Abstract
Background/Objectives: Childhood cancer is the leading cause of death among children, although medical advances are improving the prognosis. During cancer treatment, nausea or vomiting may occur and eating habits may become irregular; therefore, it is important to prevent the development of oral diseases. We encountered a childhood cancer patient with rapidly progressive multiple dental caries, and this report describes the progress. Methods: A boy aged 2 years 9 months was referred for perioperative oral management. No caries were detected in the oral cavity at the initial visit. Results: As the patient had difficulty eating because of nausea and vomiting during cancer treatment, he began to consume probiotic drinks frequently. At 8-month follow-up, dental caries localized to the primary molars was detected. However, caries had occurred in all erupted teeth by 9 months later, confirming the diagnosis of severe early childhood caries. Dental treatment and long-term oral management contributed to good oral health except for dental abnormalities caused by chemotherapy. Conclusions: Childhood cancer patients, particularly at an early age, are at risk of rapid deterioration of oral disease even over a short time period. It is important to cooperate with medical or dental professionals from other hospitals to provide dietary and oral health instruction and continue long-term oral management to improve patients’ quality of life.
1. Introduction
Dental caries is one of the most common oral diseases alongside periodontal disease, both being caused by oral bacteria [1,2,3]. These diseases affect the majority of people worldwide, and treatment costs place a significant burden on health services [4]. Although the prevalence of dental caries has decreased significantly over the past few decades, mainly in developed countries, it still affects many people, especially young children and disadvantaged communities [5,6]. Early childhood caries (ECC) is a chronic disease that affects a child’s general state of health, and the American Academy of Pediatric Dentistry defines ECC as the presence of one or more decayed (i.e., having noncavitated or cavitated lesions), missing (because of caries), or filled tooth surfaces in any primary tooth in a child 71 months of age or younger [7,8]. In addition, in children younger than 3 years of age, any sign of smooth-surface caries is indicative of severe early childhood caries (S-ECC). From ages 3 to 5 years, one or more cavitated, missing (because of caries), or filled smooth surfaces in primary maxillary anterior teeth or a decayed, missing, or filled score of ≥4 (age 3), ≥5 (age 4), or ≥6 (age 5) surfaces constitute S-ECC [8].
Although childhood cancer is rare among childhood diseases, it is the leading cause of mortality in children between 1 and 14 years of age [9,10]. According to the 2015 World Health Organization (WHO) report, each year more than 200,000 children are diagnosed with cancer globally, and it is projected that an estimated 21 million people will be diagnosed by 2030 [11,12,13]. Revolutionary advances in cancer diagnosis and treatment have dramatically improved the 5-year survival rate for children with cancer, reaching approximately 80% in developed countries [14,15]. There are currently four treatments for cancer: surgical removal, immunotherapy, radiation therapy, and chemotherapy [16,17]. All interventions carry risks of different complications and side effects, as do the treatments used to manage these adverse events [18]. The side effects of treatment are mainly classified as infectious complications, oral mucositis, nausea and vomiting, or graft-versus-host disease [18]. In addition, the incidence of oral complications during chemotherapy such as ulcers, gingivitis, or xerostomia is higher in children than in adults [19,20,21]. Bonnaure-Mallet et al. investigated oral complications during chemotherapy and reported that tooth brushing significantly reduced the number of affected children [21]. By contrast, in a survey of adults, Kim et al. reported that the frequency and duration of tooth brushing during hospitalization was lower and the use of oral care products decreased in comparison to daily life [22]. Although these reports highlight the importance of oral care during hospitalization, they also suggest a risk of poorer oral hygiene in hospital than in daily life.
Neuroblastoma is the most common extracranial solid tumor in early childhood [23,24]. The prevalence is 7–8 cases per million per year in the UK, around 100 cases per year (8% of childhood malignancy) [25,26]. In Japan, neuroblastoma occurs in approximately 150–200 children each year [27]. Ninety percent of the cases occur in those <5 years of age, with the average age at diagnosis being 2 years [28]. Neuroblastoma originates from neural crest tissue and most commonly manifests on the adrenal glands or thoracic, abdominal, or cervical paraspinal ganglia within the first few years of life, with two-thirds of patients experiencing metastasis to the regional lymph nodes [29,30,31]. The most prevalent locations of metastases are bone marrow and bone, with some cases occurring in mandibular bone [32]. Treatment for patients with neuroblastoma includes a combination of chemotherapy, surgical tumor resection, stem cell transplantation, radiotherapy, and immunotherapy, with the intensity of treatment adapted to risk [31,33]. Therefore, as with other cancers, there is a risk of side effects, and oral management during cancer treatment is important.
We encountered a childhood cancer patient with rapidly progressive multiple dental caries who was diagnosed with S-ECC. This report describes the oral condition and long-term oral management of the patient. Informed consent was obtained from the patient’s guardian to publish this report.
2. Detailed Case Description
A boy aged 2 years 9 months was referred from the pediatric department of our hospital with a chief complaint of perioperative oral management. He was born at 28 weeks’ gestation with a height of 35.2 cm and weight of 852 g. Because he was an extremely-low-birth-weight-infant, he was hospitalized in the pediatrics department of our hospital from birth for two months. At the age of 2 years and 8 months, the patient was admitted to the hospital with pain as the chief complaint and was diagnosed with neuroblastoma in the retroperitoneal region and the stage was IV. There was no family history. Chemotherapy, which includes cisplatin, cyclophosphamide, etoposide, and pirarubicin, was performed for six days.
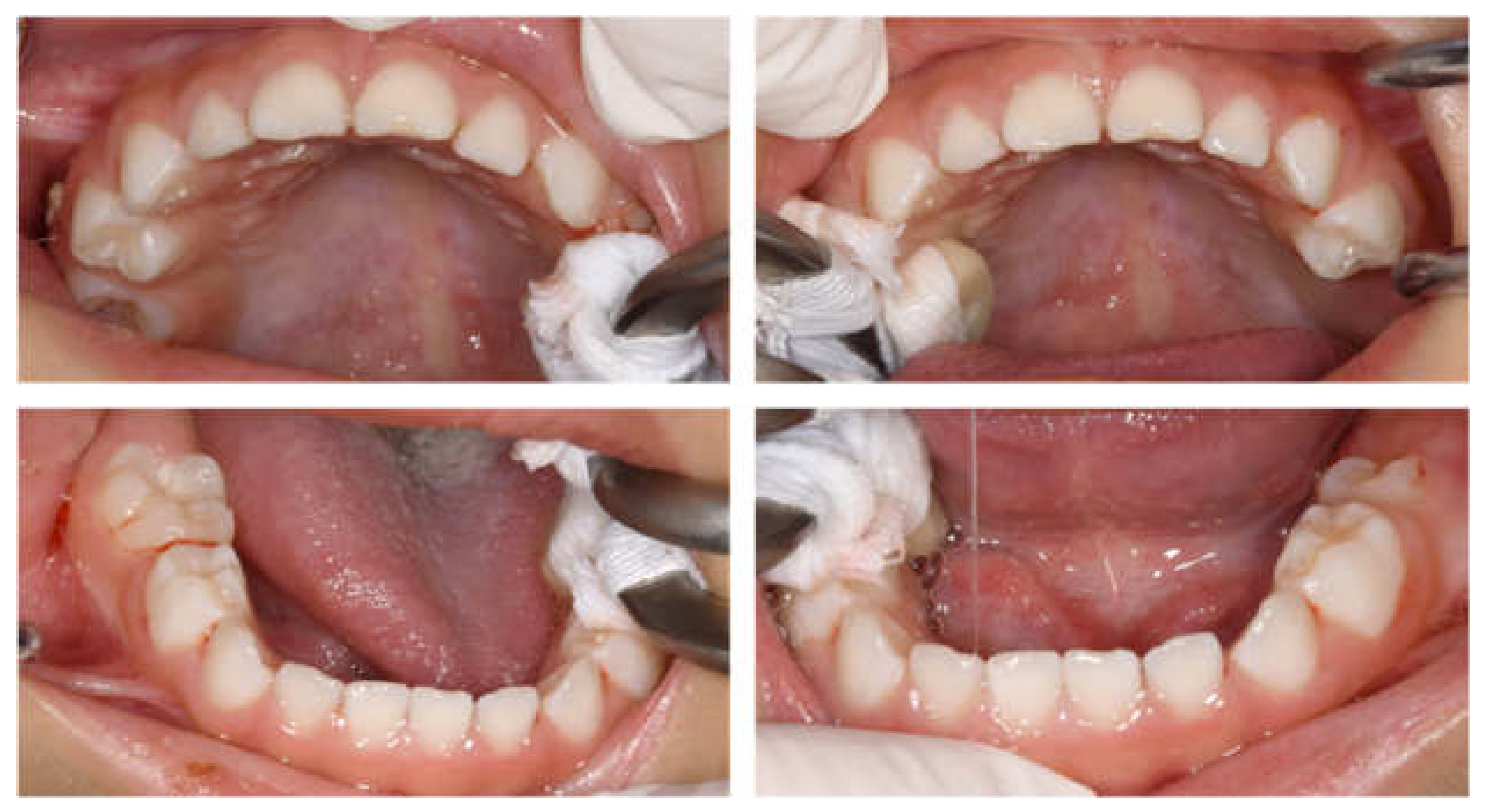
Intraoral examinations were performed by a pediatric specialist. At the first visit, 19 primary teeth except the left maxillary second primary molar erupted in the oral cavity (Figure 1). The maxillary left central primary incisor was chipped; however, no dental caries required dental treatment. Three primary second molars had erupted, and the gums were generally swollen and bled easily. The evaluation was performed according to the diagnostic criteria of the Japanese Society of Periodontology, and the patient was diagnosed with plaque-induced gingivitis [34]. He usually had his teeth brushed by his guardian at night, but this had not been possible since being hospitalized. The only drink he took was milk; owing to the effects of cancer treatment, he had difficulty eating meals and did not eat snacks. We conducted oral health instruction or fluoride application and decided to continue with follow-up during hospitalization.
