Early-Life Events and the Prevalence of Gut–Brain Interaction Disorders in Children
Highlights
- •
- Environmental factors in the first 3 years of life could influence postnatal development and lifelong health and well-being.
- •
- Breastfeeding, particularly for ≥3 months, is the most important protective factor against DGBI, whereas antibiotic/antiviral exposure, particularly in the first year of life, may increase the risk of DGBI development.
- •
- Exclusive breastfeeding should be continuously promoted to prevent the occurrence of DGBI.
- •
- The rational use of antibiotics/antivirals should be advocated to decrease the risk of developing DGBI.
Abstract
1. Introduction
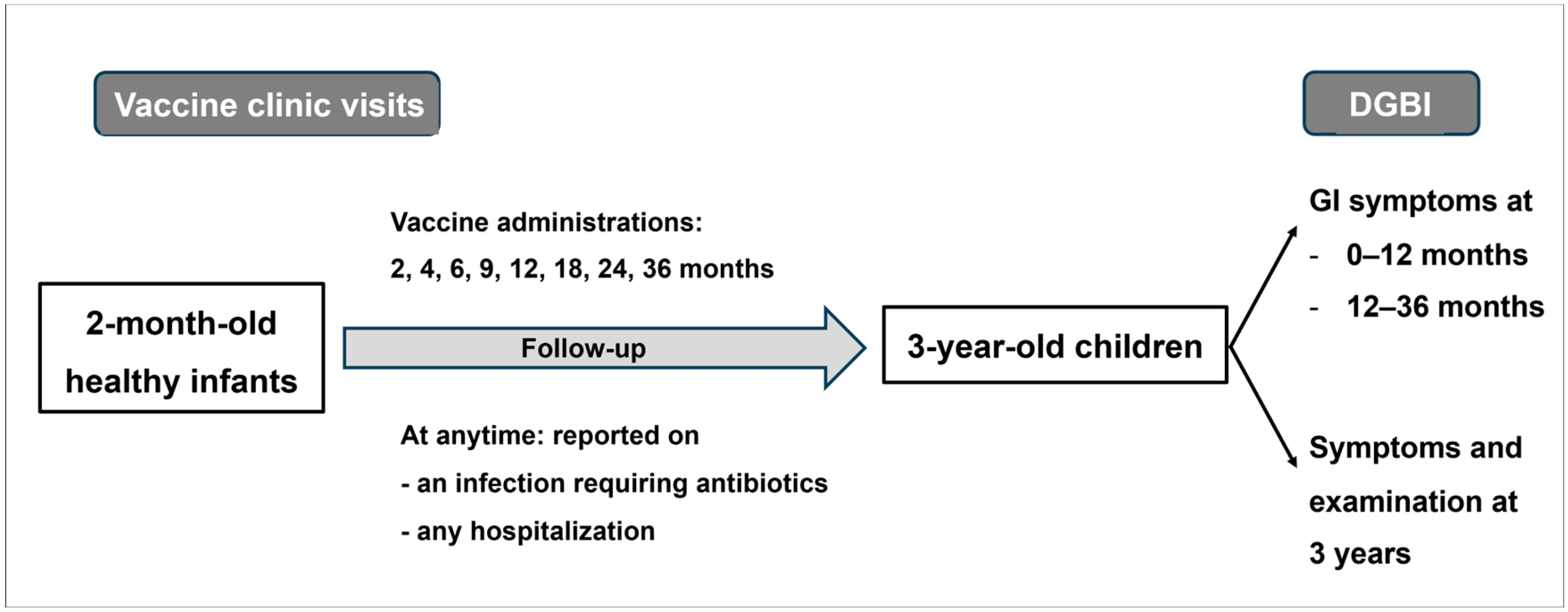
2. Materials and Methods
3. Results
3.1. Early-Life Factors
3.2. Prevalence of DGBI in Children Aged 3 Years
3.3. Association Between Early-Life Factors and DGBI Within 3 Years of Age
3.4. Functional Constipation in 3-Year-Old Children
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| DGBI | Disorders of gut–brain interaction |
| GI | Gastrointestinal |
| HMO | Human milk oligosaccharides |
| IQR | Interquartile range |
| OR | Odds ratio |
References
- Jena, A.; Montoya, C.A.; Mullaney, J.A.; Dilger, R.N.; Young, W.; McNabb, W.C.; Roy, N.C. Gut-Brain Axis in the Early Postnatal Years of Life: A Developmental Perspective. Front. Integr. Neurosci. 2020, 14, 44. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Chanpong, A.; Borrelli, O.; Thapar, N. Recent Advances in Understanding the Roles of the Enteric Nervous System. Fac. Rev. 2022, 11, 7. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural History of the Infant Gut Microbiome and Impact of Antibiotic Treatment on Bacterial Strain Diversity and Stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.Y.; Boonma, P.; Unterweger, P.; Parathan, P.; Haag, A.; Luna, R.A.; Bornstein, J.C.; Savidge, T.C.; Foong, J.P.P. Neonatal Antibiotics Disrupt Motility and Enteric Neural Circuits in Mouse Colon. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 298. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased Gut Microbiota Diversity, Delayed Bacteroidetes Colonisation and Reduced Th1 Responses in Infants Delivered by Caesarean Section. Gut 2014, 63, 559. [Google Scholar] [CrossRef]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.-A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal Factors Affect the Gut Microbiota up to Four Years after Birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Fichter, M.; Klotz, M.; Hirschberg, D.L.; Waldura, B.; Schofer, O.; Ehnert, S.; Schwarz, L.K.; Ginneken, C.V.; Schäfer, K.-H. Breast Milk Contains Relevant Neurotrophic Factors and Cytokines for Enteric Nervous System Development. Mol. Nutr. Food Res. 2011, 55, 1592. [Google Scholar] [CrossRef] [PubMed]
