Mechanism and Treatment of Right Ventricular Failure Due to Pulmonary Hypertension in Children
Abstract
1. Introduction
2. Methodology
3. Definition and Hemodynamic Classification of PH
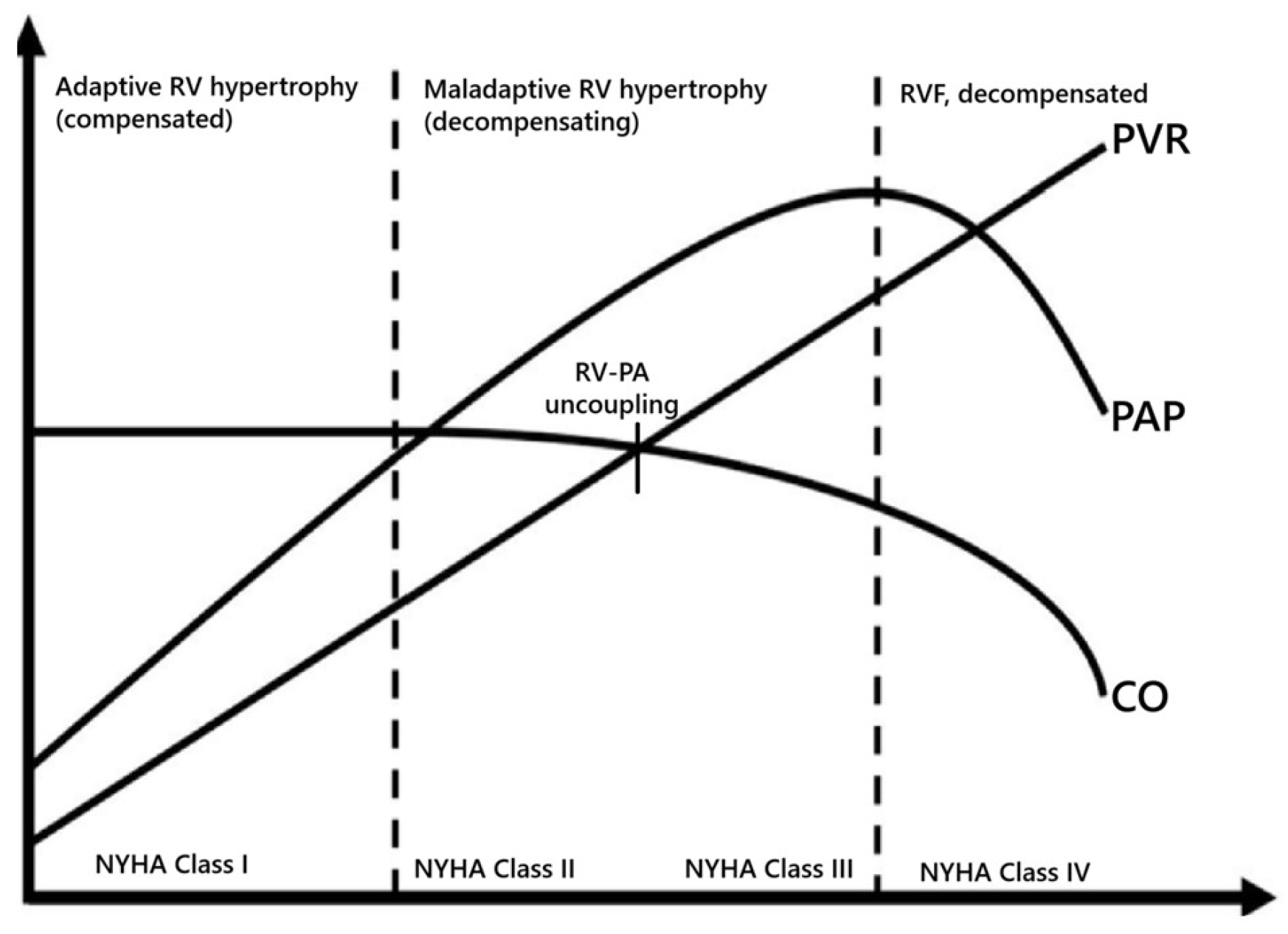
4. Pathophysiology of RVF Due to PH
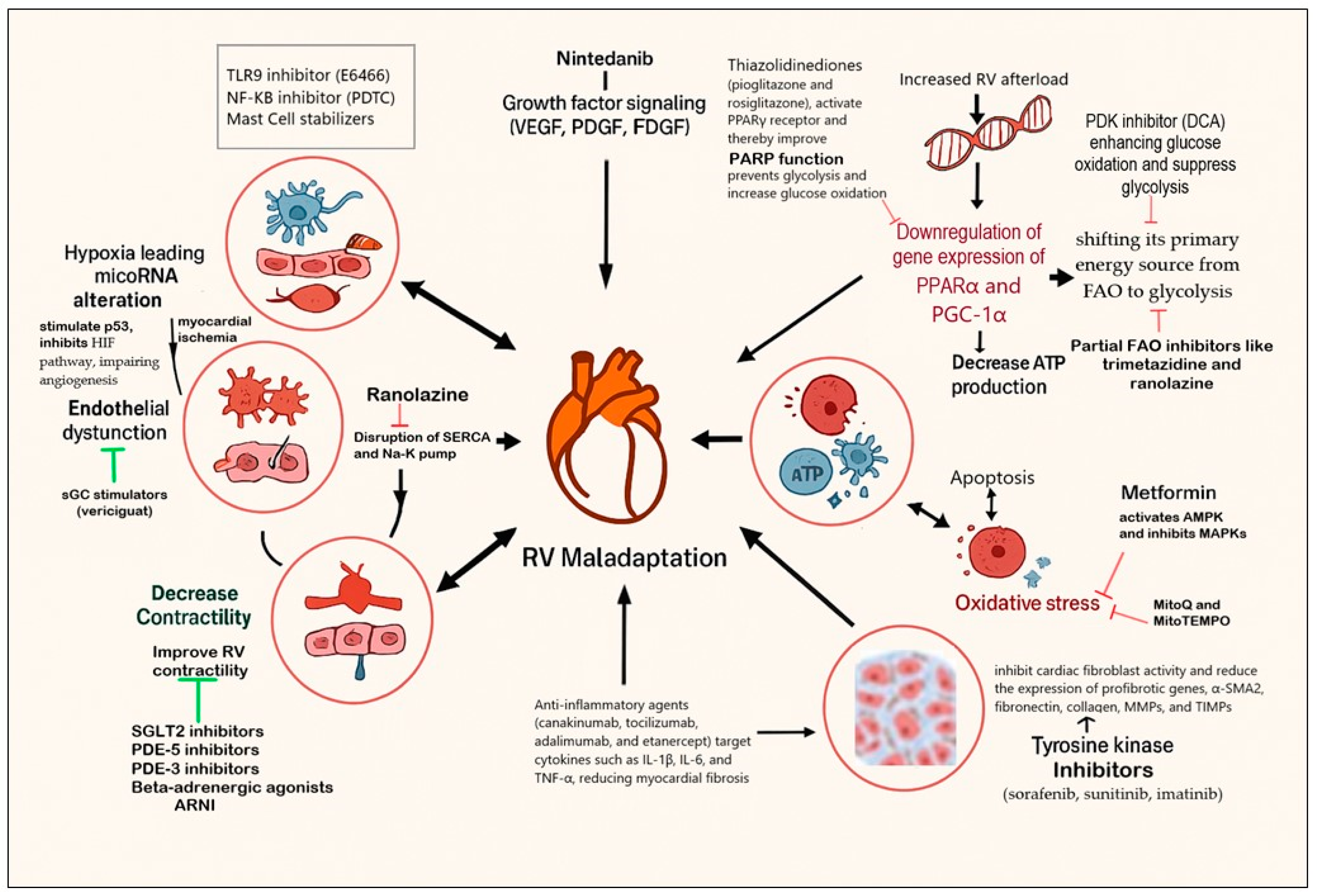
5. Experimental Therapies Targeting RV Remodeling
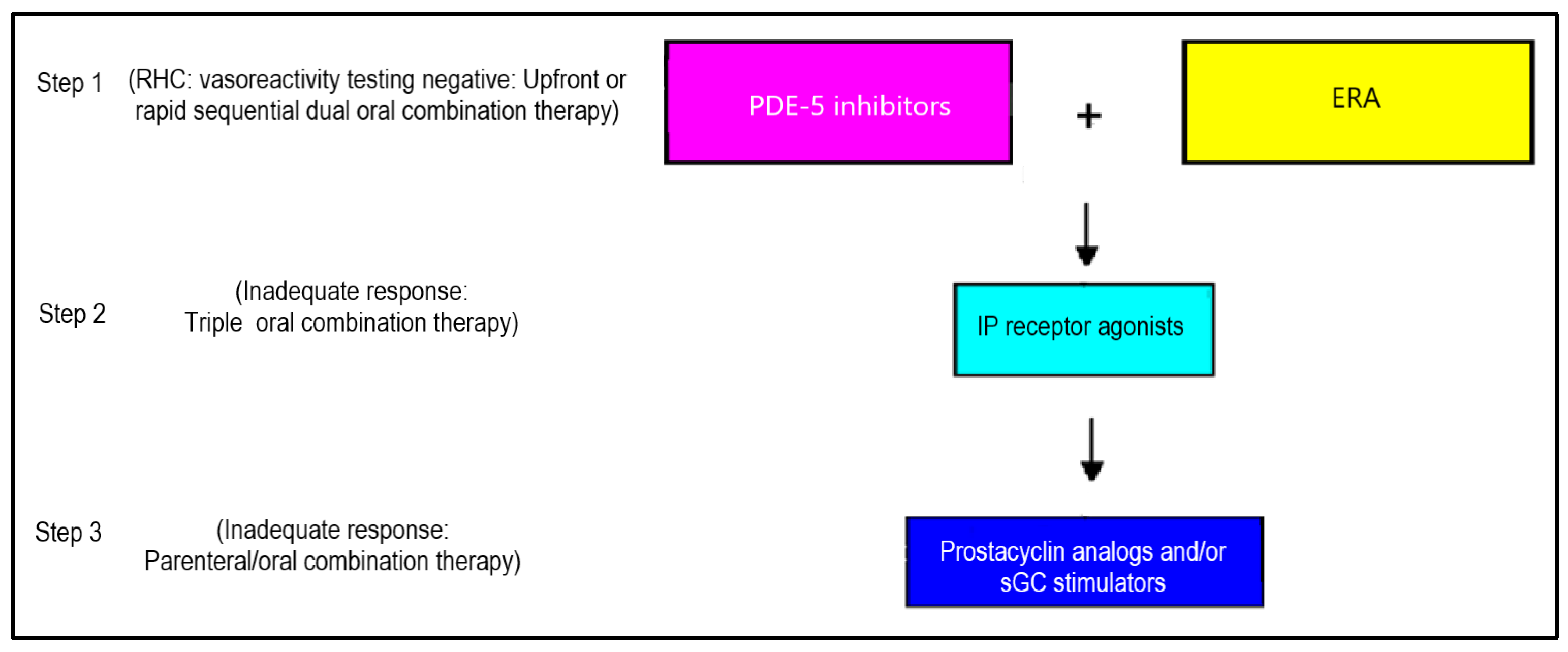
6. Combination Therapy for RVF
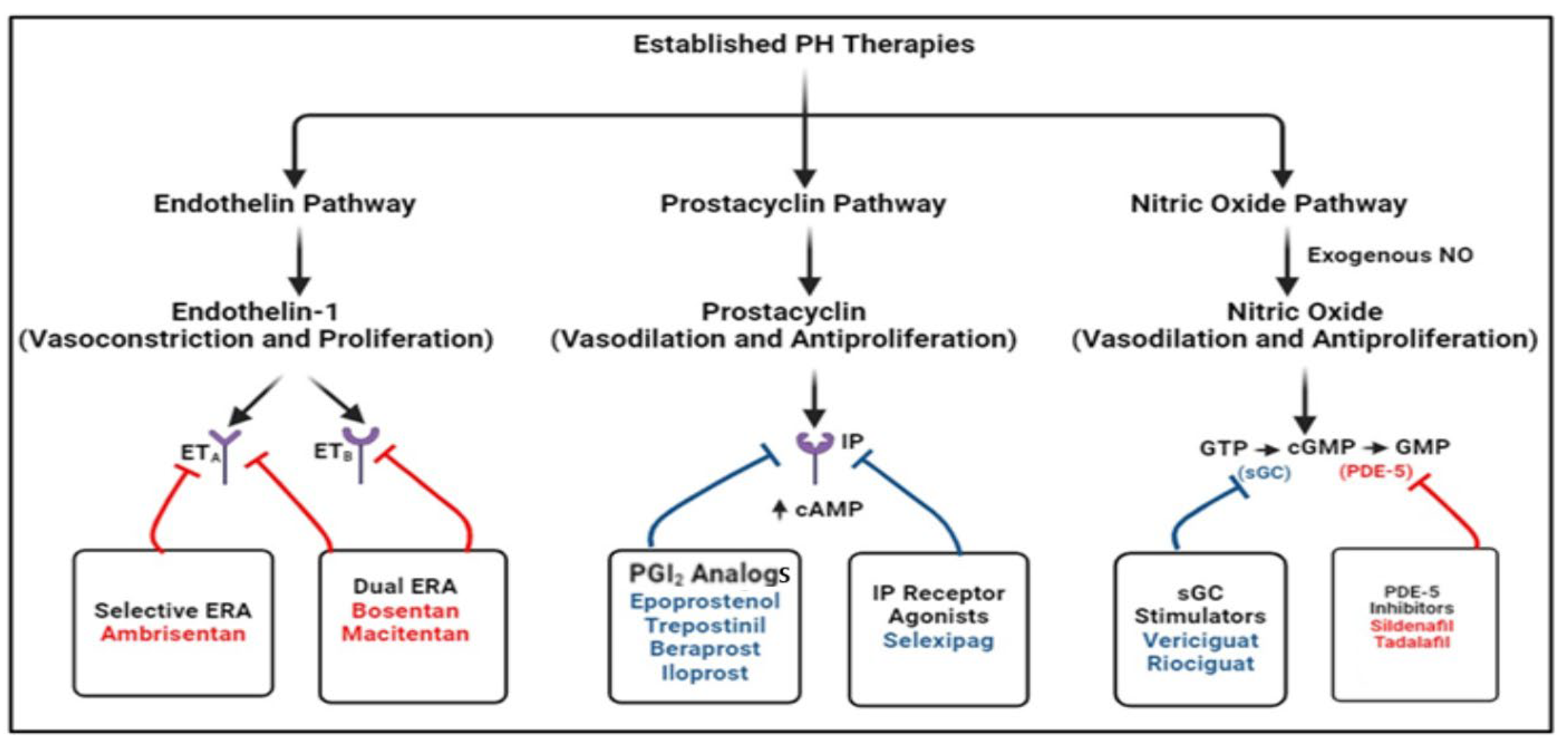
7. Current Treatment Approach to PH
8. Disease-Modifying Agents Based on Preclinical and Clinical Trials
9. Interventions for Children with Advanced Pulmonary Hypertension
9.1. Creation of Atrial-Level Communication
9.2. Pulmonary-to-Systemic Shunt (Reverse Potts Shunt)
9.3. Lung Transplantation
10. Future Directions
11. Conclusions
Funding
Conflicts of Interest
Abbreviations
| RVD | Right ventricular dysfunction |
| RVF | Right ventricular failure |
| MAP | Mean arterial pressure |
| mPAP | Mean pulmonary artery pressure |
| PH | Pulmonary hypertension |
| ACEi | Angiotensin-converting enzyme inhibitors |
| ARBs | Angiotensin II receptor blockers |
| BBs | Beta-blockers |
| ARNI | Angiotensin receptor–neprilysin inhibitor |
| iNO | Inhaled nitric oxide |
| CO | Cardiac output |
| SW | Stroke work |
| SV | Stroke volume |
| RV-PA coupling | Right ventricle–pulmonary artery coupling |
| LIPUS | Low-intensity pulsed ultrasound |
References
- Mehra, M.R.; Park, M.H.; Landzberg, M.J.; Lala, A.; Waxman, A.B.; International Right Heart Failure Foundation Scientific Working Group. Right heart failure: Toward a common language. J. Heart Lung Transplant. 2014, 33, 123–126. [Google Scholar] [PubMed]
- Mcdonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Boehm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar]
- Das, B.; Raj, S. Contemporary Treatment of Right Ventricular Failure. JHLT Open 2024, 7, 100203. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.M.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar]
- Rosenzweig, E.B.; Abman, S.H.; Adatia, I.; Beghetti, M.; Bonnet, D.; Haworth, S.; Ivy, D.D.; Berger, R.M. Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019, 53, 1801916. [Google Scholar]
- Opitz, C.F.; Hoeper, M.M.; Gibbs, J.S.R.; Kaemmerer, H.; Pepke-Zaba, J.; Coghlan, J.G.; Scelsi, L.; D’Alto, M.; Olsson, K.M.; Ulrich, S.; et al. Pre-Capillary, Combined, and Post-Capillary Pulmonary Hypertension: A Pathophysiological Continuum. J. Am. Coll. Cardiol. 2016, 68, 368–378. [Google Scholar]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar]
- Hsu, S.; Simpson, C.E.; Houston, B.A.; Wand, A.; Sato, T.; Kolb, T.M.; Mathai, S.C.; Kass, D.A.; Hassoun, P.M.; Damico, R.L.; et al. Multi-beat right ventricular-arterial coupling predicts clinical worsening in pulmonary arterial hypertension. J. Am. Heart Assoc. 2020, 9, 016031. [Google Scholar]
- Burkhoff, D.; Sagawa, K. Ventricular efficiency predicted by an analytical model. Am. J. Physiol. 1986, 250, R1021–R1027. [Google Scholar] [PubMed]
- Brener, M.I.; Burkhoff, D.; Sunagawa, K. Effective arterial elastance in the pulmonary artery circulation. Circ. Heart Fail. 2020, 13, e006591. [Google Scholar] [PubMed]
- Janowski, A.M.; Ravellette, K.S.; Insel, M.; Garcia, J.G.; Rischard, F.P.; Vanderpool, R.R. Advanced hemodynamic and cluster analysis for identifying novel RV function subphenotypes in patients with pulmonary hypertension. J. Heart Lung Transplant. 2024, 43, 755–770. [Google Scholar]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar]
- Sack, M.N.; Rader, T.A.; Park, S.; Bastin, J.; McCune, S.A.; Kelly, D.P. Fatty acid oxidation enzyme-encoding gene expression is downregulated in the failing heart. Circulation 1996, 94, 2837–2842. [Google Scholar] [PubMed]
- Wang, H.; Shen, M.; Shu, X.; Guo, B.; Jia, T.; Feng, J.; Lu, Z.; Chen, Y.; Lin, J.; Liu, Y.; et al. Cardiac metabolism, rerogramming and diseases. J. Cardiovasc. Transl. Res. 2023, 17, 71–84. [Google Scholar]
- Sydykov, A.; Mamazhakypov, A.; Petrovic, A.; Kosanovic, D.; Sarybaev, A.S.; Weissmann, N.; Ghofrani, H.A.; Schermuly, R.T. Inflammatory mediators drive adverse right ventricular remodeling and dysfunction and serve as potential biomarkers. Front. Physiol. 2018, 9, 00609. [Google Scholar]