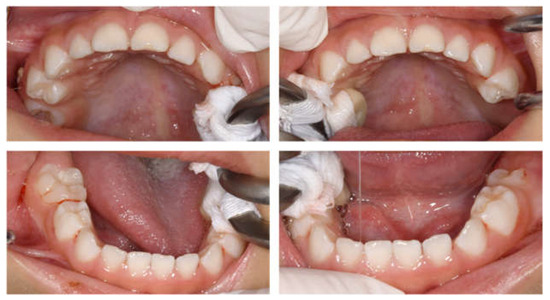
Figure 1.
Intraoral photographs at the age of 2 years and 9 months.
This was followed by four cycles of chemotherapy, and he received autoperipheral blood stem cell transplantation at the age of 3 years and 2 months. Six days after transplantation, mild gingivitis was observed, although no other pathological findings were detected. The guardian had continued to brush his teeth once a day. The left retroperitoneal tumor was removed the next month, followed during the next month by 2 weeks of radiation therapy (19.8 Gy/11 Fr.).
At the dental visit at the age of 3 years 4 months, the progression of dental caries was C2 in the mandibular bilateral primary second molar [35]. As the patient often vomited after eating meals, he frequently drank milk and liquid probiotics. Considering his physical condition, we applied a glass ionomer cement (FUJI IX GP, GC Corporate Center, Tokyo, Japan) filling. We decided to monitor the patient’s progress and continue with dietary advice; however, he was discharged from our institution for treatment at another hospital that month.
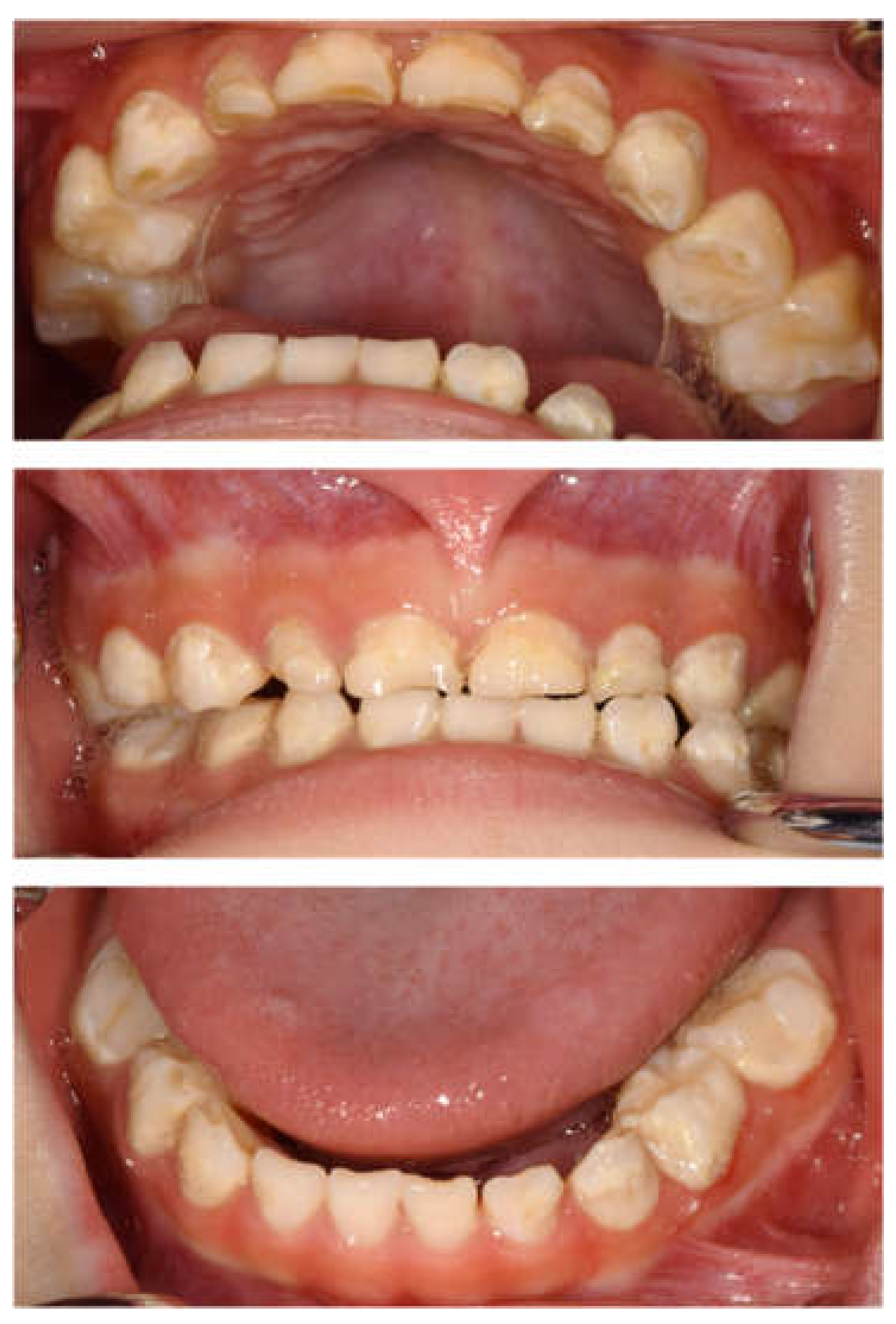