- Thapar, N.; Benninga, M.A.; Crowell, M.D.; Di Lorenzo, C.; Mack, I.; Nurko, S.; Saps, M.; Shulman, R.J.; Szajewska, H.; van Tilburg, M.A.L.; et al. Paediatric Functional Abdominal Pain Disorders. Nat. Rev. Dis. Primers 2020, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, K.; Van Daele, E.; Vlieger, A.M.; Daams, J.G.; Knol, J.; van Elburg, R.M. Early Life Antibiotics and Childhood Gastrointestinal Disorders: A Systematic Review. BMJ Paediatr. Open 2021, 5, e001028. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal Microbiota Influence the Early Postnatal Development of the Enteric Nervous System. Neurogastroenterol. Motil. 2014, 26, 98. [Google Scholar] [CrossRef]
- Nurgali, K.; Qu, Z.; Hunne, B.; Thacker, M.; Pontell, L.; Furness, J.B. Morphological and Functional Changes in Guinea-Pig Neurons Projecting to the Ileal Mucosa at Early Stages after Inflammatory Damage. J. Physiol. 2011, 589, 325. [Google Scholar] [CrossRef]
- Pensabene, L.; Salvatore, S.; D’Auria, E.; Parisi, F.; Concolino, D.; Borrelli, O.; Thapar, N.; Staiano, A.; Vandenplas, Y.; Saps, M. Cow’s Milk Protein Allergy in Infancy: A Risk Factor for Functional Gastrointestinal Disorders in Children? Nutrients 2018, 10, 1716. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263. [Google Scholar] [CrossRef]
- Usui, N.; Matsuzaki, H.; Shimada, S. Characterization of Early Life Stress-Affected Gut Microbiota. Brain Sci. 2021, 11, 913. [Google Scholar] [CrossRef]
- Zhu, J.; Zhong, Z.; Shi, L.; Huang, L.; Lin, C.; He, Y.; Xia, X.; Zhang, T.; Ding, W.; Yang, Y. Gut Microbiota Mediate Early Life Stress-Induced Social Dysfunction and Anxiety-Like Behaviors by Impairing Amino Acid Transport at the Gut. Gut Microbes 2024, 16, 2401939. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, J.L.; Whittemore, A.S.; Evans, A.S.; Thompson, W.D. Methods in Observational Epidemiology, Tables 12–15 Fleiss, Statistical Methods for Rates and Proportions, Formulas 3.18 & 3.19, 2nd ed.; Oxford University Press Inc.: Oxford, UK, 1996. [Google Scholar]
- Chia, L.W.; Nguyen, T.V.H.; Phan, V.N.; Luu, T.T.N.; Nguyen, G.K.; Tan, S.Y.; Rajindrajith, S.; Benninga, M.A. Prevalence and Risk Factors of Functional Gastrointestinal Disorders in Vietnamese Infants and Young Children. BMC Pediatr. 2022, 22, 315. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455.e2. [Google Scholar] [CrossRef] [PubMed]
