Kinetics and Kinematics of Shape Tracing in Children with Probable Developmental Coordination Disorder (pDCD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Research Tools
2.3. Procedure
3. Results
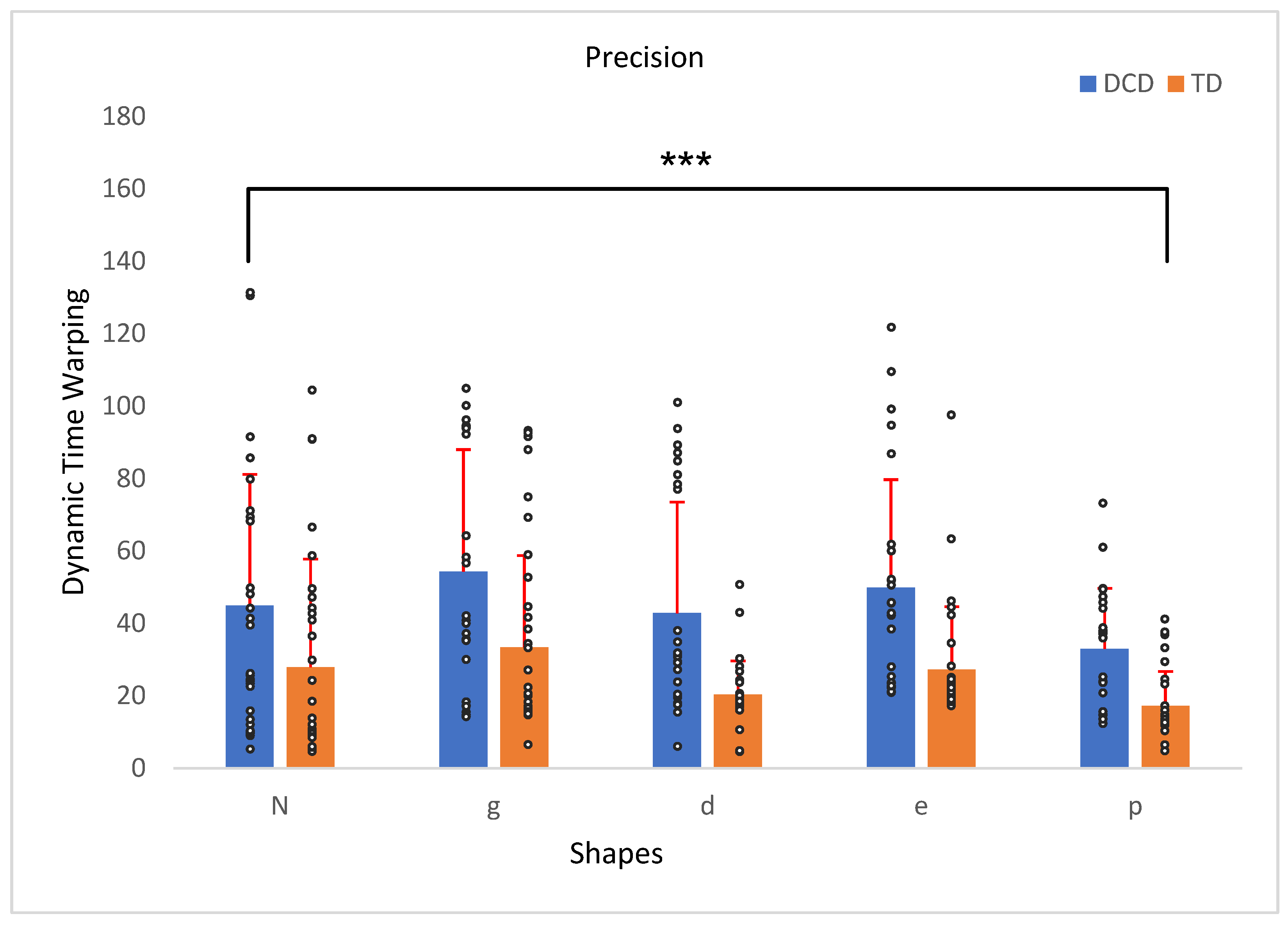
3.1. Shape Precision
3.2. Duration of Tracing
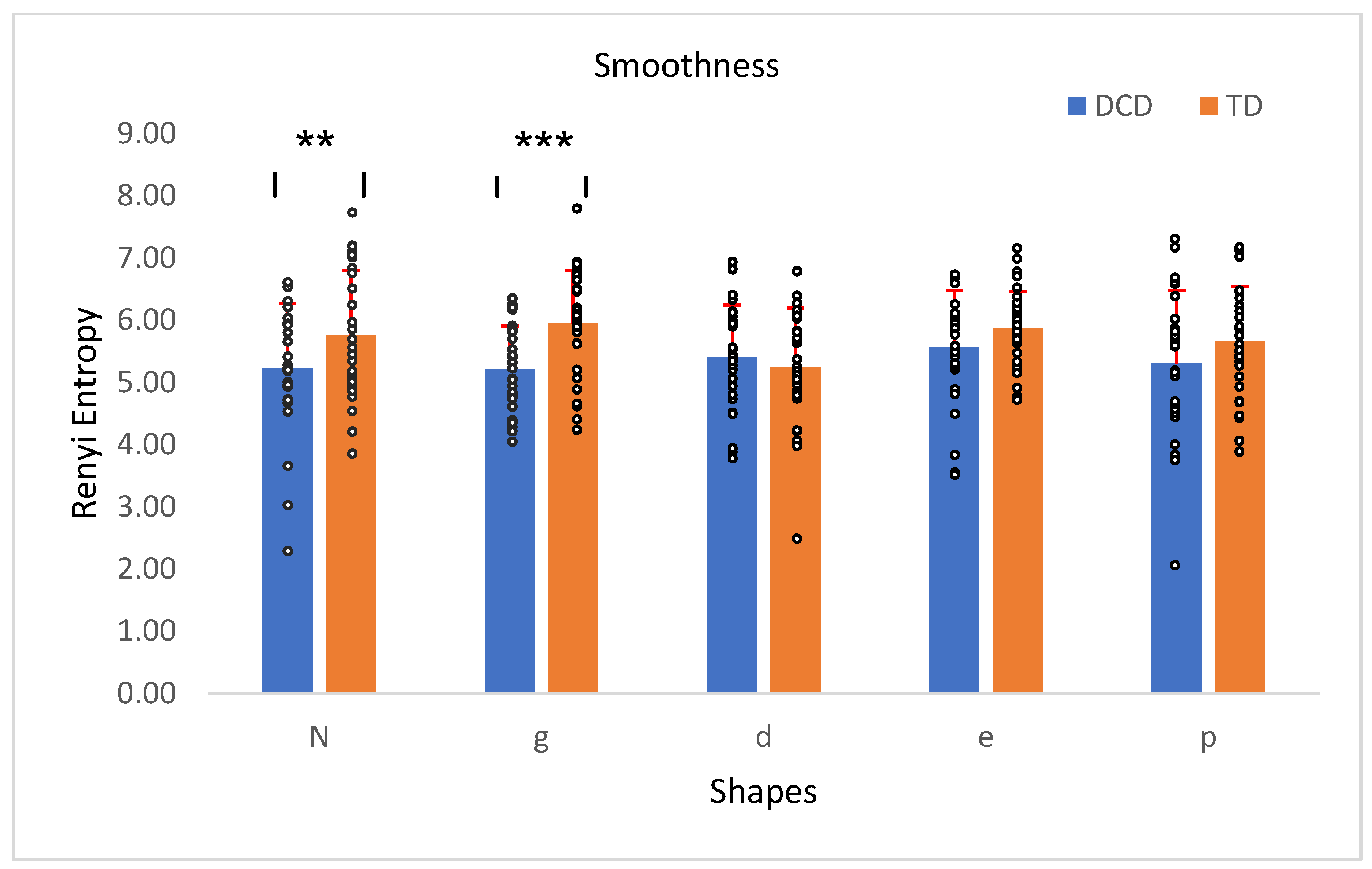
3.3. Smoothness (vs. Jerkiness)
3.4. Velocity
3.5. Pressure
4. Discussion
 or
or  , which require precise and corrective actions. Thus, the higher entropy values reflect consistency introduced by their corrective precision rather than a lack of smoothness.
, which require precise and corrective actions. Thus, the higher entropy values reflect consistency introduced by their corrective precision rather than a lack of smoothness.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lachambre, C.; Proteau-Lemieux, M.; Lepage, J.-F.; Bussières, E.-L.; Lippé, S. Attentional and executive functions in children and adolescents with developmental coordination disorder and the influence of comorbid disorders: A systematic review of the literature. PLoS ONE 2021, 16, e0252043. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013.