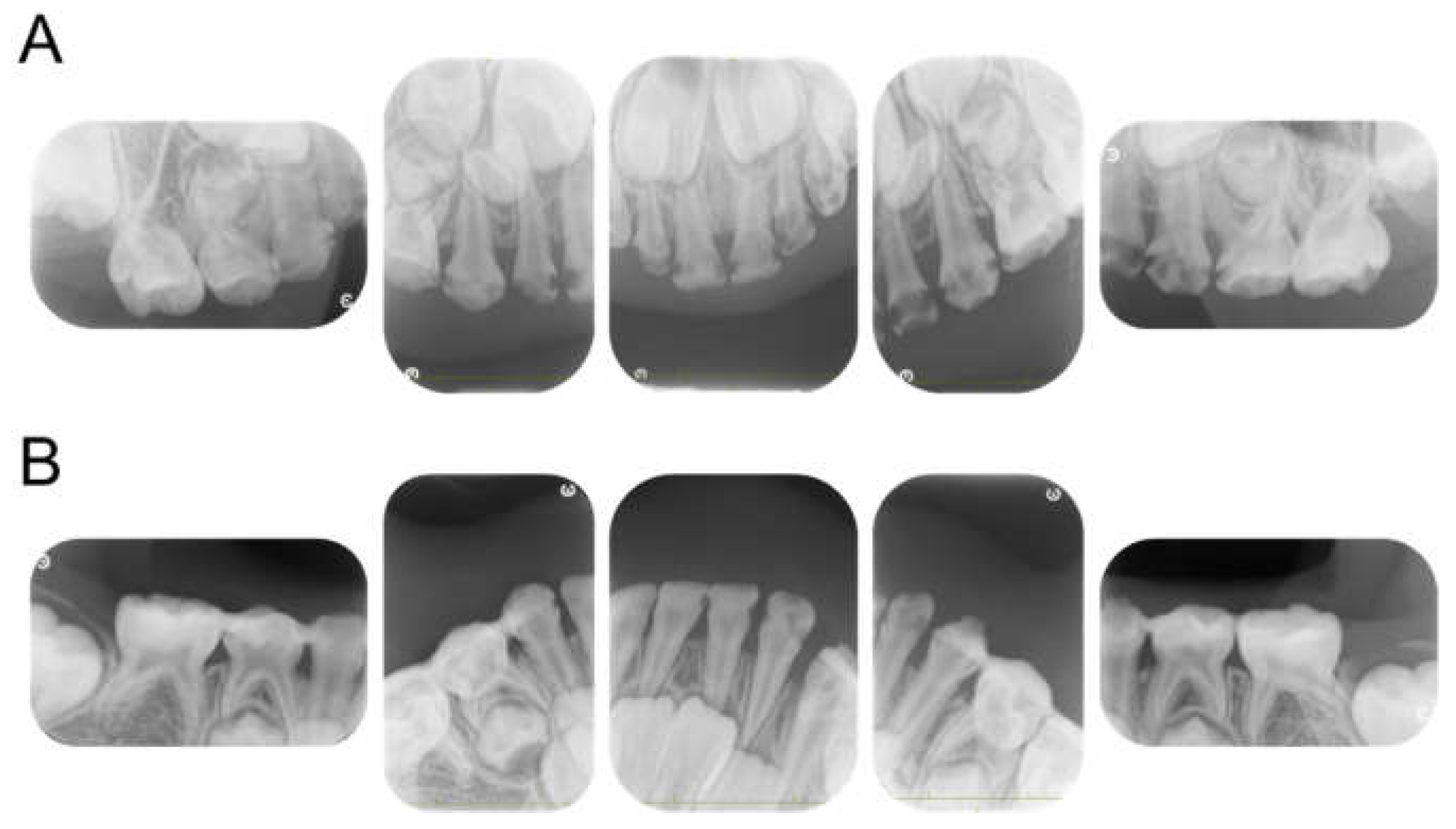
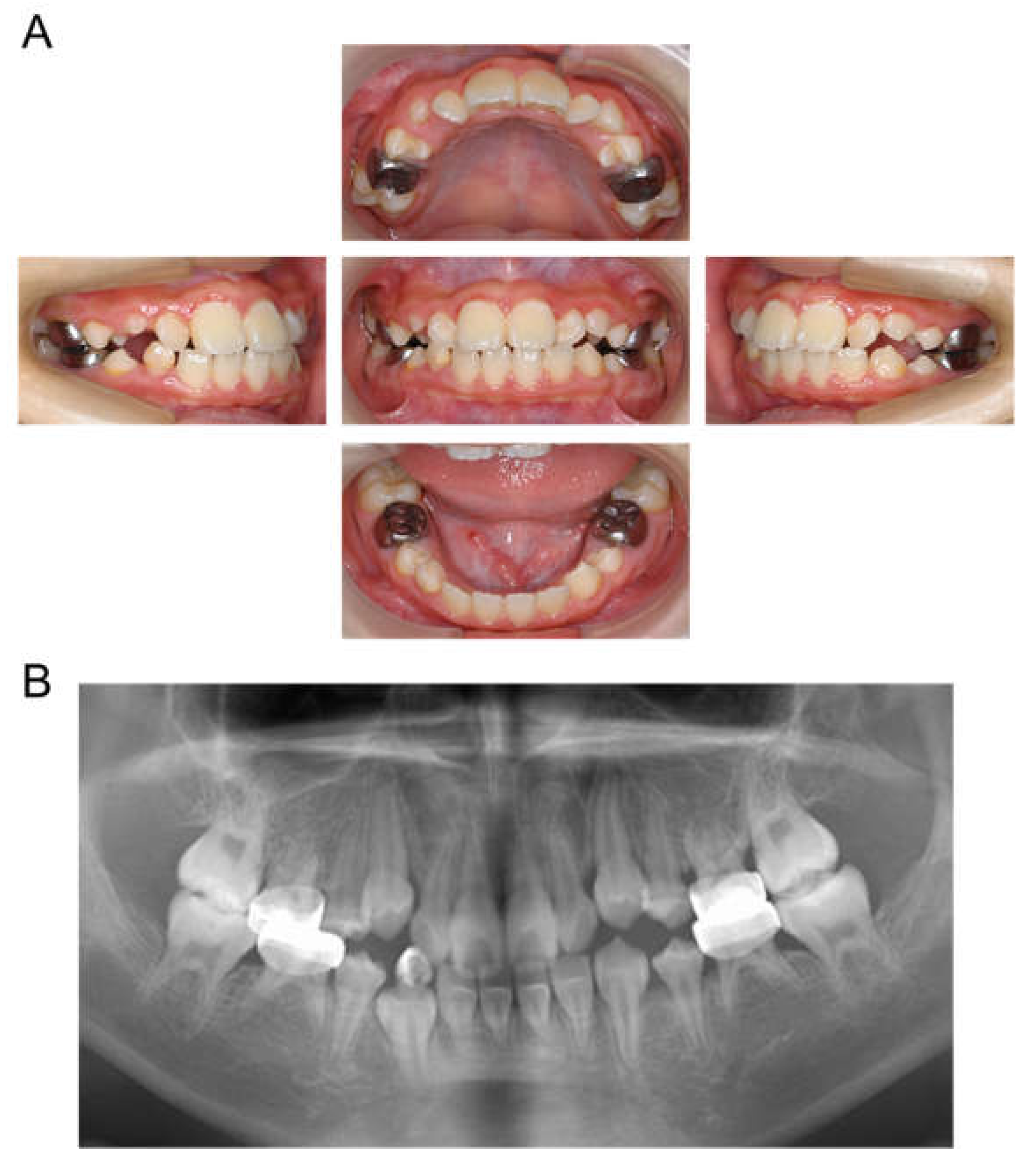
The patient returned to our department 9 months later, at the age of 4 years 2 months, for a pediatric consultation. Intraoral photographs revealed multiple severe dental caries (Figure 2). Considering the timing of treatment, radiographic examination was divided into two days (Figure 3A). Radiographic examination of the mandible was carried out 4 months later, and a temporary glass ionomer filling was applied until then (Figure 3B). Two types of glass ionomer cement (FUJI IX GP, GC Corporate Center, Tokyo, Japan, and Vitrebond, 3 M Dental Products, St. Paul, MN, USA) were used. C2 was found in all 20 erupted teeth, confirming the diagnosis of S-ECC. Given the patient’s young age and wider pulp chamber, we chose stepwise removal to avoid pulp exposure [36]. The diagnoses and final restorations are shown in Table 1, and details of the diagnosis of each tooth are given in Figure S1. The mandibular primary central incisors were kept under observation because they represent the site least likely to develop dental caries.
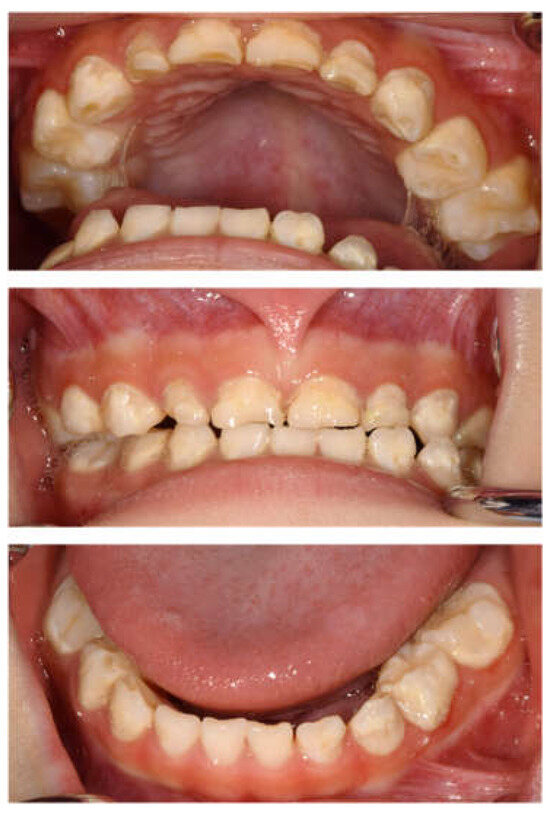
Figure 2.