- Scarpato, E.; Salvatore, S.; Romano, C.; Bruzzese, D.; Ferrara, D.; Inferrera, R.; Zeevenhooven, J.; Steutel, N.F.; Benninga, M.A.; Staiano, A. Prevalence and Risk Factors of Functional Gastrointestinal Disorders: A Cross-Sectional Study in Italian Infants and Young Children. J. Pediatr. Gastroenterol. Nutr. 2023, 76, e27. [Google Scholar] [CrossRef]
- Natividad, J.M.; Marsaux, B.; Rodenas, C.L.G.; Rytz, A.; Vandevijver, G.; Marzorati, M.; Van den Abbeele, P.; Calatayud, M.; Rochat, F. Human Milk Oligosaccharides and Lactose Differentially Affect Infant Gut Microbiota and Intestinal Barrier In Vitro. Nutrients 2022, 14, 2546. [Google Scholar] [CrossRef]
- Ferrier, L.; Eutamène, H.; Siegwald, L.; Marquard, A.M.; Tondereau, V.; Chevalier, J.; Jacot, G.E.; Favre, L.; Theodorou, V.; Vicario, M.; et al. Human Milk Oligosaccharides Alleviate Stress-Induced Visceral Hypersensitivity and Associated Microbiota Dysbiosis. J. Nutr. Biochem. 2022, 99, 108865. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.L.; Palsson, O.S.; Whitehead, W.E.; van Tilburg, M.A.L. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents. J. Pediatr. 2016, 177, 39. [Google Scholar] [CrossRef] [PubMed]
- Scarpato, E.; Kolacek, S.; Jojkic-Pavkov, D.; Konjik, V.; Živković, N.; Roman, E.; Kostovski, A.; Zdraveska, N.; Altamimi, E.; Papadopoulou, A.; et al. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents in the Mediterranean Region of Europe. Clin. Gastroenterol. Hepatol. 2018, 16, 870. [Google Scholar] [CrossRef] [PubMed]
- Cenni, S.; Pensabene, L.; Dolce, P.; Campanozzi, A.; Salvatore, S.; Pujia, R.; Serra, M.R.; Scarpato, E.; Miele, E.; Staiano, A.; et al. Prevalence of Functional Gastrointestinal Disorders in Italian Children Living in Different Regions: Analysis of the Difference and the Role of Diet. Dig. Liver Dis. 2023, 55, 1640. [Google Scholar] [CrossRef]
- Garcia, T.M.; van Roest, M.; Vermeulen, J.L.M.; Meisner, S.; Smit, W.L.; Silva, J.; Koelink, P.J.; Koster, J.; Faller, W.J.; Wildenberg, M.E.; et al. Early Life Antibiotics Influence In Vivo and In Vitro Mouse Intestinal Epithelium Maturation and Functioning. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Long-Lasting Effects of Early-Life Antibiotic Treatment and Routine Animal Handling on Gut Microbiota Composition and Immune System in Pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; van Houten, M.A.; Watson, R.L.; Chu, M.L.J.N.; Arp, K.; de Waal, W.J.; Schiering, I.; Plötz, F.B.; Willems, R.J.L.; van Schaik, W.; et al. Effects of Early-Life Antibiotics on the Developing Infant Gut Microbiome and Resistome: A Randomized Trial. Nat. Commun. 2022, 13, 893. [Google Scholar] [CrossRef]