- Blank, R.; Barnett, A.L.; Cairney, J.; Green, D.; Kirby, A.; Polatajko, H.; Rosenblum, S.; Smits-Engelsman, B.; Sugden, D.; Wilson, P.; et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 2019, 61, 242–285. [Google Scholar] [CrossRef] [PubMed]
- Prunty, M.; Barnett, A.L.; Wilmut, K.; Plumb, M.S. The impact of handwriting difficulties on compositional quality in children with developmental coordination disorder. Br. J. Occup. Ther. 2016, 79, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Bartov, R.; Wagner, M.; Shvalb, N.; Hochhauser, M. Enhancing Handwriting Performance of Children with Developmental Coordination Disorder (DCD) Using Computerized Visual Feedback. Children 2023, 10, 1534. [Google Scholar] [CrossRef]
- Prunty, M.; Barnett, A.L. Accuracy and Consistency of Letter Formation in Children with Developmental Coordination Disorder. J. Learn. Disabil. 2020, 53, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, S.; Livneh-Zirinski, M. Handwriting process and product characteristics of children diagnosed with developmental coordination disorder. Hum. Mov. Sci. 2008, 27, 200–214. [Google Scholar] [CrossRef]
- Barnett, A.L.; Prunty, M. Handwriting Difficulties in Developmental Coordination Disorder (DCD). Curr. Dev. Disord. Rep. 2021, 8, 6–14. [Google Scholar] [CrossRef]
- Park, W.; Babushkin, V.; Tahir, S.; Eid, M. Haptic Guidance to Support Handwriting for Children with Cognitive and Fine Motor Delays. IEEE Trans. Haptics 2021, 14, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Van Galen, G.P. Handwriting: Issues for a psychomotor theory. Hum. Mov. Sci. 1991, 10, 165–191. [Google Scholar] [CrossRef]
- Adams, I.L.J.; Lust, J.M.; Wilson, P.H.; Steenbergen, B. Testing predictive control of movement in children with developmental coordination disorder using converging operations. Br. J. Psychol. 2017, 108, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Van Hartingsveldt, M.J.; Cup, E.H.C.; Hendriks, J.C.M.; De Vries, L.; De Groot, I.J.M.; Der Sanden, M.W.G.N.-V. Predictive validity of kindergarten assessments on handwriting readiness. Res. Dev. Disabil. 2015, 36, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.J.; Kelley, G.T.; Krauss, A. Relationship Between Visual-Motor Integration and Handwriting Skills of Children in Kindergarten: A Modified Replication Study. Am. J. Occup. Ther. 2003, 57, 459–462. [Google Scholar] [CrossRef]
- Volman, M.J.M.; Van Schendel, B.M.; Jongmans, M.J. Handwriting Difficulties in Primary School Children: A Search for Underlying Mechanisms. Am. J. Occup. Ther. 2006, 60, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Dinehart, L.H. Handwriting in early childhood education: Current research and future implications. J. Early Child. Lit. 2015, 15, 97–118. [Google Scholar] [CrossRef]
- Adi-Japha, E.; Strulovich-Schwartz, O.; Julius, M. Delayed motor skill acquisition in kindergarten children with language impairment. Res. Dev. Disabil. 2011, 32, 2963–2971. [Google Scholar] [CrossRef]
- Julius, M.S.; Adi-Japha, E. Learning of a simple grapho-motor task by young children and adults: Similar acquisition but age-dependent retention. Front. Psychol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Zwicker, J.G.; Lee, E.J. Early intervention for children with/at risk of developmental coordination disorder: A scoping review. Dev. Med. Child Neurol. 2021, 63, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Beery, K.; Buktenica, N.; Beery, N.; Keith, E. Developmental Test of Visual-Motor Integration, 6th ed.; NSC Pearson: Minneap, MN, USA, 2010. [Google Scholar]
- Bruininks, R. Bruininks-Oseretsky Test of Motor Proficiency Second Edition (BOT2); AGS Publishing: Circle Pines, MN, USA, 2005. [Google Scholar]
- Chu, V.; Krishnan, K. Quantitative Assessment of Prewriting Skills in Children: The Development and Validation of a Tablet Assessment Tool. Percept. Mot. Ski. 2022, 129, 554–569. [Google Scholar] [CrossRef]
- Thorsson, M.; Galazka, M.A.; Hajjari, P.; Fernell, E.; Delafield-Butt, J.; Gillberg, C.; Johnson, M.; Johnels, J.; Hadjikhani, N. A novel tablet-based motor coordination test performs on par with the Beery VMI subtest and offers superior temporal metrics: Findings from children with pediatric acute-onset neuropsychiatric syndrome. Exp. Brain Res. 2023, 241, 5. [Google Scholar] [CrossRef]