- Egemnazarov, B.; Crnkovic, S.; Nagy, B.M.; Olschewski, H.; Kwapiszewska, G. Right ventricular fibrosis and dysfunction: Actual concepts and common misconceptions. Matrix Biol. 2018, 68, 507–521. [Google Scholar]
- Roe, A.; Frisk, M.; Louch, W. Targeting cardiomyocyte Ca2+ homeostasis in heart failure. Curr. Pharm. Des. 2014, 21, 431–448. [Google Scholar]
- Dupont, M.; Tang, W.W. Right Ventricular Afterload and the Role of Nitric Oxide Metabolism in Left-Sided Heart Failure. J. Card. Fail. 2013, 19, 712–721. [Google Scholar]
- Frangogiannis, N.G. Transforming growth factor-beta in myocardial disease. Nat. Rev. Cardiol. 2022, 19, 435–455. [Google Scholar] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 312, 312. [Google Scholar]
- Hautbergue, T.; Antigny, F.; Boët, A.; Haddad, F.; Masson, B.; Lambert, M.; Delaporte, A.; Menager, J.-B.; Savale, L.; Le Pavec, J.; et al. Right Ventricle Remodeling Metabolic Signature in Experimental Pulmonary Hypertension Models of Chronic Hypoxia and Monocrotaline Exposure. Cells 2021, 10, 1559. [Google Scholar] [CrossRef]
- Sun, X.-Q.; Zhang, R.; Zhang, H.-D.; Yuan, P.; Wang, X.-J.; Zhao, Q.-H.; Wang, L.; Jiang, R.; Bogaard, H.J.; Jing, Z.-C. Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis. Hypertens. Res. 2016, 39, 302–311. [Google Scholar] [PubMed]
- Fang, Y.-H.; Piao, L.; Hong, Z.; Toth, P.T.; Marsboom, G.; Bache-Wiig, P.; Rehman, J.; Archer, S.L. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: Exploiting Randle’s cycle. J. Mol. Med. 2012, 90, 31–43. [Google Scholar] [PubMed]
- Han, Y.; Forfia, P.; Vaidya, A.; Mazurek, J.A.; Park, M.H.; Ramani, G.; Chan, S.Y.; Waxman, A.B. Ranolazine improves right ventricular function in patients with precapillary pulmonary hypertension: Results from a double-blind, randomized, placebo-controlled trial. J. Card. Fail. 2021, 27, 253–257. [Google Scholar]
- Hansmann, G.; Calvier, L.; Risbano, M.G.; Chan, S.Y. Activation of the Metabolic Master Regulator PPARgamma: A Potential PIOneering Therapy for Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 62, 143–156. [Google Scholar]
- Liang, S.; Yegambaram, M.; Wang, T.; Wang, J.; Black, S.M.; Tang, H. Mitochondrial metabolism, redox, and calcium homeostasis in pulmonary arterial hypertension. Biomedicines 2022, 10, 341. [Google Scholar] [CrossRef]
- Sharp, J.; Farha, S.; Park, M.M.; Comhair, S.A.; Lundgrin, E.L.; Tang, W.W.; Bongard, R.D.; Merker, M.P.; Erzurum, S.C. Coenzyme Q supplementation in pulmonary arterial hypertension. Redox Biol. 2014, 2, 884–891. [Google Scholar]
- Brittain, E.L.; Niswender, K.; Agrawal, V.; Chen, X.; Fan, R.; Pugh, M.E.; Rice, T.W.; Robbins, I.M.; Song, H.; Thompson, C.; et al. Mechanistic Phase II Clinical Trial of Metformin in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2020, 9, 018349. [Google Scholar] [CrossRef]
- Wu, Y. Metformin inhibits mitochondrial dysfunction and apoptosis in cardiomyocytes induced by high glucose by upregulating AMPK activity. Exp. Biol. Med. 2023, 248, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Li, Y.R.; Danelisen, I. Molecular mechanisms of action of metformin: Latest advances and therapeutic implications. Clin. Exp. Med. 2023, 23, 2941–2951. [Google Scholar] [CrossRef]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-κB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar]
- Mamazhakypov, A.; Maripov, A.; Sarybaev, A.S.; Schermuly, R.T.; Sydykov, A. Mast Cells in Cardiac Remodeling: Focus on the Right Ventricle. J. Cardiovasc. Dev. Dis. 2024, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Mamazhakypov, A.; Sommer, N.; Assmus, B.; Tello, K.; Schermuly, R.T.; Kosanovic, D.; Sarybaev, A.S.; Weissmann, N.; Pak, O. Novel Therapeutic Targets for the Treatment of Right Ventricular Remodeling: Insights from the Pulmonary Artery Banding Model. Int. J. Environ. Res. Public Health 2021, 18, 8297. [Google Scholar] [CrossRef]
- Sun, X.-Q.; Abbate, A.; Bogaard, H.-J. Role of cardiac inflammation in right ventricular failure. Cardiovasc. Res. 2017, 113, 1441–1452. [Google Scholar] [CrossRef]
- Oknińska, M.; Zajda, K.; Zambrowska, Z.; Grzanka, M.; Paterek, A.; Mackiewicz, U.; Szczylik, C.; Kurzyna, M.; Piekiełko-Witkowska, A.; Torbicki, A.; et al. Role of Oxygen Starvation in Right Ventricular Decompensation and Failure in Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. Heart Fail. 2024, 12, 235–247. [Google Scholar] [CrossRef]