- Salvatore, S.; Baldassarre, M.E.; Di Mauro, A.; Laforgia, N.; Tafuri, S.; Bianchi, F.P.; Dattoli, E.; Morando, L.; Pensabene, L.; Meneghin, F.; et al. Neonatal Antibiotics and Prematurity Are Associated with an Increased Risk of Functional Gastrointestinal Disorders in the First Year of Life. J. Pediatr. 2019, 212, 44. [Google Scholar] [CrossRef]
- Chanpong, A.; Borrelli, O.; Thapar, N. The Potential Role of Microorganisms on Enteric Nervous System Development and Disease. Biomolecules 2023, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Foong, J.P.P.; Hung, L.Y.; Poon, S.; Savidge, T.C.; Bornstein, J.C. Early Life Interaction between the Microbiota and the Enteric Nervous System. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G541. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Sencio, V.; Gallerand, A.; Gomes Machado, M.; Deruyter, L.; Heumel, S.; Soulard, D.; Barthelemy, J.; Cuinat, C.; Vieira, A.T.; Barthelemy, A.; et al. Influenza Virus Infection Impairs the Gut’s Barrier Properties and Favors Secondary Enteric Bacterial Infection through Reduced Production of Short-Chain Fatty Acids. Infect. Immun. 2021, 89, e00734-20. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | No DGBI (n = 429) | DGBI (n = 139) | p-Value |
|---|---|---|---|
| Male sex, n (%) | 219 (51.0) | 57 (41.0) | 0.050 |
| Weight, kg (IQR) | 13.4 (12.2–14.7) | 13.8 (12.2–15.0) | 0.482 |
| Height, cm (IQR) | 93.5 (91.0–96.0) | 93.0 (91.0–96.0) | 0.850 |
| Infection history during pregnancy, n (%) | 11 (2.6) | 3 (2.2) | 1.000 |
| Cesarean section, n (%) | 174 (41.0) | 54 (38.8) | 0.721 |
| Low Apgar score (≤7) at 1 min, n (%) | 6 (1.4) | 3 (2.2) | 0.463 |
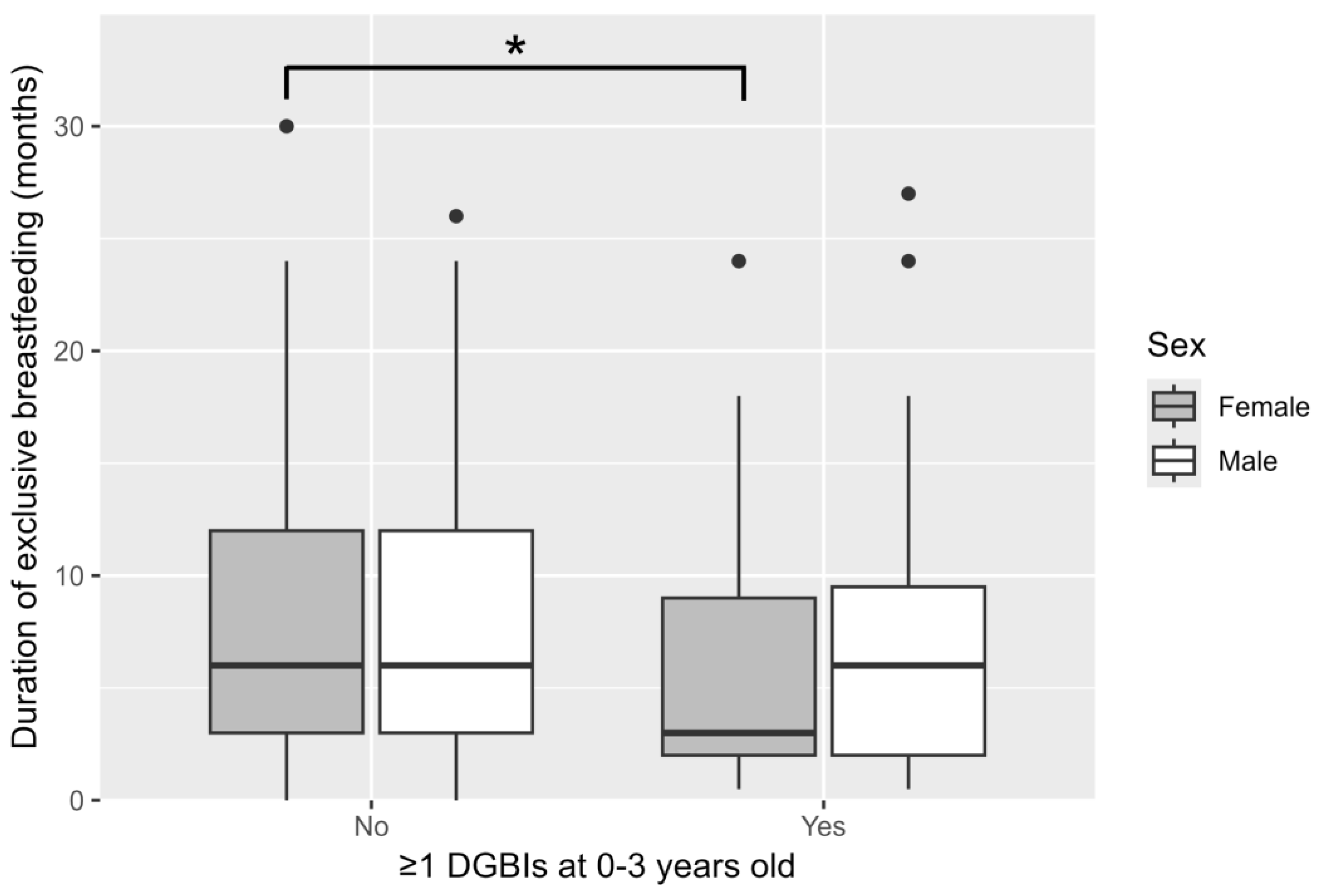
| Duration of exclusive breastfeeding, months (IQR) | 6.0 (3.0–12.0) | 4.0 (2.0–9.0) | 0.001 |
| ≥3 months of exclusive breastfeeding, n (%) | 295 (68.8) | 81 (58.3) | 0.030 |
| 1-year breastfeeding, n (%) | 108 (25.2) | 19 (13.8) | 0.007 |
| Bottle feeding, n (%) | 365 (85.1) | 117 (84.2) | 0.902 |
| Age at first antibiotics/antiviral exposure, months (IQR) | 17.0 (11.0–23.0) | 18.0 (11.0–24.0) | 0.227 |
| Age at first antibiotics/antiviral exposure, n (%) | 0.611 | ||
| No exposure | 93 (21.7) | 31 (22.3) | |
| ≤6 months | 37 (8.6) | 7 (5.0) | |
| >6–12 months | 55 (12.8) | 21 (15.1) | |
| >12–24 months | 169 (39.4) | 52 (37.4) | |