- Schott, N.; El-Rajab, I.; Klotzbier, T. Cognitive-motor interference during fine and gross motor tasks in children with Developmental Coordination Disorder (DCD). Res. Dev. Disabil. 2016, 57, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.G.; Missiuna, C.; Harris, S.R.; Boyd, L.A. Brain activation associated with motor skill practice in children with developmental coordination disorder: An fMRI study. Int. J. Dev. Neurosci. 2011, 29, 145–152. [Google Scholar] [CrossRef]
- Snapp-Childs, W.; Casserly, E.; Mon-Williams, M.; Bingham, G.P. Active Prospective Control Is Required for Effective Sensorimotor Learning. PLoS ONE 2013, 8, e77609. [Google Scholar] [CrossRef]
- Snapp-Childs, W.; Flatters, I.; Fath, A.; Mon-Williams, M.; Bingham, G.P. Training Compliance Control Yields Improvements in Drawing as a Function of Beery Scores. PLoS ONE 2014, 9, e92464. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.L.; Prunty, M.; Rosenblum, S. Development of the Handwriting Legibility Scale (HLS): A preliminary examination of Reliability and Validity. Res. Dev. Disabil. 2018, 72, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.N.; Roebers, C.M. New insights into visual-motor integration exploring process measures during copying shapes. Psychol. Sport Exerc. 2021, 55, 101954. [Google Scholar] [CrossRef]
- Bo, J.; Colbert, A.; Lee, C.-M.; Schaffert, J.; Oswald, K.; Neill, R. Examining the relationship between motor assessments and handwriting consistency in children with and without probable Developmental Coordination Disorder. Res. Dev. Disabil. 2014, 35, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Mergl, R.; Tigges, P.; Schröter, A.; Möller, H.-J.; Hegerl, U. Digitized analysis of handwriting and drawing movements in healthy subjects: Methods, results and perspectives. J. Neurosci. Methods 1999, 90, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Mekyska, J.; Faundez-Zanuy, M.; Mzourek, Z.; Galaz, Z.; Smekal, Z.; Rosenblum, S. Identification and Rating of Developmental Dysgraphia by Handwriting Analysis. IEEE Trans. Hum. Mach. Syst. 2017, 47, 235–248. [Google Scholar] [CrossRef]
- Henderson, S.E.; Sugden, D.; Barnett, A.L. Movement Assessment Battery for Children: Manual, 2nd ed.; Psychol. Corp.: London, UK, 2007; Available online: https://cir.nii.ac.jp/crid/1370565169420927365 (accessed on 14 November 2024).
- Di Brina, C.; Niels, R.; Overvelde, A.; Levi, G.; Hulstijn, W. Dynamic time warping: A new method in the study of poor handwriting. Hum. Mov. Sci. 2008, 27, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Drotár, P.; Mekyska, J.; Rektorová, I.; Masarová, L.; Smékal, Z.; Faundez-Zanuy, M. Decision Support Framework for Parkinson’s Disease Based on Novel Handwriting Markers. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 508–516. [Google Scholar] [CrossRef]
- Chang, S.-H.; Yu, N.-Y. Characterization of motor control in handwriting difficulties in children with or without developmental coordination disorder. Dev. Med. Child Neurol. 2010, 52, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.H.; Smits-Engelsman, B.; Caeyenberghs, K.; Steenbergen, B.; Sugden, D.; Clark, J.; Mumford, N.; Blank, R. Cognitive and neuroimaging findings in developmental coordination disorder: New insights from a systematic review of recent research. Dev. Med. Child Neurol. 2017, 59, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Adi-Japha, E.; Brestel, G. Motor skill learning with impaired transfer by children with developmental coordination disorder. Res. Dev. Disabil. 2020, 103, 103671. [Google Scholar] [CrossRef]
- Berger, S.E.; Adolph, K.E.; Kavookjian, A.E. Bridging the Gap: Solving Spatial Means–Ends Relations in a Locomotor Task. Child Dev. 2010, 81, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-T.; Tsai, C.-L.; Chen, F.-C.; Konczak, J. Wrist position sense acuity and its relation to motor dysfunction in children with developmental coordination disorder. Neurosci. Lett. 2018, 674, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.G.; Taylor, A.J. Children’s errors in copying angles: Perpendicular error or bisection error? Perception 1982, 11, 163–171. [Google Scholar]