- Klinke, A.; Schubert, T.; Müller, M.; Legchenko, E.; Zelt, J.G.E.; Shimauchi, T.; Napp, L.C.; Rothman, A.M.K.; Bonnet, S.; Stewart, D.J.; et al. Emerging therapies for right ventricular dysfunction and failure. Cardiovasc. Diagn. Ther. 2020, 10, 1735–1767. [Google Scholar] [CrossRef]
- Ramalingam, A.; Budin, S.B.; Fauzi, N.M.; Ritchie, R.H.; Zainalabidin, S. Targeting mitochondrial reactive oxygen species-mediated oxidative stress attenuates nicotine-induced cardiac remodeling and dysfunction. Sci. Rep. 2021, 11, 13845. [Google Scholar]
- Makkaoui, N.; Prasad, V.; Bagchi, P.; Carmona, T.; Li, K.; Latham, O.L.; Zhang, Y.; Lee, J.; Furdui, C.M.; Maxwell, J.T. Cell-based therapies reverse the heart failure-altered right ventricular proteome towards a pre-disease state. Stem Cell Res. Ther. 2024, 15, 420. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Lin, Y.; Li, S.; Liu, C.; Cai, A.; Li, W.; Zhang, W.; Gao, X.; Ren, Z.; et al. Cardiac repair using regenerating neonatal heart tissue-derived extracellular vesicles. Nat. Commun. 2025, 16, 1292. [Google Scholar] [CrossRef]
- Tan, J.S.; Wei, Y.; Chong, L.; Yang, Y.; Hu, S.; Wang, Y. SGLT2 inhibitors as a potential therapeutic option for pulmonary hypertension: Mechanisms and clinical perspectives. Crit. Rev. Clin. Lab. Sci. 2024, 1, 2361012. [Google Scholar]
- Lin, W.; Poh, A.-L.; Tang, W.H.W. Novel Insights and Treatment Strategies for Right Heart Failure. Curr. Heart Fail. Rep. 2018, 15, 141–155. [Google Scholar]
- Das, B.B. Unlocking the Potential: Angiotensin Receptor Neprilysin and Sodium Glucose Co-Transporter 2 Inhibitors for Right Ventricle Dysfunction in Heart Failure. Medicina 2024, 60, 1112. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-L.; Lin, Y.; Lin, M.-S.; Tsai, T.-H.; Yang, N.-I.; Wang, C.-Y.; Hsieh, I.-C.; Hung, M.-J.; Chen, T.-H. Comparing angiotensin receptor–neprilysin inhibitors with sodium–glucose cotransporter 2 inhibitors for heart failure with diabetes mellitus. Diabetol. Metab. Syndr. 2023, 15, 01081. [Google Scholar]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar]
- Giannattasio, S.; Citarella, A.; Trocchianesi, S.; Filardi, T.; Morano, S.; Lenzi, A.; Ferretti, E.; Crescioli, C. Cell-Target-Specific Anti-Inflammatory Effect of Empagliflozin: In Vitro Evidence in Human Cardiomyocytes. Front. Mol. Biosci. 2022, 9, 879522. [Google Scholar]
- Sano, R.; Shinozaki, Y.; Ohta, T. Sodium–glucose cotransporters: Functional properties and pharmaceutical potential. J. Diabetes Investig. 2020, 11, 770–782. [Google Scholar]
- Benes, J.; Kotrc, M.; Wohlfahrt, P.; Kroupova, K.; Tupy, M.; Kautzner, J.; Melenovsky, V. Right ventricular global dysfunction score: A new concept of right ventricular function assessment in patients with heart failure with reduced ejection fraction (HFrEF). Front. Cardiovasc. Med. 2023, 10, 1194174. [Google Scholar]
- Iborra-Egea, O.; Santiago-Vacas, E.; Yurista, S.R.; Lupón, J.; Packer, M.; Heymans, S.; Zannad, F.; Butler, J.; Pascual-Figal, D.; Lax, A.; et al. Unraveling the Molecular Mechanism of Action of Empagliflozin in Heart Failure with Reduced Ejection Fraction with or Without Diabetes. J. Am. Coll. Cardiol. Basic Transl. Sci. 2019, 4, 831–840. [Google Scholar]
- Xie, Y.; Wei, Y.; Li, D.; Pu, J.; Ding, H.; Zhang, X. Mechanisms of SGLT2 Inhibitors in Heart Failure and Their Clinical Value. J. Cardiovasc. Pharmacol. 2023, 81, 4–14. [Google Scholar]
- Vardeny, O.; Claggett, B.; Packer, M.; Zile, M.R.; Rouleau, J.; Swedberg, K.; Teerlink, J.R.; Desai, A.S.; Lefkowitz, M.; Shi, V.; et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: The PARADIGM-HF trial. Eur. J. Heart Fail. 2016, 18, 1228–1234. [Google Scholar] [PubMed]
- Hechter, S.J.; Fredriksen, P.M.; Liu, P.; Veldtman, G.; Merchant, N.; Freeman, M.; Therrien, J.; Benson, L.; Siu, S.; Webb, G. Angiotensin-converting enzyme inhibitors in adults after the Mustard procedure. Am. J. Cardiol. 2001, 87, 660–663. [Google Scholar]
- Tutarel, O.; Meyer, G.P.; Bertram, H.; Wessel, A.; Schieffer, B.; Westhoff-Bleck, M. Safety and efficiency of chronic ACE inhibition in symptomatic heart failure patients with a systemic right ventricle. Int. J. Cardiol. 2012, 154, 14–16. [Google Scholar]