| >24–36 months | 75 (17.5) | 28 (20.1) | |
| Exposure to antibiotics within the 1st year of life, n (%) | 75 (17.5) | 16 (11.5) | 0.125 |
| Age at 1st exposure to antibiotics within the 1st year of life, months * | 6.4 ± 2.4 | 7.8 ± 2.1 | 0.035 |
| Exposure to antivirals within the 1st year of life, n (%) | 20 (4.7) | 12 (8.6) | 0.120 |
| Age at 1st exposure to antivirals within the 1st year of life, months * | 10.2 ± 2.0 | 8.0 ± 2.4 | 0.008 |
| Exposure to secondhand smoke, n (%) | 260 (60.6) | 94 (67.6) | 0.166 |
| History of gastroenteritis requiring hospitalization, n (%) | 40 (9.3) | 14 (10.1) | 0.924 |
| Influenza infection within 3 years, n (%) | 12 (2.8) | 9 (6.5) | 0.083 |
| COVID-19 infection within 1st 6-month, n (%) | 0 (0) | 2 (1.4) | 0.060 |
| History of food allergy, n (%) | 29 (7.4) | 8 (6.3) | 0.831 |
| Asthma, n (%) | 11 (2.8) | 2 (1.6) | 0.744 |
| Atopic dermatitis, n (%) | 24 (6.1) | 7 (5.5) | 0.960 |
| Family history of allergic diseases | |||
| - Father, n (%) | 56 (13.1) | 19 (13.7) | 0.966 |
| - Mother, n (%) | 50 (11.7) | 23 (16.5) | 0.176 |
| - Sibling, n (%) | 173 (40.3) | 60 (43.2) | 0.623 |
| Socioeconomic status | |||
| - Paternal income, Baht/month (IQR) | 15,000 (9500–20,000) | 15,000 (9000–20,000) | 0.906 |
| - Maternal income, Baht/month (IQR) | 9000 (0–15,000) | 10,000 (0–15,000) | 0.867 |
| Paternal education, n (%) | 0.807 | ||
| - Bachelor’s degree | 98 (23.5) | 33 (24.4) | |
| - Higher than bachelor’s degree | 8 (1.9) | 2 (1.5) | |
| - Less than or equal to high school | 235 (56.4) | 71 (52.6) | |
| - Vocational training | 76 (18.2) | 29 (21.5) | |
| Maternal education, n (%) | 0.700 | ||
| - Bachelor’s degree | 185 (43.1) | 53 (38.1) | |
| - Higher than bachelor’s degree | 13 (3.0) | 4 (2.9) | |
| - Less than or equal to high school | 173 (40.3) | 59 (42.4) | |
| - Vocational training | 58 (13.5) | 23 (16.5) |
| Factor | Crude OR (95% CI) | Adj. OR (95% CI) | p-Value (Wald’s Test) | p-Value (LR-Test) |
|---|---|---|---|---|
| Exclusive breastfeeding for ≥3 months: Yes vs. No | 0.63 (0.43, 0.94) | 0.66 (0.44, 0.99) | 0.043 | 0.044 |
| Sex: Male vs. Female | 0.67 (0.45, 0.98) | 0.66 (0.44, 0.97) | 0.038 | 0.037 |
| Maternal history of allergy: Yes vs. No | 1.50 (0.87, 2.54) | 1.65 (0.94, 2.83) | 0.073 | 0.080 |
| Exposure to secondhand smoke: Yes vs. No | 1.36 (0.91, 2.05) | 1.29 (0.86, 1.96) | 0.231 | 0.228 |
| Exposure to antibiotics within 1 year: Yes vs. No | 0.61 (0.33, 1.07) | 0.60 (0.32, 1.05) | 0.086 | 0.074 |
| Exposure to antivirals within 1 year: Yes vs. No | 1.93 (0.89, 4.01) | 2.02 (0.92, 4.30) | 0.071 | 0.079 |