- Ibbotson, A.; Bryant, P.E. The perpendicular error and the vertical effect in children’s drawing. Perception 1976, 5, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Yu, N.-Y. Visual and Haptic Perception Training to Improve Handwriting Skills in Children with Dysgraphia. Am. J. Occup. Ther. 2017, 71, 7102220030p1–7102220030p10. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, M.M.; Van Der Wees, M.; Flapper, B.; Verheij-Jansen, N.; Scholten-Jaegers, S.; Geuze, R.H. Perceptual skills of children with developmental coordination disorder. Hum. Mov. Sci. 2001, 20, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Prunty, M.; Barnett, A.L.; Wilmut, K.; Plumb, M.S. An examination of writing pauses in the handwriting of children with Developmental Coordination Disorder. Res. Dev. Disabil. 2014, 35, 2894–2905. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Hadjiosif, A.M.; Xu, J.; Wong, A.L.; Haith, A.M. Motor learning. Compr. Physiol. 2019, 9, 613–663. [Google Scholar]
- Pless, M.; Carlsson, M. Effects of Motor Skill Intervention on Developmental Coordination Disorder: A Meta-Analysis. Adapt. Phys. Act. Q. 2000, 17, 381–401. [Google Scholar] [CrossRef]
- Wilson, P.H.; Ruddock, S.; Smits-Engelsman, B.; Polatajko, H.; Blank, R. Understanding performance deficits in developmental coordination disorder: A meta-analysis of recent research. Dev. Med. Child Neurol. 2013, 55, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.P.; Skinner, R.A. Timing and force control during a sequential tapping task in children with and without motor coordination problems. J. Int. Neuropsychol. Soc. 1999, 5, 320–329. [Google Scholar] [CrossRef]
- Missiuna, C.; Rivard, L.; Pollock, N. Children with Developmental Coordination Disorder; CanChild Centre for Childhood Disability Research, School of Rehabilitation Science: Hamilton, ON, Canada, 2011. [Google Scholar]
- Bartov, R.; Wagner, M.; Shvalb, N.; Hochhauser, M. Evaluating handwriting in children with developmental coordination disorder (DCD): Temporal, spatial, pressure and grip-force measures. Res. Dev. Disabil. 2024, 151, 104765. [Google Scholar] [CrossRef] [PubMed]


| Variable | pDCD Group N = 27 | TD Group N = 31 | Ӽ2 | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | 0.6 | ||||
| Boys | 17 | 63 | 17 | 55 | |
| Girls | 10 | 37 | 14 | 45 | |
| M | SD | M | SD | t(56), p | |
| Age (year) | 9.64 | 1.03 | 9.97 | 1.18 | 1.14, 0.26 |
| pDCD Group N = 27 | TD Group N = 31 | ||||
| MABC-2 | M | SD | M | SD | t(56), p |
| Manual dexterity | 14.19 | 3.3 | 55.48 | 27.79 | 9.68, <0.001 |
| Variable | pDCD Group n = 27 | TD Group n = 31 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | df | F | p | η2 | |
| Precision 1 | 45.9 | 21.78 | 28.2 | 15.53 | 1, 56 | 12.96 | 0.001 | 0.19 |
| Duration (sec) 2 | 19.5 | 1.09 | 17.43 | 2.94 | 1, 56 | 11.78 | 0.001 | 0.17 |
| Smoothness 3 | 5.34 | 0.61 | 5.7 | 0.63 | 1, 56 | 4.67 | 0.04 | 0.08 |
| Velocity 4 | 0.11 | 0.07 | 0.17 | 0.07 | 1, 56 | 9.23 | 0.004 | 0.14 |
| Pressure 5 | 0.63 | 0.09 | 0.69 | 0.1 | 1, 56 | 5.46 | 0.02 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochhauser, M.; Ben Refael, Y.; Adi-Japha, E.; Bartov, R. Kinetics and Kinematics of Shape Tracing in Children with Probable Developmental Coordination Disorder (pDCD). Children 2025, 12, 90. https://doi.org/10.3390/children12010090
Hochhauser M, Ben Refael Y, Adi-Japha E, Bartov R. Kinetics and Kinematics of Shape Tracing in Children with Probable Developmental Coordination Disorder (pDCD). Children. 2025; 12(1):90. https://doi.org/10.3390/children12010090
Chicago/Turabian StyleHochhauser, Michal, Yfat Ben Refael, Esther Adi-Japha, and Rachel Bartov. 2025. "Kinetics and Kinematics of Shape Tracing in Children with Probable Developmental Coordination Disorder (pDCD)" Children 12, no. 1: 90. https://doi.org/10.3390/children12010090
APA StyleHochhauser, M., Ben Refael, Y., Adi-Japha, E., & Bartov, R. (2025). Kinetics and Kinematics of Shape Tracing in Children with Probable Developmental Coordination Disorder (pDCD). Children, 12(1), 90. https://doi.org/10.3390/children12010090