- Pieske, B.; Wachter, R.; Shah, S.J.; Baldridge, A.; Szeczoedy, P.; Ibram, G.; Shi, V.; Zhao, Z.; Cowie, M.R.; Prado, A.C.; et al. Effect of Sacubitril/Valsartan vs. Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients with Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial. JAMA 2021, 326, 1919–1929. [Google Scholar]
- Januzzi, J.L.; Prescott, M.F.; Butler, J.; Felker, G.M.; Maisel, A.S.; McCague, K.; Camacho, A.; Piña, I.L.; Rocha, R.A.; Shah, A.M.; et al. Association of Change in N-Terminal Pro–B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment with Cardiac Structure and Function in Patients with Heart Failure with Reduced Ejection Fraction. JAMA 2019, 322, 12821. [Google Scholar]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar]
- Lin, Y.; Zhang, H.; Zhao, S.; Chen, L.; Li, J.; Wang, X.; Tian, W. The Efficacy and Safety of the Combined Therapy of Sodium-Glucose Co-Transporter-2 Inhibitors and Angiotensin Receptor-Neprilysin Inhibitor in Patients with Heart Failure with Reduced Ejection Fraction: A Meta-Analysis of the EMPEROR-Reduced and DAPA-HF Sub-Analysis. Front. Cardiovasc. Med. 2022, 9, 882089. [Google Scholar]
- Mo, X.; Lu, P.; Yang, X. Efficacy of sacubitril-valsartan and SGLT2 inhibitors in heart failure with reduced ejection fraction: A systematic review and meta-analysis. Clin. Cardiol. 2023, 46, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Ayalasomayajula, S.; Blaustein, R.O.; Gheyas, F. Vericiguat, a novel sGC stimulator: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2023, 16, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Bronzwaer, J.G.F. Nitric oxide’s role in the heart: Control of beating or breathing? Am. J. Physiol.-Heart Circ. Physiol. 2004, 287, H8–H13. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Langleben, D.; Hemnes, A.R.; Noordegraaf, A.V.; Rosenkranz, S.; Thenappan, T.; Hassoun, P.M.; Preston, I.R.; Ghio, S.; Badagliacca, R.; et al. Riociguat and the right ventricle in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2022, 31, 220061. [Google Scholar] [CrossRef]
- Norre, T.; Grimm, D.; Simonsen, U. Sacubitril/valsartan, sodium-glucose cotransporter 2 inhibitors and vericiguat for congestive heart failure therapy. Basic Clin. Pharmacol. Toxicol. 2022, 130, 425–438. [Google Scholar] [CrossRef]
- Jiménez-Blanco, B.M.; Valle, A.; Gayán, O.J.; Del Prado Díaz, S.; Cordero, P.D.; Morillas, C.H.; Bascompte, C.R.; Seller, M.J.; Zamorano Gómez, J.L.; Alonso Salinas, G.L. Safety and Efficacy of the Combination of Sacubitril/Valsartan and SGLT2i in HFrEF Patients (SECSI Registry). J. Cardiovasc. Pharmacol. 2021, 78, e662–e668. [Google Scholar] [CrossRef]
- Barst, R.J.; Beghetti, M.; Pulido, T.; Layton, G.; Konourina, I.; Zhang, M.; Ivy, D.D. STARTS-2: Long-term survival with oral sildenafil monotherapy in treatment-naïve pediatric pulmonary arterial hypertension. Circulation 2014, 129, 1914–1923. [Google Scholar] [CrossRef]
- Barst, R.J.; Ivy, D.D.; Gaitan, G.; Szatmari, A.; Rudzinski, A.; Garcia, A.E.; Sastry, B.K.S.; Pulido, T.; Layton, G.R.; Serdarevic-Pehar, M.; et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naïve children with pulmonary arterial hypertension. Circulation 2012, 125, 324–334. [Google Scholar] [CrossRef]
- Ivy, D.; Bonnet, D.; Berger, R.; Meyer, G.; Baygani, S.; Li, B. Efficacy and safety of tadalafil in a pediatric population with pulmonary arterial hypertension: Phase3 randomized, double-blind placebo-controlled study. Pulm. Circ. 2021, 11, 20458940211024955. [Google Scholar] [CrossRef]
- Small, D.; Ferguson-Sells, L.; Dahdah, N.; Bonnet, D.; Landry, J.; Li, B. Pharmacokinetics and safety of tadalafil in a paediatric population with pulmonary arterial hypertension: A multiple ascending-dose study. Br. J. Clin. Pharmacol. 2019, 85, 2302–2309. [Google Scholar] [CrossRef]
- Berger, R.M.; Haworth, S.G.; Bonnet, D.; Dulac, Y.; Fraisse, A.; Galiè, N.; Ivy, D.D.; Jaïs, X.; Miera, O.; Rosenzweig, E.B.; et al. FUTURE-2: Results from an open-label, long-term safety and tolerability extension study using the pediatric FormUlation of bosenTan in pUlmonary arterial hypeRtEnsion. Int. J. Cardiol. 2016, 202, 52–58. [Google Scholar]