Intraoral photographs at the age of 4 years and 2 months.
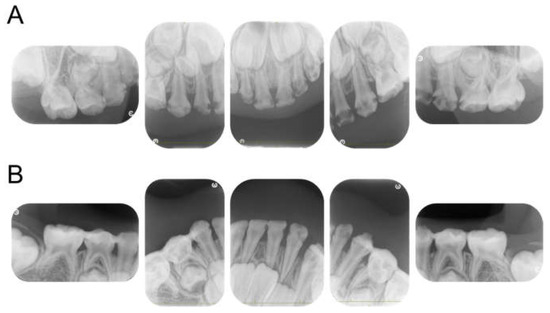
Figure 3.
Periapical photographs. (A) At 4 years and 2 months. (B) At 4 years and 6 months.

Table 1.
Diagnosis and final restoration.
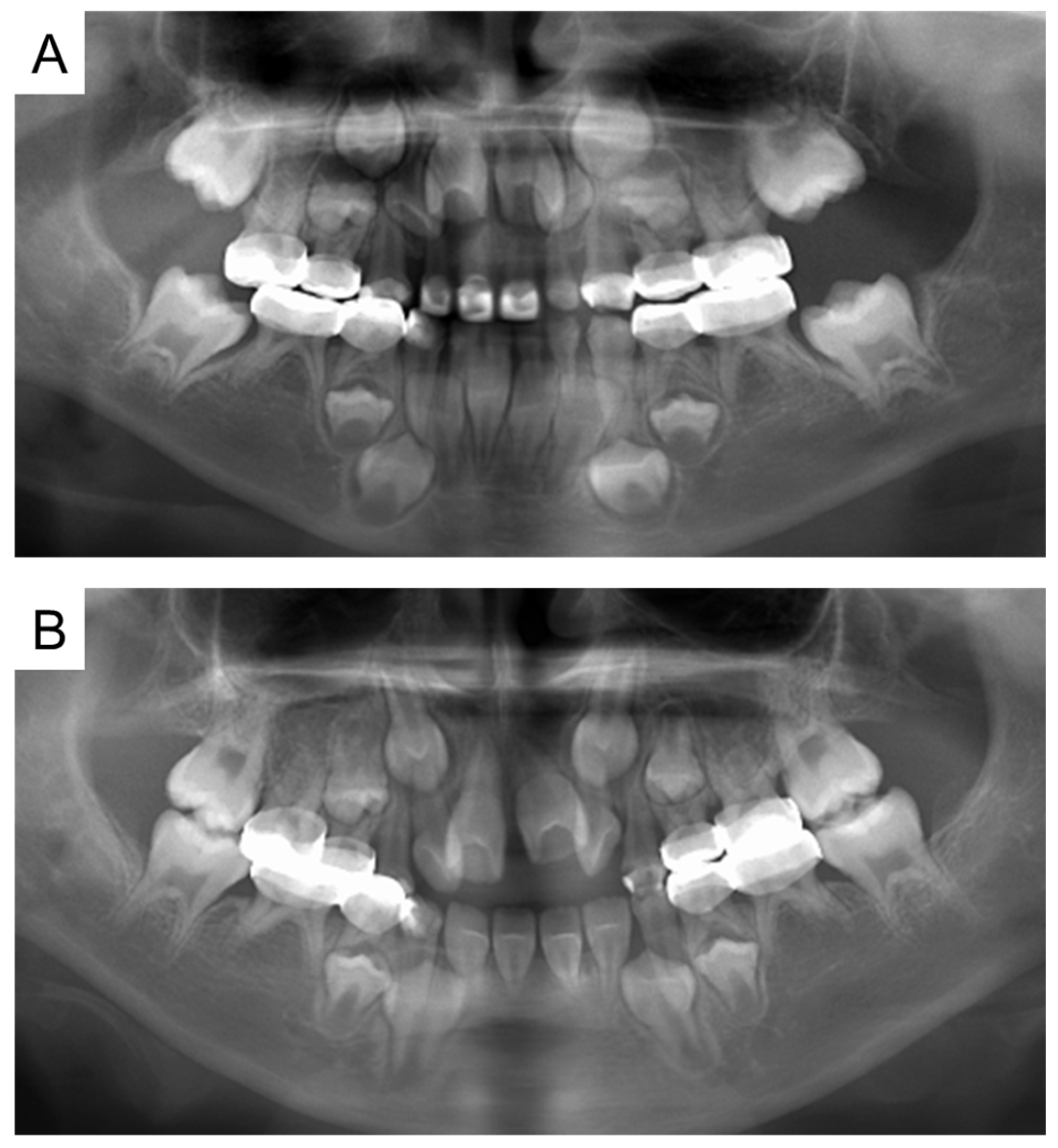
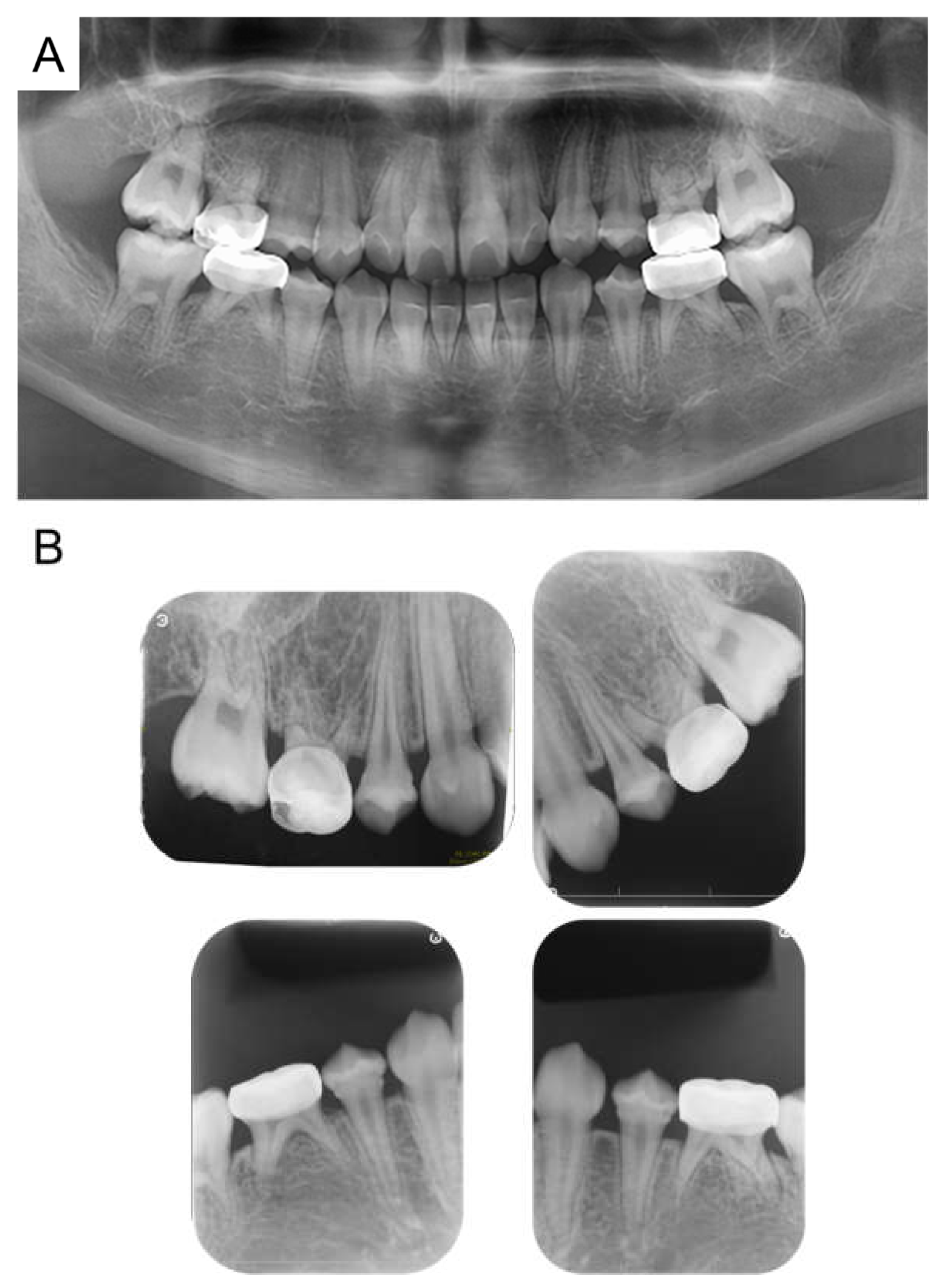
At the age of 5 years 11 months, the patient’s primary central incisors had mobility. Panoramic examination led to suspected congenital absence of second premolars and second molars; however, there were no dental caries requiring dental treatment. The patient continued to undergo regular checkups and dental treatment as needed. Periodic radiographic examinations confirmed the congenital absence of second premolars and second molars (Figure 4). Short roots were detected in incisors and first molar without mobility and other symptoms, and we continued to observe them. At the age of 10 years 2 months, intraoral examination revealed tooth demineralization and gingivitis, but teeth requiring treatment were not found, and there were no pathological findings on panoramic examination (Figure 5).
