Biomech. 2013, 46, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.T.; Burden, A.; Payton, C.; Onambélé-Pearson, G.L.; Morse, C.I. A Quantitative Description of Self-Selected Walking in Adults with Achondroplasia Using the Gait Profile Score. Gait Posture 2019, 68, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.B.; Ounpuu, S.; Tyburski, D.; Gage, J.R. A Gait Analysis Data Collection and Reduction Technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Broström, E.; Gutierrez-Farewik, E.M.; Örtqvist, M.; Hagenäs, L.; Neumeyer, L.; Rozumalski, A.; Schwartz, M.H. A comparison of functional and regression-based hip joint centers in persons with achondroplasia. Gait Posture 2009, 30, S81–S82. [Google Scholar] [CrossRef]
- Ehrig, R.M.; Taylor, W.R.; Duda, G.N.; Heller, M.O. A Survey of Formal Methods for Determining Functional Joint Axes. J. Biomech. 2007, 40, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Naili, J.E.; Broström, E.W.; Clausen, B.; Holsgaard-Larsen, A. Measures of Knee and Gait Function and Radiographic Severity of Knee Osteoarthritis—A Cross-Sectional Study. Gait Posture 2019, 74, 20–26. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, K.M.; Han, H.; Koo, S.; Kang, S.B.; Chang, C.B. Relationship Between Radiographic Measurements and Knee Adduction Moment Using 3D Gait Analysis. Gait Posture 2021, 90, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Stief, F.; Böhm, H.; Dussa, C.U.; Multerer, C.; Schwirtz, A.; Imhoff, A.B.; Döderlein, L. Effect of Lower Limb Malalignment in the Frontal Plane on Transverse Plane Mechanics During Gait in Young Individuals with Varus Knee Alignment. Knee 2014, 21, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.; Kranzl, A.; Hahne, J.; Ganger, R. Rotational Gait Patterns in Children and Adolescents Following Tension Band Plating of Idiopathic Genua Valga. J. Orthop. Res. 2017, 35, 1617–1624. [Google Scholar] [CrossRef]
- Wink, A.E.; Gross, K.D.; Brown, C.A.; Guermazi, A.; Roemer, F.; Niu, J.; Torner, J.; Lewis, C.E.; Nevitt, M.C.; Tolstykh, I.; et al. Varus Thrust During Walking and the Risk of Incident and Worsening Medial Tibiofemoral MRI Lesions: The Multicenter Osteoarthritis Study. Osteoarthr. Cartil. 2017, 25, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, L.; Grayson, J.; Hiller, C.; D’Souza, N.; Kobayashi, S.; Simic, M. Relationship Between Knee Biomechanics and Pain in People with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2023, 75, 1351–1361. [Google Scholar] [CrossRef]
- Palad, Y.Y.; Leaver, A.M.; McKay, M.J.; Baldwin, J.N.; Lunar, F.R.M.; Caube, F.D.M.; Burns, J.; Simic, M. Knee Thrust Prevalence and Normative Hip-Knee-Ankle Angle Deviation Values among Healthy Individuals Across the Lifespan. Osteoarthr. Cartil. 2018, 26, 1326–1332. [Google Scholar] [CrossRef]
- Espinosa, S.E.; Costello, K.E.; Souza, R.B.; Kumar, D. Lower Knee Extensor and Flexor Strength is Associated with Varus Thrust in People with Knee Osteoarthritis. J. Biomech. 2020, 107, 109865. [Google Scholar] [CrossRef]
- Romeo, D.M.; Pironi, V.; Velli, C.; Sforza, E.; Rigante, D.; Giorgio, V.; Leoni, C.; De Rose, C.; Kuczynska, E.M.; Limongelli, D.; et al. Ligamentous Laxity in Children with Achondroplasia: Prevalence, Joint Involvement, and Implications for Early Intervention Strategies. Eur. J. Med. Genet. 2024, 68, 104930. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.J. Hypermobility Disorders in Children and Adolescents. Best Pract. Res. Clin. Rheumatol. 2006, 20, 329–351. [Google Scholar] [CrossRef]
- De Courtiviron, B.; Bonneau, S.; Odent, T.; Finidori, G. Achondroplasie: Déviations Axiales des Membres Inféreurs. Soc. Fr. D’orthop. Pediatr. 2022, 119–123. [Google Scholar] [CrossRef]
- Takamine, Y.; Kitoh, H.; Ito, H.; Yazaki, S.; Oki, T. Patellar Dislocation in Achondroplasia. J. Pediatr. Orthop. B 2008, 17, 47–49. [Google Scholar] [CrossRef]
- Akyol, Y.; Averill, L.W.; Atanda, A.; Kecskemethy, H.H.; Bober, M.B.; Mackenzie, W.G. Magnetic Resonance Evaluation of the Knee in Children and Adolescents with Achondroplasia. Pediatr. Radiol. 2015, 45, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T.; Bernholt, D.L.; Tran, K.V.; Ain, M.C. The Tibial Slope in Patients with Achondroplasia: Its Characterization and Possible Role in Genu Recurvatum Development. J. Pediatr. Orthop. 2016, 36, 349–354. [Google Scholar] [CrossRef]