- Berger, R.M.; Gehin, M.; Beghetti, M.; Ivy, D.; Kusic-Pajic, A.; Cornelisse, P.; Grill, S.; Bonnet, D.; FUTURE-3 Investigators. A bosentan pharmacokinetic study to investigate dosing regimens in paediatric patients with pulmonary arterial hypertension: FUTURE-3. Br. J. Clin. Pharmacol. 2017, 83, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Ivy, D.; Beghetti, M.; Juaneda-Simian, E.; Miller, D.; Lukas, M.A.; Ioannou, C.; Okour, M.; Narita, J.; Berger, R.M. A randomized study of safety and efficacy of two doses of ambrisentan to treat pulmonary arterial hypertension in pediatric patients aged 8years up to 18 years. J. Pediatr. 2020, 5, 100055. [Google Scholar]
- Takatsuki, S.; Rosenzweig, E.B.; Zuckerman, W.; Brady, D.; Calderbank, M.; Ivy, D.D. Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr. Pulmonol. 2013, 48, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hopper, R.K.; Wang, Y.; DeMatteo, V.; Santo, A.; Kawut, S.M.; Elci, O.U.; Hanna, B.D.; Mercer-Rosa, L. Right ventricular function mirrors clinical improvement with use of prostacyclin analogues in pediatric pulmonary hypertension. Pulm. Circ. 2018, 8, 2045894018759247. [Google Scholar]
- Lammers, A.E.; Hislop, A.A.; Flynn, Y.; Haworth, S.G. Epoprostenol treatment in children with severe pulmonary hypertension. Heart 2007, 93, 739–743. [Google Scholar]
- Tella, J.B.; Kulik, T.J.; McSweeney, J.E.; Sleeper, L.A.; Lu, M.; Mullen, M.P. Prostanoids in pediatric pulmonary hypertension: Clinical response, time-to-effect, and dose-response. Pulm. Circ. 2020, 10, 2045894020944858. [Google Scholar]
- Cassady, S.J.; Almario, J.A.N.; Ramani, G.V. Therapeutic Potential of Treprostinil Inhalation Powder for Patients with Pulmonary Arterial Hypertension: Evidence to Date. Drug Healthc. Patient Saf. 2024, 16, 51–59. [Google Scholar]
- Ivy, D.D.; Rosenzweig, E.B.; Lemarié, J.-C.; Brand, M.; Rosenberg, D.; Barst, R.J. Long-Term Outcomes in Children with Pulmonary Arterial Hypertension Treated with Bosentan in Real-World Clinical Settings. Am. J. Cardiol. 2010, 106, 1332–1338. [Google Scholar]
- Ploegstra, M.-J.; Ivy, D.D.; Beghetti, M.; Bonnet, D.; Alehan, D.; Ablonczy, L.; Mattos, S.; Bowers, D.; Humpl, T.; Berger, R.M.F. Long-term outcome of children with newly diagnosed pulmonary arterial hypertension: Results from the global TOPP registry. Eur. Heart J.-Qual. Care Clin. Outcomes 2024, 10, 66–76. [Google Scholar]
- Bai, Y.; Sun, L.; Hu, S.; Wei, Y. Combination therapy in pulmonary arterial hypertension: A meta-analysis. Cardiology 2011, 120, 157–165. [Google Scholar] [PubMed]
- Fox, B.D.; Shtraichman, O.; Langleben, D.; Shimony, A.; Kramer, M.R. Combination Therapy for Pulmonary Arterial Hypertension: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2016, 32, 1520–1530. [Google Scholar]
- Wang, R.; Wei, M.; Wang, J.; Huang, X.; Yan, Q.; Wang, S.; Wu, Y. A Network Meta-analysis of the Efficacy and Safety of Targeted Drug Combinations in the Treatment of Pulmonary Arterial Hypertension. Cardiol. Discov. 2023, 3, 249–260. [Google Scholar]
- Wilkins, M.R.; Mckie, M.A.; Law, M.; Roussakis, A.A.; Harbaum, L.; Church, C.; Coghlan, J.G.; Condliffe, R.; Howard, L.S.; Kiely, D.G.; et al. Positioning imatinib for pulmonary arterial hypertension: A phase I/II design comprising dose finding and single-arm efficacy. Pulm. Circ. 2021, 11, 20458940211052823. [Google Scholar] [PubMed]
- Gillies, H.; Chakinala, M.M.; Dake, B.T.; Feldman, J.P.; Hoeper, M.M.; Humbert, M.; Jing, Z.; Langley, J.; McLaughlin, V.V.; Niven, R.W.; et al. IMPAHCT: A randomized phase 2b/3 study of inhaled imatinib for pulmonary arterial hypertension. Pulm. Circ. 2024, 14, e12352. [Google Scholar] [CrossRef]
- Frantz, R.P.; McLaughlin, V.V.; Sahay, S.; Subías, P.E.; Zolty, R.L.; Benza, R.L.; Channick, R.N.; Chin, K.M.; Hemnes, A.R.; Howard, L.S.; et al. Seralutinib in adults with pulmonary arterial hypertension (TORREY): A randomized, double-blind, placebo-controlled phase 2 trial. Lancet Respir. Med. 2024, 12, 523–534. [Google Scholar]
- Humbert, M.; Mclaughlin, V.; Gibbs, S.; Gomberg-Maitland, M.; Hoeper, M.; Preston, I.; Souza, R.; Waxman, A.; Ghofrani, H.; Subias, P.E.; et al. Sotatercept for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grünig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar]