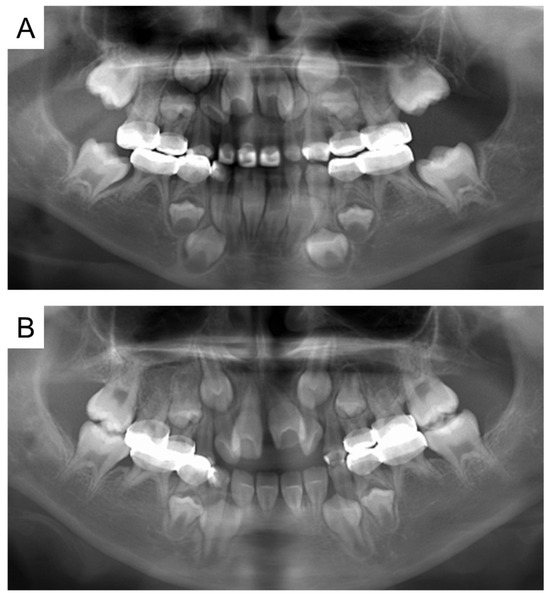
Figure 4.
Panoramic examination. (A) At 5 years and 11 months. (B) At 8 years and 1 month.
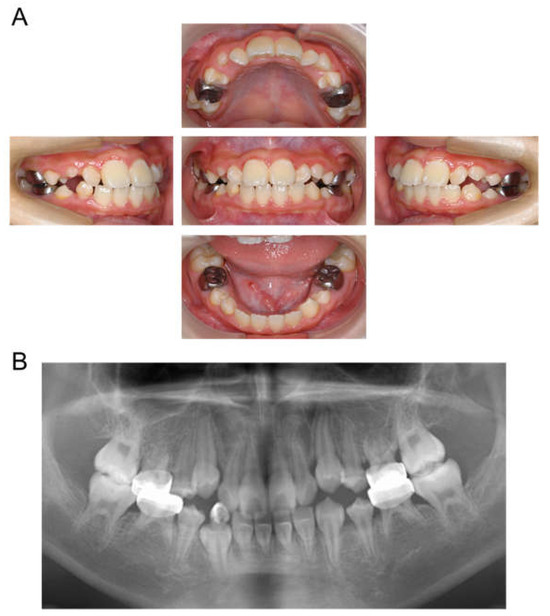
Figure 5.
Images at the age of 10 years and 2 months. (A) Intraoral photographs. (B) Panoramic radiograph.
At age 11 years 6 months, there was no evidence of recurrence of neuroblastoma. All permanent teeth had completed eruption into the oral cavity, and although radiographic examination revealed morphological abnormalities in the first premolar, no teeth showed any pathological symptoms (Figure 6). A hole developed in the preformed stainless-steel crown in the maxillary right primary second molar, which was treated by a replacement. Currently the patient continues to undergo follow-up observation.
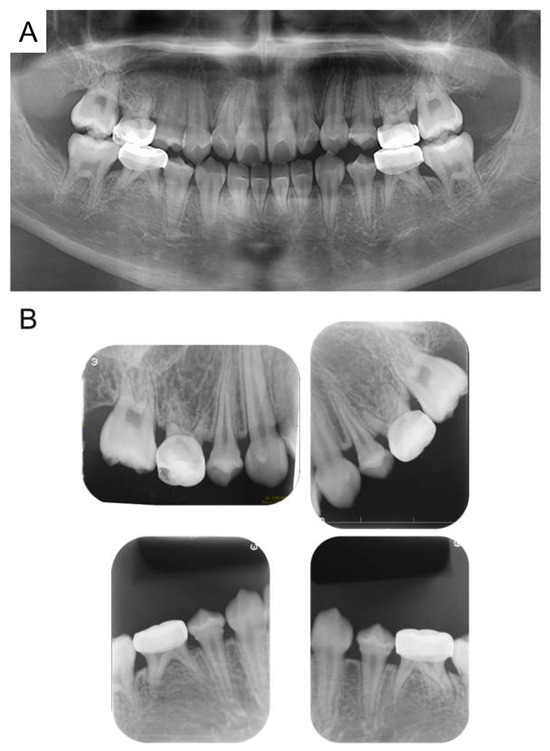
Figure 6.
Images at the age of 11 years and 6 months. (A) Panoramic radiograph. (B) Periapical radiographs.
3. Discussion
Oral function is extremely important not only for chewing and speaking but also psychological well-being [37,38]. Recently, it has been reported that good oral health leads to overall health and well-being, and the importance of oral care has been highlighted [39,40]. It has also been reported that the oral condition of hospitalized patients affects the prognosis of the treatment site and the occurrence of adverse events during hospitalization [41,42]. These results suggest the importance of oral care during hospitalization, particularly by dental professionals. In the present case, perioperative management, including oral care by dental professionals, oral hygiene instruction, and fluoride application, started from the month chemotherapy was administered.
As mentioned above, cancer treatment also causes various side effects in the oral cavity, and oral mucositis is one of the most common adverse effects of radiotherapy and cytotoxic therapy for cancer [43]. Chemotherapy-induced mucositis usually develops within 4–7 days after initiation of treatment and peaks within 2 weeks [43]. On the other hand, radiotherapy has a more gradual clinical course since it is most often administered in small fractions over weeks [43]. In the present case, this patient did not develop oral mucositis during hospitalization, but he had gingivitis and dental caries.
Shayani et al. (2022) reported a significantly greater frequency of gingivitis, and a mean of new caries lesions were observed in children with acute lymphoblastic leukemia who received chemotherapy [44]. Gingivitis has been described as one of the most frequent oral manifestations that affects patients undergoing chemotherapy because of secondary saliva dysfunction and the accumulation of plaque [44,45,46]. On the other hand, dental caries does not occur due to the effect of the chemotherapy itself [44,45,46]. Occurrence of dental caries relates to reduced saliva production, habitual consumption of sugary drinks, or cases of vomiting affect oral hygiene [44]. In addition, during chemotherapy, patients with cancer present a change in oral microbiota with a significant increase in Gram-positive cariogenic bacteria (Streptococcus mutans and Lactobacillus spp.) [47,48,49].
In the present case, during the 8-month follow-up period, chemotherapy was administered as well as stem cell transplantation, tumor removal, and radiation therapy, while, thanks to oral care and oral hygiene instruction by dental professionals, only mild caries occurred in the primary molars. However, during the 9-month period when the patient did not visit the dentist, the dental caries progressed and he was subsequently diagnosed with S-ECC.