| Characteristics | No Constipation (n = 437) | Constipation (n = 116) | p-Value |
|---|---|---|---|
| Male sex, n (%) | 228 (50.4) | 48 (41.4) | 0.101 |
| Weight, kg (IQR) | 13.4 (12.2–14.7) | 13.8 (12.3–15.0) | 0.414 |
| Height, cm (IQR) | 93.5 (91.0–96.0) | 93.0 (91.0–96.0) | 0.918 |
| Infection history during pregnancy, n (%) | 13 (2.9) | 1 (0.9) | 0.320 |
| Cesarean section, n (%) | 183 (40.9) | 45 (38.8) | 0.754 |
| Low Apgar score (≤7) at 1 min, n (%) | 8 (1.8) | 1 (0.9) | 0.694 |
| Duration of exclusive breastfeeding, months (IQR) | 6.0 (3.0–12.0) | 4.0 (2.0–8.0) | 0.001 |
| ≥6 months of exclusive breastfeeding, n (%) | 228 (50.4) | 45 (38.8) | 0.033 |
| 1-year breastfeeding, n (%) | 111 (24.7) | 16 (13.8) | 0.017 |
| Bottle feeding, n (%) | 383 (84.7) | 99 (85.3) | 0.985 |
| Age at first antibiotics/antiviral exposure, months (IQR) | 17.0 (11.0–23.0) | 18.0 (11.8–23.2) | 0.271 |
| Days of antibiotics/antiviral exposure within 6 months, days (IQR) | 7.0 (3.0–10.0) | 7.0 (5.0–11.0) | 0.494 |
| Days of antibiotics/antiviral exposure within 12 months, days (IQR) | 7.0 (5.0–10.0) | 5.0 (5.0–10.0) | 0.281 |
| Exposure to antibiotics within the 1st year of life, n (%) | 77 (17.0) | 14 (12.1) | 0.246 |
| Age at 1st exposure to antibiotics within the 1st year of life, months * | 6.4 ± 2.4 | 7.8 ± 2.3 | 0.040 |
| Exposure to antivirals within the 1st year of life, n (%) | 25 (5.5) | 7 (6.0) | 1.000 |
| Age at 1st exposure to antivirals within the 1st year of life, months * | 10.0 ± 2.0 | 7.2 ± 2.5 | 0.004 |
| Exposure to secondhand smoke, n (%) | 275 (60.8) | 79 (68.1) | 0.183 |
| Influenza infection within 3 years, n (%) | 13 (2.9) | 8 (6.9) | 0.053 |
| COVID-19 infection within 1st 6-month, n (%) | 0 | 2 (1.7) | 0.041 |
| COVID-19 infection within 1st year, n (%) | 25 (5.5) | 7 (6.0) | 1.000 |
| Toilet training, n (%) | 395 (87.4) | 93 (80.2) | 0.065 |
| Successful toilet training, n (%) | 333 (84.3) | 74 (80.4) | 0.456 |
| History of food allergy, n (%) | 33 (8.0) | 4 (3.8) | 0.207 |
| Asthma, n (%) | 12 (2.9) | 1 (1.0) | 0.482 |
| Atopic dermatitis, n (%) | 26 (6.3) | 5 (4.7) | 0.710 |
| Family history of allergic diseases | |||
| - Father, n (%) | 61 (13.5) | 14 (12.1) | 0.802 |
| - Mother, n (%) | 54 (11.9) | 19 (16.4) | 0.264 |
| - Sibling, n (%) | 183 (40.5) | 50 (43.1) | 0.685 |
| Socioeconomic status | |||
| - Paternal income, Baht/month (IQR) | 15,000 (9500–20,000) | 14,000 (9000–20,000) | 0.857 |
| - Maternal income, Baht/month (IQR) | 9000 (0–15,000) | 10,000 (0–15,000) | 0.689 |