- Hodgson, J.; Swietlik, E.M.; Salmon, R.M.; Hadinnapola, C.; Nikolic, I.; Wharton, J.; Guo, J.; Liley, J.; Haimel, M.; Bleda, M.; et al. Characterization of GDF2 Mutations and Levels of BMP9 and BMP10 in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 575–585. [Google Scholar]
- Wood, J.H.; Guo, J.; Morrell, N.W.; Li, W. Advances in the molecular regulation of endothelial BMP9 signaling complexes and implications for cardiovascular disease. Biochem. Soc. Trans. 2019, 47, 779–791. [Google Scholar]
- Chen, X.; Austin, E.D.; Talati, M.; Fessel, J.P.; Farber-Eger, E.H.; Brittain, E.L.; Hemnes, A.R.; Loyd, J.E.; West, J. Estrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur. Respir. J. 2017, 50, 1602337. [Google Scholar] [PubMed]
- Alzoubi, A.; Toba, M.; Abe, K.; Bauer, N.N.; Fagan, K.; McMurtry, I.F.; Oka, M. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H1708–H1718. [Google Scholar]
- McMurtry, M.S.; Bonnet, S.; Wu, X.; Dyck, J.R.; Haromy, A.; Hashimoto, K.; Michelakis, E.D. Dichloroacetate Prevents and Reverses Pulmonary Hypertension by Inducing Pulmonary Artery Smooth Muscle Cell Apoptosis. Circ. Res. 2004, 95, 830–840. [Google Scholar]
- Khan, F.A.; Stewart, I.; Fabbri, L.; Moss, S.; Robinson, K.; Smyth, A.R.; Jenkins, G. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021, 76, 907–919. [Google Scholar] [PubMed]
- Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. JAK-STAT signaling in inflammation and stress-related diseases: Implications for therapeutic interventions. Mol. Biomed. 2023, 4, 00151. [Google Scholar]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Rodríguez-Penas, D.; Portolés, M.; Roselló-Lletí, E.; Rivera, M.; González-Juanatey, J.R.; Lago, F. Relaxin-2 in Cardiometabolic Diseases: Mechanisms of Action and Future Perspectives. Front. Physiol. 2017, 8, 00599. [Google Scholar]
- Wei, H.; Zhang, D.; Liu, L.; Xia, W.; Li, F. Rho signaling pathway enhances proliferation of PASMCs by suppressing nuclear translocation of Smad1 in PAH. Exp. Ther. Med. 2019, 17, 6942. [Google Scholar]
- Mouchaers, K.; Schalij, I.; de Boer, M.; Postmus, P.; van Hinsbergh, V.; Amerongen, G.v.N.; Noordegraaf, A.V.; van der Laarse, W. Fasudil reduces monocrotaline-induced pulmonary arterial hypertension: Comparison with bosentan and sildenafil. Eur. Respir. J. 2010, 36, 800–807. [Google Scholar]
- Laidlaw, T.M.; Buchheit, K.M.; Cahill, K.N.; Hacker, J.; Cho, L.; Cui, J.; Feng, C.; Chen, C.C.; Le, M.; Israel, E.; et al. Trial of thromboxane receptor inhibition with ifetroban: TP receptors regulate eicosanoid homeostasis in aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2023, 152, 700–710. [Google Scholar]
- Lou, G.; Li, A.; Cen, Y.; Yang, Q.; Zhang, T.; Qi, J.; Chen, Z.; Liu, Y. Selonsertib, a potential drug for liver failure therapy by rescuing the mitochondrial dysfunction of macrophage via ASK1–JNK–DRP1 pathway. Cell Biosci. 2021, 11, 00525. [Google Scholar]
- Zolty, R. Pulmonary arterial hypertension specific therapy: The old and the new. Pharmacol. Ther. 2020, 214, 107576. [Google Scholar] [CrossRef] [PubMed]
- Cassady, S.J.; Soldin, D.; Ramani, G.V. Novel and emerging therapies in pulmonary arterial hypertension. Front. Drug Discov. 2022, 2, 1022971. [Google Scholar]
- Lazarus, H.M.; Denning, J.; Wring, S.; Palacios, M.; Hoffman, S.; Crizer, K.; Kamau-Kelley, W.; Symonds, W.; Feldman, J. A trial design to maximize knowledge of the effects of rodatristat ethyl in the treatment of pulmonary arterial hypertension (ELEVATE 2). Pulm. Circ. 2022, 12, e12088. [Google Scholar] [CrossRef]
- Leuchte, H.H.; Baezner, C.; Baumgartner, R.A.; Bevec, D.; Bacher, G.; Neurohr, C.; Behr, J. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur. Respir. 2008, 32, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; MacLean, M.R.; Khanin, R.; McClure, J.; Soon, E.; Southgate, M.; MacDonald, R.A.; Greig, J.A.; Robertson, K.E.; Masson, R.; et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arter. Thromb. Vasc. Biol. 2010, 30, 716–723. [Google Scholar] [CrossRef]
- Yang, S.; Banerjee, S.; de Freitas, A.; Cui, H.; Xie, N.; Abraham, E.; Liu, G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, L521–L529. [Google Scholar] [CrossRef]