- Levinger, P.; Menz, H.B.; Morrow, A.D.; Bartlett, J.R.; Feller, J.A.; Bergman, N.R. Relationship Between Foot Function and Medial Knee Joint Loading in People with Medial Compartment Knee Osteoarthritis. J. Foot Ankle Res. 2013, 6, 33. [Google Scholar] [CrossRef]
- Ohi, H.; Iijima, H.; Fukutani, N.; Aoyama, T.; Kaneda, E.; Ohi, K.; Ito, H.; Matsuda, S.; Kaoru, A. Varus Thrust Visualized During Gait was Associated with Inverted Foot in Patients with Knee Osteoarthritis: An Exploratory Study. Gait Posture 2018, 61, 269–275. [Google Scholar] [CrossRef]




| TD (n = 31, 62 Knees) | ACH (n = 31, 62 Knees) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| Age (years) | 11.1 | 4.3 | 11.7 | 3.9 | 0.606 |
| Height (cm) | 145.9 | 24.5 | 112.9 | 13.9 | 0.027 |
| Leg length (cm) | 76.5 | 14.5 | 44.7 | 7.0 | <0.001 |
| Weight (kg) | 39.1 | 17.7 | 31.7 | 11.1 | <0.001 |
| BMI (kg/m2) | 17.3 | 2.4 | 24.0 | 3.9 | <0.001 |
| Gender [Nr of sub.]—[m/f] | 14/17 | 13/18 | 0.797 | ||
| Pain [Nr of knees]—[y/n] | 0/62 | 34/28 | <0.001 | ||
| 8-Plates [Nr of knees] (current/previous/none) | 0 | 11/8/43 | n.a. | ||
| Prediction of KAM-0 | ||||
| Predictor Variables | β | p | SE | Δ Adj. R² |
| mTFA [°] (v1) | 0.000846 | <0.001 | 0.000201 | 0.412 |
| foot progression angle [°] (v2) | 0.000663 | 0.024 | 0.000286 | 0.039 |
| Prediction equation | Total R² | |||
| KAM-0 = −0.0181 + 0.000846 × v1 + 0.000663 × v2 | 0.451 | |||
| Prediction of KAM-1 | ||||
| mTFA [°] (v1) | 0.002682 | <0.001 | 0.000292 | 0.548 |
| ipsilateral trunk lean [°] (v2) | 0.002333 | 0.032 | 0.001063 | 0.027 |
| Prediction equation | Total R² | |||
| KAM-1 = 0.080071 + 0.002682 × v1 + 0.002333 × v2 | 0.575 | |||
| Prediction of KAMmidstance | ||||
| mTFA [°] (v1) | 0.002133 | <0.001 | 0.000553 | 0.747 |
| Foot progession angle [°] (v3) | 0.000869 | 0.006 | 0.006117 | 0.026 |
| Prediction equation | Total R² | |||
| KAMmidstance = 0.04737 + 0.002133 × v1 + 0.000869 × v2 | 0.773 | |||
| Prediction of KAM-2 | ||||
| mTFA [°] (v1) | 0.002345 | <0.001 | 0.000203 | 0.671 |
| Hip rotation [°] (v2) | −0.000455 | 0.025 | 0.000197 | 0.022 |
| Prediction equation | Total R² | |||
| KAM-2 = 0.043897 + 0.002345 × v1 − 0.000455 × v2 | 0.693 | |||
| TD | ACH no pain | ACH pain | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | p-Value | |
| v (m/s) | 1.25 | 0.14 | 0.95 | 0.13 ** | 0.97 | 0.11 ** | <0.001 |
| cadence (steps/min) | 129.05 | 19.03 | 144.72 | 20.42 ** | 143.63 | 15.26 ** | <0.001 |
| step length (m) | 0.58 | 0.10 | 0.39 | 0.06 ** | 0.40 | 0.06 ** | <0.001 |
| non-dim velocity | 0.46 | 0.06 | 0.46 | 0.06 | 0.47 | 0.05 | 0.058 |
| non-dim cadence | 0.59 | 0.04 | 0.51 | 0.05 ** | 0.51 | 0.03 ** | <0.001 |
| norm step length [% LL] | 76.57 | 7.13 | 88.98 | 8.20 ** | 90.31 | 9.94 **,† | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hösl, M.; Thamm, A.; Afifi, F.K.; Nader, S. Frontal Plane Knee Kinematics and Kinetics During Gait in Children and Youth with Achondroplasia—Correspondence with Static X-Ray Images and Relevance to Symptoms. Children 2025, 12, 78. https://doi.org/10.3390/children12010078
Hösl M, Thamm A, Afifi FK, Nader S. Frontal Plane Knee Kinematics and Kinetics During Gait in Children and Youth with Achondroplasia—Correspondence with Static X-Ray Images and Relevance to Symptoms. Children. 2025; 12(1):78. https://doi.org/10.3390/children12010078
Chicago/Turabian StyleHösl, Matthias, Antonia Thamm, Faik Kamel Afifi, and Sean Nader. 2025. "Frontal Plane Knee Kinematics and Kinetics During Gait in Children and Youth with Achondroplasia—Correspondence with Static X-Ray Images and Relevance to Symptoms" Children 12, no. 1: 78. https://doi.org/10.3390/children12010078
APA StyleHösl, M., Thamm, A., Afifi, F. K., & Nader, S. (2025). Frontal Plane Knee Kinematics and Kinetics During Gait in Children and Youth with Achondroplasia—Correspondence with Static X-Ray Images and Relevance to Symptoms. Children, 12(1), 78. https://doi.org/10.3390/children12010078