| Paternal education, n (%) | 0.608 | ||
| - Bachelor’s degree | 102 (23.2) | 29 (25.9) | |
| - Higher than bachelor’s degree | 8 (1.8) | 2 (1.8) | |
| - Less than or equal to high school | 250 (56.8) | 56 (50.0) | |
| - Vocational training | 80 (18.2) | 25 (22.3) | |
| Maternal education, n (%) | 0.988 | ||
| - Bachelor’s degree | 190 (42.0) | 48 (41.4) | |
| - Higher than bachelor’s degree | 13 (2.9) | 4 (3.4) | |
| - Less than or equal to high school | 185 (40.9) | 47 (40.5) | |
| - Vocational training | 64 (14.2) | 17 (14.7) |
| Factor | Crude OR (95% CI) | Adj. OR (95% CI) | p-Value (Wald’s Test) | p-Value (LR-Test) |
|---|---|---|---|---|
| Exclusive breastfeeding for ≥6 months: Yes vs. No | 0.62 (0.41, 0.94) | 0.65 (0.43, 0.99) | 0.047 | 0.046 |
| Sex: Male vs. Female | 0.69 (0.46, 1.05) | 0.69 (0.45, 1.05) | 0.085 | 0.083 |
| Exposure to secondhand smoke: Yes vs. No | 1.37 (0.90, 2.14) | 1.28 (0.83, 2.00) | 0.277 | 0.273 |
| Lack of toilet training: Yes vs. No | 1.71 (0.99, 2.89) | 1.65 (0.95, 2.82) | 0.070 | 0.077 |
| Exposure to antibiotics within 1 year: Yes vs. No | 0.67 (0.35, 1.20) | 0.68 (0.35, 1.22) | 0.213 | 0.199 |
| Exposure to antivirals within 1 year: Yes vs. No | 1.10 (0.43, 2.48) | 1.13 (0.44, 2.59) | 0.787 | 0.789 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanpong, A.; Ponjorn, N.; Tassanakijpanich, N.; Koosakulchai, V.; Rachatawiriyakul, P.; Kittivisuit, S.; Khantee, P.; Laoprasopwattana, K. Early-Life Events and the Prevalence of Gut–Brain Interaction Disorders in Children. Children 2025, 12, 1430. https://doi.org/10.3390/children12111430
Chanpong A, Ponjorn N, Tassanakijpanich N, Koosakulchai V, Rachatawiriyakul P, Kittivisuit S, Khantee P, Laoprasopwattana K. Early-Life Events and the Prevalence of Gut–Brain Interaction Disorders in Children. Children. 2025; 12(11):1430. https://doi.org/10.3390/children12111430
Chicago/Turabian StyleChanpong, Atchariya, Natchayada Ponjorn, Nattaporn Tassanakijpanich, Vanlaya Koosakulchai, Pornruedee Rachatawiriyakul, Sirinthip Kittivisuit, Puttichart Khantee, and Kamolwish Laoprasopwattana. 2025. "Early-Life Events and the Prevalence of Gut–Brain Interaction Disorders in Children" Children 12, no. 11: 1430. https://doi.org/10.3390/children12111430
APA StyleChanpong, A., Ponjorn, N., Tassanakijpanich, N., Koosakulchai, V., Rachatawiriyakul, P., Kittivisuit, S., Khantee, P., & Laoprasopwattana, K. (2025). Early-Life Events and the Prevalence of Gut–Brain Interaction Disorders in Children. Children, 12(11), 1430. https://doi.org/10.3390/children12111430