- Qin, W.; Zhao, B.; Shi, Y.; Yao, C.; Jin, L.; Jin, Y. BMPRII is a direct target of miR-21. Acta Biochim. Biophys. Sin. 2009, 41, 618–623. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, T.; Raj, J.U. MicroRNAs in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2015, 52, 139–151. [Google Scholar]
- Pattathu, J.; Michel, S.; Tengler, A.I.; Mandilaras, G.; Jakob, A.; Pozza, R.D.; Haas, N.A. Case report: Beneficial longterm effect of the atrial-flow-regulator device in a pediatric patient with idiopathic pulmonary arterial hypertension and recurring syncope. Front. Cardiovasc. Med. 2023, 10, 1197985. [Google Scholar]
- Youssef, D.E.; Averin, K.; Richards, S.; Sheppard, C.; Seaman, C.; Pietrosanu, M.; Bates, A. North American, single center experience implanting fenestrated atrial devices and atrial flow regulators into a heterogeneous group of pediatric pulmonary hypertension patients. Front. Pediatr. 2023, 11, 1073336. [Google Scholar] [CrossRef]
- Butera, G.; Piccinelli, E.; Kolesnik, A.; Averin, K.; Seaman, C.; Castaldi, B.; Cuppini, E.; Fraisse, A.; Bautista-Rodriguez, C.; Hascoet, S.; et al. Implantation of atrial flow regulator devices in patients with congenital heart disease and children with severe pulmonary hypertension or cardiomyopathy—An international multicenter case series. Front. Cardiovasc. Med. 2024, 10, 1332395. [Google Scholar]
- Hayes, D.; Jennerich, A.L.; Coleman, R.D.; Abston, E.; Adamson, G.T.; Berger, J.T.; Cohen, S.P.; Cooper, D.S.; Eghtesady, P.; Fynn-Thompson, F.; et al. Interventional Strategies for Children with Progressive Pulmonary Hypertension Despite Optimal Therapy. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2024, 211, 157–173. [Google Scholar] [CrossRef]
- Rosenzweig, E.B.; Ankola, A.; Krishnan, U.; Middlesworth, W.; Bacha, E.; Bacchetta, M. A novel unidirectional-valved shunt approach for end-stage pulmonary arterial hypertension: Early experience in adolescents and adults. J. Thorac. Cardiovasc. Surg. 2021, 161, 1438–1446.e1432. [Google Scholar]
- Hayes, D.; Cherikh, W.S.; Harhay, M.O.; Perch, M.; Hsich, E.; Potena, L.; Sadavarte, A.; Zehner, A.; Singh, T.P.; Zuckermann, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-fifth pediatric lung transplantation report–2022; focus on pulmonary vascular diseases. J. Heart Lung Transplant. 2022, 41, 1348–1356. [Google Scholar] [PubMed]
- Carvajal, H.G.; Merritt, T.C.; Canter, M.W.; Abarbanell, A.M.; Nath, D.S.; Eghtesady, P. Improved Outcomes of Infant Lung Transplantation Over 3 Decades. Ann. Thorac. Surg. 2022, 114, 184–192. [Google Scholar]
- Critser, P.J.; Boyer, D.; Visner, G.A.; Collins, S.L.; Fynn-Thompson, F.; Mullen, M.P. Recovery of right ventricular function after bilateral lung transplantation for pediatric pulmonary hypertension. Pediatr. Transplant. 2022, 26, e14236. [Google Scholar]
- Griffiths, M.; Yang, J.; Nies, M.; Vaidya, D.; Brandal, S.; Williams, M.; Matsui, E.C.; Grant, T.; Damico, R.; Ivy, D.; et al. Pediatric pulmonary hypertension: Insulin-like growth factor-binding protein 2 is a novel marker associated with disease severity and survival. Pediatr. Res. 2020, 88, 850–856. [Google Scholar] [CrossRef]
- Chen, J.Y.; Griffiths, M.; Yang, J.; Nies, M.K.; Damico, R.L.; Simpson, C.E.; Vaidya, R.D.; Brandal, S.; Ivy, D.D.; Austin, E.D.; et al. Elevated interleukin-6 levels predict clinical worsening in pediatric pulmonary arterial hypertension. J. Pediatr. 2020, 223, 164–169. [Google Scholar] [PubMed]
- Griffiths, M.; Yang, J.; Simpson, C.E.; Vaidya, D.; Nies, M.; Brandal, S.; Damico, R.; Ivy, D.D.; Austin, E.D.; Pauciulo, M.W.; et al. ST2 is a biomarker of pediatric pulmonary arterial hypertension severity and clinical worsening. Chest 2021, 160, 297–306. [Google Scholar]
- Li, G.; Zhang, H.; Zhao, L.; Zhang, Y.; Yan, D.; Liu, Y.; Su, J.; Fan, X. The expression of survivin in irreversible pulmonary arterial hypertension rats and its value in evaluating the reversibility of pulmonary arterial hypertension secondary to congenital heart disease. Pulm. Circ. 2019, 9, 2045894019859480. [Google Scholar]
- Kheyfets, V.O.; Sucharov, C.C.; Truong, U.; Dunning, J.; Hunter, K.; Ivy, D.; Miyamoto, S.; Shandas, R. Circulating miRNAs in pediatric pulmonary hypertension show promise as biomarkers of vascular function. Oxidative Med. Cell. Longev. 2017, 2017, 4957147. [Google Scholar]