There are two reasons to explain why dental caries progressed so rapidly. First, the patient lived through a period when nausea and vomiting made it difficult for him to eat, and he began drinking liquid probiotics more frequently. Nausea and vomiting are common side effects of many cancer treatments, and there is also often a psychological impact; in particular, nausea is commonly identified as being a distressing aspect of chemotherapy treatment [18,50]. In addition, in children with cancer, malnutrition is common because of tumors, treatment-related factors, and long-term changes in diet, leaving parents concerned about their child’s nutritional status and appropriate food choices for their children [51]. In the present case, the patient had difficulty eating because of vomiting; therefore, although milk was previously given mostly, the frequency of providing prebiotic drinks gradually increased. The etiology of dental erosion is divided into two categories: endogenous, caused by acids entering the oral cavity through reflux or vomiting, and exogenous, caused by environmental factors such as diet, drugs, and lifestyle habits [52,53]. At pH levels below the critical pH for enamel (pH 5.5), tooth minerals are demineralized, and it has been reported that soft drinks with pH values below the critical pH level cause greater loss of surface enamel [53,54]. Recently, promotion of oral health with probiotics has gained considerable attention, and there are some reports on inhibition of S. mutans, which is a major pathogen of dental caries [55,56,57]. By contrast, however, Jitpukdeebodintra et al. reported that enamel exposed to drinkable yogurt showed uniform etching and this caused enamel dissolution [58]. The primary dentition is thought to be more susceptible to erosion than the permanent dentition because of its thinner and less mineralized enamel [59]. In addition, Schab et al. pointed out that consumption of milk, dairy products, vegetables, and fruits in children throughout cancer treatment is too low, while the consumption of sugar often exceeds recommendations [60]. Sugar supplementation lowers the pH of the environment, which subsequently increases the number of key bacteria and leads to a shift in microflora, pushing the ecosystem toward demineralization [61,62,63]. Frequent consumption of liquid probiotics may affect the occurrence and progression of dental caries, resulting in S-ECC. Nutritional and dietary guidance during cancer treatment is thus important in the prevention of oral disease.
The other reason is related to the fact that the patient did not visit the dentist during the period when he was receiving treatment at another hospital. Regular dental checkups are important for the early detection of disease [64,65,66]. It is unclear whether the reason for not visiting the dentist was related to a problem with his physical condition or with the medical system, although it is possible that there was a lack of coordination between dental professionals. The arisal of such a situation confirms the importance of cooperation between dental professionals with regard to childhood cancer patients, especially those of a young age.
In recent years, it has been reported that the oral condition in acute ischemic stroke patients at admission is associated with good outcomes and a decreased incidence of hospital-acquired pneumonia, suggesting the importance of oral care for the patient during hospitalization [41]. Regular evaluation for oral mucositis and brushing of the teeth during cancer treatment are common recommendations in several oral care protocols for oral mucositis [67]. In addition, there are also other oral care protocols for the patient during hospitalization [68,69]. In the case of pediatric patients, the American Academy of Pediatric Dentistry strongly recommends that dental professionals assist pediatric cancer patients closely, from diagnosis to follow-up, with the multidisciplinary oncology team [70,71]. On the other hand, Ribeiro et al. (2023) reported that the greatest difficulties of the oral healthcare team were communicating with the medical team and understanding the importance of oral care for the patient’s systemic condition [71]. Oral diseases such as dental caries can progress rapidly during cancer treatment, as in the present case. Dental professionals need to provide proper information and establish close communication among patients, the guardian, and all medical staff involved during hospitalization.
In the present case, pulp preservation was achieved by stepwise removal. In addition, after consultation with a pediatrician, the primary molar region was restored with preformed stainless-steel crowns. A stainless-steel crown protects the dental crown from fracture, reduces the possibility of leakage, and ensures a biological seal [72,73]. During the follow-up period, there were neither tooth fractures nor pulp infection. Patients who have undergone chemotherapy during childhood may develop dental abnormalities, including tooth agenesis, microdontism, and disturbed root development [74,75,76]. Childhood cancer treatment can cause dental abnormalities at an earlier age, the effects of which vary depending on tooth formation [77]. Our patient had tooth agenesis of the second premolar and second molar, microdontia of first premolar, and short roots in the anterior teeth. In childhood cancer patients who have undergone chemotherapy at a young age and in whom no successor permanent teeth have been identified, the importance of restoring the teeth with durable stainless-steel crowns to preserve the dental pulp is evident. Long-term follow-up is important in patients with dental abnormalities [78]. We continue to follow our patient and strive to improve his quality of life.
4. Conclusions
In the present case, rapid caries progression was observed in a childhood cancer patient, resulting in a diagnosis of S-ECC. The importance of oral care during hospitalization is well known, but oral diseases can sometimes rapidly become severe. This report provides all healthcare professionals involved in cancer treatment with new risks that pediatric patients face during treatment. With proper treatment and long-term oral management, the patient can live without any oral disease requiring dental treatment. This case highlights the importance of long-term cooperation and intervention between medical and dental professionals during and after hospitalization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children12030261/s1, Figure S1: The detailing the diagnosis of each tooth.
Author Contributions
Conceptualization, T.A. and N.N.; attending dentists, T.A. and N.N.; intraoral photography, S.I., Y.I. and E.T.; writing—original draft preparation, T.A.; writing—review and editing, R.N.; supervision, C.M. and R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was granted an exemption from requiring ethics approval by the “Ethical Guidelines for Medical and Biological Research Involving Human Subjects Guidance” (https://www.mhlw.go.jp/content/001237478.pdf, accessed on 12 January 2025) formulated by the Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare, and Ministry of Economy, Trade, and Industry (Japan).
Informed Consent Statement
Written informed consent was obtained from the parents of the patients to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank the Department of Pediatrics (Shuhei Karakawa, Yoko Mizoguchi and Satoshi Okada) at Hiroshima University for their cooperation with this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Usuda, M.; Kametani, M.; Hamada, M.; Suehiro, Y.; Matayoshi, S.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Akitomo, T.; Mitsuhata, C.; et al. Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite. Int. J. Mol. Sci. 2023, 24, 11371. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, J.; Yasuda, H.; Nomura, R.; Matayoshi, S.; Inaba, H.; Gongora, E.; Iwashita, N.; Shirahata, S.; Kaji, N.; Akitomo, T.; et al. Investigation of periodontal disease development and Porphyromonas gulae FimA genotype distribution in small dogs. Sci. Rep. 2024, 14, 5360. [Google Scholar] [CrossRef] [PubMed]
- Worthington, H.V.; MacDonald, L.; Poklepovic Pericic, T.; Sambunjak, D.; Johnson, T.M.; Imai, P.; Clarkson, J.E. Home use of interdental cleaning devices, in addition to toothbrushing, for preventing and controlling periodontal diseases and dental caries. Cochrane Database Syst. Rev. 2019, 4, CD012018. [Google Scholar] [CrossRef] [PubMed]
- Koberová Ivančaková, R.; Radochová, V.; Kovácsová, F.; Merglová, V. Exogenous Intake of Fluorides in Caries Prevention: Benefits and Risks. Acta Medica 2021, 64, 71–76. [Google Scholar] [CrossRef]
- Veneri, F.; Vinceti, S.R.; Filippini, T. Fluoride and caries prevention: A scoping review of public health policies. Ann. Ig. 2024, 36, 270–280. [Google Scholar]
- Branger, B.; Camelot, F.; Droz, D.; Houbiers, B.; Marchalot, A.; Bruel, H.; Laczny, E.; Clement, C. Breastfeeding and early childhood caries. Review of the literature, recommendations, and prevention. Arch. Pediatr. 2019, 26, 497–503. [Google Scholar] [CrossRef]
- Definition of Early Childhood Caries (ECC). American Academy of Pediatric Dentistry. Available online: https://www.aapd.org/assets/1/7/d_ecc.pdf (accessed on 12 January 2025).