- Colvin, K.L.; Dufva, M.J.; Delaney, R.P.; Ivy, D.D.; Stenmark, K.R.; Yeager, M.E. Biomarkers for pediatric pulmonary arterial hypertension—A call to collaborate. Front Pediatr. 2014, 2, 7. [Google Scholar] [PubMed]
- García Aguilar, H.; Gorenflo, M.; Ivy, D.D.; Moledina, S.; Castaldi, B.; Ishida, H.; Cześniewicz, P.; Kusa, J.; Miera, O.; Pattathu, J.; et al. Riociguat in children with pulmonary arterial hypertension: The PATENT-CHILD study. Pulm. Circ. 2022, 12, e12133. [Google Scholar] [CrossRef]
- Domingo, L.T.; Ivy, D.D.; Abman, S.H.; Grenolds, A.M.; MacLean, J.T.; Breaux, J.A.; Minford, K.J.; Frank, B.S. Novel use of riociguat in infants with severe pulmonary arterial hypertension unable to wean from inhaled nitric oxide. Front. Pediatr. 2022, 10, 1014922. [Google Scholar]
- Pu, X.; Du, L.; Hu, Y.; Fan, Y.; Xu, Q. Stem/Progenitor Cells and Pulmonary Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 167–178. [Google Scholar] [PubMed]
- Sun, Q.W.; Sun, Z. Stem cell therapy for pulmonary arterial hypertension: An update. J. Heart Lung Transplant. 2022, 41, 692–703. [Google Scholar]
- Haarman, M.G.; Kerstjens-Frederikse, W.S.; Vissia-Kazemier, T.R.; Breeman, K.T.; Timens, W.; Vos, Y.J.; Roofthooft, M.T.; Hillege, H.L.; Berger, R.M. The genetic epidemiology of pediatric pulmonary arterial hypertension. J. Pediatr. 2020, 225, 65–73. [Google Scholar]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar]
- Shindo, T.; Shimokawa, H. Therapeutic angiogenesis with sound waves. Ann. Vasc. Dis. 2020, 13, 116–125. [Google Scholar]
- Nakata, T.; Shindo, T.; Ito, K.; Eguchi, K.; Monma, Y.; Ichijo, S.; Ryoke, R.; Satoh, W.; Kumasaka, K.; Sato, H.; et al. Beneficial Effects of Low-Intensity Pulsed Ultrasound Therapy on Right Ventricular Dysfunction in Animal Models. JACC Basic Transl. Sci. 2023, 8, 283–297. [Google Scholar]



| Group 1 (PAH: CHD-PAH, iPAH, HPAH) | Group 2 (PH Due to LHD) | Group 3 (PH Due to Lung Disease) | Group 4 (CTEPH) | Group 5 (Miscellaneous) | |
|---|---|---|---|---|---|
| Common Pediatric Etiologies | CHD (e.g, ASD, VSD, AVSD), idiopathic PAH, BMPR2 mutations | Rare; congenital mitral/aortic lesions, cardiomyopathies | BPD, CDH, alveolar capillary dysplasia pediatric ILD | Exceptionally rare in pediatrics | Sarcoidosis, storage disorders, hematologic diseases |
| Incidence of RV Failure | High, especially in iPAH and Eisenmenger syndrome | Low to moderate, depending on LHD severity | Moderate to high in severe developmental lung disease | Variable, based on clot burden and RV adaptation | Variable |
| Medical Management | Targeted therapies (PDE5i, ERA, prostacyclins); CCB if vasoreactive; sotatercept in trials | Optimize LHD; PH therapies typically ineffective or harmful | Supportive care (oxygen, diuretics); PH therapies selectively in elevated PVR | Anticoagulation; surgical consideration if operable | Treat underlying cause; supportive care |
| Surgical/Intervent ional Role | CHD repair if operable; lung/heart-lung transplant in advanced disease | Valve repair/replacement; heart transplant in end-stage | Ventilatory support; transplant in refractory cases | Pulmonary thromboendarterectomy (rarely performed in children) | Individualized; etiology—dependent |
| Long-Term Prognosis | Variable; improved with advanced therapies; Eisenmenger may have stable course | Better if LHD is correctable; poor with progressive myocardial disease | Guarded; prognosis tied to underlying lung pathology | Unclear due to rarity | Heterogeneous outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.B. Mechanism and Treatment of Right Ventricular Failure Due to Pulmonary Hypertension in Children. Children 2025, 12, 476. https://doi.org/10.3390/children12040476
Das BB. Mechanism and Treatment of Right Ventricular Failure Due to Pulmonary Hypertension in Children. Children. 2025; 12(4):476. https://doi.org/10.3390/children12040476
Chicago/Turabian StyleDas, Bibhuti B. 2025. "Mechanism and Treatment of Right Ventricular Failure Due to Pulmonary Hypertension in Children" Children 12, no. 4: 476. https://doi.org/10.3390/children12040476
APA StyleDas, B. B. (2025). Mechanism and Treatment of Right Ventricular Failure Due to Pulmonary Hypertension in Children. Children, 12(4), 476. https://doi.org/10.3390/children12040476