- Radhi, M.; Fulbright, J.M.; Ginn, K.F.; Guest, E.M. Childhood cancer for the primary care physician. Prim. Care 2015, 42, 43–55. [Google Scholar] [CrossRef]
- Aleassa, E.M.; Xing, M.; Keijzer, R. Nanomedicine as an innovative therapeutic strategy for pediatric cancer. Pediatr. Surg. Int. 2015, 31, 611–616. [Google Scholar] [CrossRef]
- Kingham, T.P.; Alatise, O.I.; Vanderpuye, V.; Casper, C.; Abantanga, F.A.; Kamara, T.B.; Olopade, O.I.; Habeebu, M.; Abdulkareem, F.B.; Denny, L. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013, 14, e158–e167. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S. International incidence of childhood cancer, 2001–10: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Endalamaw, A.; Assimamaw, N.T.; Ayele, T.A.; Muche, A.A.; Zeleke, E.G.; Wondim, A.; Belay, G.M.; Birhanu, Y.; Tazebew, A.; Techane, M.A.; et al. Prevalence of childhood Cancer among children attending referral hospitals of outpatient Department in Ethiopia. BMC Cancer 2021, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, Y.; Wei, B.; Xiang, D.; Hu, J.; Zhao, P.; Lin, S.; Zheng, Y.; Yao, J.; Zhai, Z.; et al. Global, regional, and national childhood cancer burden, 1990–2019: An analysis based on the Global Burden of Disease Study 2019. J. Adv. Res. 2022, 40, 233–247. [Google Scholar] [CrossRef]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef]
- Akitomo, T.; Ogawa, M.; Kaneki, A.; Nishimura, T.; Usuda, M.; Kametani, M.; Kusaka, S.; Asao, Y.; Iwamoto, Y.; Tachikake, M.; et al. Dental Abnormalities in Pediatric Patients Receiving Chemotherapy. J. Clin. Med. 2024, 13, 2877. [Google Scholar] [CrossRef]
- Phillips, B.; Morgan, J.; Walker, R.; Heggie, C.; Ali, S. Interventions to reduce the risk of side-effects of cancer treatments in childhood. Expert. Rev. Anticancer Ther. 2024, 24, 1117–1129. [Google Scholar] [CrossRef]
- Sonis, S.T.; Sonis, A.L.; Lieberman, A. Oral complications in patients receiving treatment for malignancies other than of the head and neck. J. Am. Dent. Assoc. 1978, 97, 468–472. [Google Scholar] [CrossRef]
- Childers, N.K.; Stinnett, E.A.; Wheeler, P.; Wright, J.T.; Castleberry, R.P.; Dasanayake, A.P. Oral complications in children with cancer. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 41–47. [Google Scholar] [CrossRef]
- Bonnaure-Mallet, M.; Bunetel, L.; Tricot-Doleux, S.; Guérin, J.; Bergeron, C.; LeGall, E. Oral complications during treatment of malignant diseases in childhood: Effects of tooth brushing. Eur. J. Cancer 1998, 34, 1588–1591. [Google Scholar] [CrossRef]
- Kim, S.M.; Noh, H.J.; Mun, S.J.; Han, S.Y. Differences in Korean adult inpatients’ oral care behaviour in daily life and hospitals. Int. J. Dent. Hyg. 2023, 21, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Jansky, S.; Sharma, A.K.; Körber, V.; Quintero, A.; Toprak, U.H.; Wecht, E.M.; Gartlgruber, M.; Greco, A.; Chomsky, E.; Grünewald, T.G.P.; et al. Single-cell transcriptomic analyses provide insights into the developmental origins of neuroblastoma. Nat. Genet. 2021, 53, 683–693. [Google Scholar] [CrossRef]
- Stiller, C.A.; Allen, M.B.; Eatock, E.M. Childhood cancer in Britain: The National Registry of Childhood Tumours and incidence rates 1978–1987. Eur. J. Cancer 1995, 31, 2028–2034. [Google Scholar] [CrossRef]
- Fisher, J.P.H.; Tweddle, D.A. Neonatal neuroblastoma. Semin. Fetal Neonatal Med. 2012, 17, 207–215. [Google Scholar] [CrossRef]
- Nakagawara, A.; Li, Y.; Izumi, H.; Muramori, K.; Inada, H.; Nishi, M. Neuroblastoma. Jpn. J. Clin. Oncol. 2018, 48, 214–241. [Google Scholar] [CrossRef]
- Qiu, B.; Matthay, K.K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Maris, J.M.; Matthay, K.K. Molecular biology of neuroblastoma. J. Clin. Oncol. 1999, 17, 2264–2279. [Google Scholar] [CrossRef]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef]
- Rivera, Z.; Escutia, C.; Madonna, M.B.; Gupta, K.H. Biological Insight and Recent Advancement in the Treatment of Neuroblastoma. Int. J. Mol. Sci. 2023, 24, 8470. [Google Scholar] [CrossRef]
- Izi, Z.; El Haddad, S.; Oubaddi, T.; Amsiguine, N.; Allali, N.; Chat, L. Mandibular Metastasis in Neuroblastoma in a 3 Year-Old Child: A Case Report. Glob. Pediatr. Health 2023, 10, 2333794X231200616. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Thiele, C.J. Immunotherapy approaches targeting neuroblastoma. Curr. Opin. Pediatr. 2021, 33, 19–25. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society of Periodontology, 2008 Guidelines for Examination, Diagnosis and Therapeutic Strategies for Periodontal Disease. (In Japanese). Available online: https://www.perio.jp/publication/upload_file/guideline_perio_plan_2008.pdf (accessed on 12 February 2025).
- Nakatani, S.; Ohara, T.; Ashihara, K.; Izumi, C.; Iwanaga, S.; Eishi, K.; Okita, Y.; Daimon, M.; Kimura, T.; Toyoda, K.; et al. JCS 2017 Guideline on Prevention and Treatment of Infective Endocarditis. Circ. J. 2019, 83, 1767–1809. [Google Scholar] [CrossRef] [PubMed]
- Giacaman, R.A.; Muñoz-Sandoval, C.; Neuhaus, K.W.; Fontana, M.; Chałas, R. Evidence-based strategies for the minimally invasive treatment of carious lesions: Review of the literature. Adv. Clin. Exp. Med. 2018, 27, 1009–1016. [Google Scholar] [CrossRef]
- Furuta, M.; Yamashita, Y. Oral Health and Swallowing Problems. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 216–222. [Google Scholar] [CrossRef]
- Akitomo, T.; Kusaka, S.; Iwamoto, Y.; Usuda, M.; Kametani, M.; Asao, Y.; Nakano, M.; Tachikake, M.; Mitsuhata, C.; Nomura, R. Five-Year Follow-Up of a Child with Non-Syndromic Oligodontia from before the Primary Dentition Stage: A Case Report. Children 2023, 10, 717. [Google Scholar] [CrossRef]
- Fiorillo, L. Oral Health: The First Step to Well-Being. Medicina 2019, 55, 676. [Google Scholar] [CrossRef]
- Kametani, M.; Akitomo, T.; Usuda, M.; Kusaka, S.; Asao, Y.; Nakano, M.; Iwamoto, Y.; Tachikake, M.; Ogawa, M.; Kaneki, A.; et al. Evaluation of periodontal status and oral health habits with continual dental support for young patients with hemophilia. Appl. Sci. 2024, 14, 1349. [Google Scholar] [CrossRef]
- Eto, F.; Nezu, T.; Nishi, H.; Aoki, S.; Tasaka, S.; Horikoshi, S.; Yano, K.; Kawaguchi, H.; Maruyama, H. Oral condition at admission predicts functional outcomes and hospital-acquired pneumonia development among acute ischemic stroke patients. Clin. Oral. Investig. 2024, 28, 434. [Google Scholar] [CrossRef]
- Sasaki, M.; Shigeishi, H.; Nishi, H.; Hamada, N.; Kitasaki, H.; Yano, K.; Kaneyasu, Y.; Horikoshi, S.; Kawaguchi, H.; Ohta, K. Periodontitis and postoperative inflammation in gastric cancer patients: Propensity score analysis. Oral. Dis. 2024, 30, 4691–4704. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral mucositis. Oral. Oncol. 2010, 46, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Shayani, A.; Aravena, P.C.; Rodríguez-Salinas, C.; Escobar-Silva, P.; Diocares-Monsálvez, Y.; Angulo-Gutiérrez, C.; Rivera, C. Chemotherapy as a risk factor for caries and gingivitis in children with acute lymphoblastic leukemia: A retrospective cohort study. Int. J. Paediatr. Dent. 2022, 32, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.F.; Lira, J.A.; Macedo, R.A.; Santos, K.S.; Elias, C.T.; Morais, M.D. Oral manifestations resulting from chemotherapy in children with acute lymphoblastic leukemia. Braz. J. Otorhinolaryngol. 2014, 80, 78–85. [Google Scholar] [PubMed]
- Valéra, M.C.; Noirrit-Esclassan, E.; Pasquet, M.; Vaysse, F. Oral complications and dental care in children with acute lymphoblastic leukaemia. J. Oral Pathol. Med. 2015, 44, 483–489. [Google Scholar] [CrossRef]
- Jensen, M.L.; Thymann, T.; Cilieborg, M.S.; Lykke, M.; Mølbak, L.; Jensen, B.B.; Schmidt, M.; Kelly, D.; Mulder, I.; Burrin, D.G.; et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G59–G71. [Google Scholar] [CrossRef]
- Olszewska, K.; Mielnik-Błaszczak, M. An Assessment of the Number of Cariogenic Bacteria in the Saliva of Children with Chemotherapy-Induced Neutropenia. Adv. Clin. Exp. Med. 2016, 25, 11–19. [Google Scholar] [CrossRef]
- Villafuerte, K.R.V.; Martinez, C.J.H.; Dantas, F.T.; Carrara, H.H.A.; Dos Reis, F.J.C.; Palioto, D.B. The impact of chemotherapeutic treatment on the oral microbiota of patients with cancer: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 552–566. [Google Scholar] [CrossRef]
- Hedström, M.; Haglund, K.; Skolin, I.; von Essen, L. Distressing events for children and adolescents with cancer: Child, parent, and nurse perceptions. J. Pediatr. Oncol. Nurs. 2003, 20, 120–132. [Google Scholar] [CrossRef]
- Arpaci, T.; Toruner, E.K.; Altay, N. Assessment of Nutritional Problems in Pediatric Patients with Cancer and the Information Needs of Their Parents: A Parental Perspective. Asia Pac. J. Oncol. Nurs. 2018, 5, 231–236. [Google Scholar] [CrossRef]
- Bhowmick, G.S. Etiological and predisposing factors for dentin hypersensitivity: An overview. IJSS 2017, 3, 16. [Google Scholar]
- Tabari, M.; Alaghemand, H.; Qujeq, D.; Mohammadi, E. Effect of Popping Chocolate and Candy on Enamel Microhardness of Primary and Permanent Teeth. J. Int. Soc. Prev. Community Dent. 2017, 7, 370–376. [Google Scholar] [PubMed]
- Wang, Y.L.; Chang, C.C.; Chi, C.W.; Chang, H.H.; Chiang, Y.C.; Chuang, Y.C.; Chang, H.H.; Huang, G.F.; Liao, Y.S.; Lin, C.P. Erosive potential of soft drinks on human enamel: An in vitro study. J. Formos. Med. Assoc. 2014, 113, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; He, S.J.; Mar, K.; Stephen Hsu, C.Y.; Hung, S.L. Inhibition of Streptococcus mutans by a commercial yogurt drink. J. Dent. Sci. 2019, 14, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef]
- Guo, M.; Wu, J.; Hung, W.; Sun, Z.; Zhao, W.; Lan, H.; Zhao, Z.; Wuri, G.; Fang, B.; Zhao, L.; et al. Lactobacillus paracasei ET-22 Suppresses Dental Caries by Regulating Microbiota of Dental Plaques and Inhibiting Biofilm Formation. Nutrients 2023, 15, 3316. [Google Scholar] [CrossRef]
- Jitpukdeebodintra, S.; Chuenarom, C.; Muttarak, C.; Khonsuphap, P.; Prasattakarn, S. Effects of 1.23% acidulated phosphate fluoride gel and drinkable yogurt on human enamel erosion, in vitro. Quintessence Int. 2010, 41, 595–604. [Google Scholar]
- Taji, S.; Seow, W.K. A literature review of dental erosion in children. Aust. Dent. J. 2010, 55, 358–367. [Google Scholar] [CrossRef]
- Schab, M.; Skoczen, S. Nutritional status, body composition and diet quality in children with cancer. Front. Oncol. 2024, 14, 1389657. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the oral microbiota in health: Mechanisms that prevent dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Z.; Zhang, Y.; Hong, Y.; Zheng, Y. Effects of a probiotic drink containing Lactobacillus casei strain Shirota on dental plaque microbiota. J. Int. Med. Res. 2019, 47, 3190–3202. [Google Scholar] [CrossRef]
- Akitomo, T.; Asao, Y.; Mitsuhata, C.; Kozai, K. A new supernumerary tooth occurring in the same region during follow-up after supernumerary tooth extraction: A case report. Pediatr. Dent. 2021, 32, 100–107. [Google Scholar] [CrossRef]
- Usuda, M.; Akitomo, T.; Kametani, M.; Kusaka, S.; Mitsuhata, C.; Nomura, R. Dens invaginatus of fourteen teeth in a pediatric patient. Pediatr. Dent. J. 2023, 33, 240–245. [Google Scholar] [CrossRef]
- Akitomo, T.; Kusaka, S.; Usuda, M.; Kametani, M.; Kaneki, A.; Nishimura, T.; Ogawa, M.; Mitsuhata, C.; Nomura, R. Fusion of a Tooth with a Supernumerary Tooth: A Case Report and Literature Review of 35 Cases. Children 2023, 11, 6. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; Kataoka, T.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef]
- Chipps, E.M.; Carr, M.; Kearney, R.; MacDermott, J.; Von Visger, T.; Calvitti, K.; Vermillion, B.; Weber, M.L.; Newton, C.; St Clair, J.; et al. Outcomes of an Oral Care Protocol in Postmechanically Ventilated Patients. Worldviews Evid. Based Nurs. 2016, 13, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.R.; Ongole, R.; Banerjee, S.; Prasad, K.; George, L.S.; George, A.; Nayak, B.S. Oral Care Protocol for Chemotherapy- and Radiation Therapy-Induced Oral Complications in Cancer Patients: Study Protocol. Asia Pac. J. Oncol. Nurs. 2019, 6, 417–423. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Dental management of pediatric patients receiving immunosuppressive therapy and/or radiation therapy. Pediatr. Dent. 2018, 40, 392–400. [Google Scholar]
- Ribeiro, I.L.A.; Caccia-Bava, M.D.C.G.G.; Sampaio, M.E.A.; Limeira, R.R.T.; de Carvalho, L.G.A.; Dos Santos, F.G.; Bezerra, P.M.M.; Sousa, S.A.; Valença, A.M.G. The Implementation of an Integrated Oral Care Protocol for Pediatric Cancer Patients: A Qualitative Study. J. Cancer Educ. 2023, 38, 940–947. [Google Scholar] [CrossRef]
- Hutcheson, C.; Seale, N.S.; McWhorter, A.; Kerins, C.; Wright, J. Multi-surface composite vs. Stainless steel crown restorations after mineral trioxide aggregate pulpotomy: A randomized controlled trial. Pediatr. Dent. 2012, 34, 460–467. [Google Scholar]
- Alharbi, M.; Sayed, A.J.; Habibullah, M.A.; Almulhim, B.; Alharthi, N.; Ravi Prakash, S.M.; Ahmad, M.Z. Preformed Stainless-Steel Crowns: Their Effects on Oral Hygiene Maintenance and Gingival Health—A Prospective Original Research. J. Pharm. Bioallied Sci. 2024, 16, S1526–S1530. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kamata, T.; Yanagisawa, R.; Morita, D.; Saito, S.; Sakashita, K.; Shiohara, M.; Kurita, H.; Koike, K.; Nakazawa, Y. Increasing Risk of Disturbed Root Development in Permanent Teeth in Childhood Cancer Survivors Undergoing Cancer Treatment at Older Age. J. Pediatr. Hematol. Oncol. 2017, 39, e150–e154. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Hahn, S.M.; Kim, H.S.; Lyu, C.J.; Lee, J.H.; Lee, J.; Han, J.W. Clinical Risk Factors Influencing Dental Developmental Disturbances in Childhood Cancer Survivors. Cancer Res. Treat. 2018, 50, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Kılınç, G.; Bulut, G.; Ertuğrul, F.; Ören, H.; Demirağ, B.; Demiral, A.; Aksoylar, S.; Kamer, E.S.; Ellidokuz, H.; Olgun, N. Long-term Dental Anomalies after Pediatric Cancer Treatment in Children. Turk. J. Haematol. 2019, 36, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Akitomo, T.; Tsuge, Y.; Mitsuhata, C.; Nomura, R. A Narrative Review of the Association between Dental Abnormalities and Chemotherapy. J. Clin. Med. 2024, 13, 4942. [Google Scholar] [CrossRef]
- Akitomo, T.; Asao, Y.; Iwamoto, Y.; Kusaka, S.; Usuda, M.; Kametani, M.; Ando, T.; Sakamoto, S.; Mitsuhata, C.; Kajiya, M.; et al. A Third Supernumerary Tooth Occurring in the Same Region: A Case Report. Dent. J. 2023, 11, 49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).