Abstract
Dental fear and anxiety are frequently identified as major contributing factors to non-compliance, uncooperativeness, and difficulties during dental procedures in pediatric patients. These issues can lead to avoidance of dental treatment, resulting in long-term negative consequences for oral health and overall well-being. The assessment and quantification of psychological functioning (i.e., dental fear, anxiety, and self-perceived stress) has traditionally relied on self-reported questionnaires validated for the pediatric population. While this approach is cost-effective and non-invasive, it relies on subjective self-reported data, oftentimes influenced by parental or guardian interaction, especially in young children. Salivary diagnostics has recently emerged as an objective method for the procurement of biological molecules that serve as biomarkers for a variety of oral and systemic conditions. This literature review aims to comprehensively summarize the available literature on the correlation between psychological and salivary physiological measurements assessing dental fear, dental anxiety, and self-perceived stress in pediatric dental patients, highlighting the advantages and disadvantages of each method of assessment. Four databases (PubMed®, PsycInfo, Dentistry & Oral Sciences Source, and Web of Science) were searched for published articles, in the English language, assessing the correlation between psychological and physiological distress in children undergoing dental procedures. Studies on pediatric patients reveal positive correlations between salivary cortisol and dental fear, stress, and anxiety, especially in returning patients. Conversely, findings on salivary alpha-amylase and secretory immunoglobulin A were inconsistent, with some studies suggesting correlations with dental fear and prior dental experiences.
1. Introduction
Tooth decay continues to be the most common disease amongst children in the U.S., and access to dental care is often out of reach for many, contributing to a growing global health problem. Numerous barriers have been identified as contributors to oral health disparities. According to Reich et al., they can be broadly categorized into structural, attitudinal, knowledge-based, and behavioral factors [1]. These barriers often derive from children being raised in situations of poverty, low parental educational attire, family demographics, lack of access to properly educated dental providers, and geographic disparities in care, among others [1]. Such inequalities particularly affect underserved populations, further intensifying the oral health disparity.
Significant behavioral factors contributing to poor oral health outcomes in children include dental anxiety and dental fear. Dental anxiety is a state of apprehension in anticipation of a negative dental experience, while dental fear is an in-the-moment response to perceived threatening stimuli and contributes significantly to the development of dental anxiety [2]. These domains are known to lead to lack of compliance, avoidance of dental treatment, and challenges during dental procedures [3]. Their prevalence has been estimated to vary considerably from 3% to 43%, according to specific populations, assessment tools, and methodological differences [4]. In underserved pediatric populations, estimates of dental anxiety are even greater [4]. Moreover, differences across age groups and sexes have been reported. For example, a growing body of literature has suggested that dental fear and dental anxiety are more frequent among young girls and tend to decrease as age progresses [5,6,7,8].
Roughly 10–20% of adults experience dental anxiety [9], which has often developed during childhood [10], due to traumatic dental experiences or indirect observation of the parents [11,12]. Notably, patients who report dental fear are suggested to be three times more likely to miss their dental appointment compared to patients who do not report fear [13]. Moreover, performing dental procedures becomes more challenging when a child exhibits dental fear and anxiety, as these are often accompanied by maladaptive and avoidance behaviors [14]. A causal model was described long ago by Berggren, whereby fear and anxiety may lead to avoidance, which in turn exacerbates oral health issues [15]. This deterioration may intensify feelings of inferiority and shame, which in turn perpetuate a vicious cycle of fear and anxiety [3]. As a result, avoidance of dental procedures due to fear and anxiety lead to aggravation of the dental pathology and deterioration of patients’ overall oral health.
Dental fear and anxiety not only impair treatment adherence but also exacerbate the oral health disparities already prevalent among racial and ethnic minority groups. Individuals are impacted daily from neglected oral care, and these impacts have resulted in decreased quality of life from missed school days and decreased work productivity [16,17]. Moreover, low-income and minority children have fewer preventive measures, such as sealants, and attend dental appointments less frequently [18], which additionally could further deteriorate their self-perceived quality of life. Despite the significant impact of dental anxiety and dental fear, few clinicians routinely address these issues during pediatric care, underscoring the need for more comprehensive approaches in dental practice.
Psychological distress related to dental procedures is usually reported as self-perceived stress, which refers to the feelings or thoughts an individual has regarding how much stress they are experiencing during a specific period [19]. The relationship between perceived stress and dental anxiety has been demonstrated in the past, and has indicated that as levels of perceived stress increase, so does dental anxiety [20]. In children, dental fear and dental anxiety are commonly measured through questionnaires adapted and validated in this specific population, including the Children’s Fear Survey Schedule—Dental Subscale (CFSS-DS) [21] and the Modified Child Dental Anxiety Scale (MCDAS) [22]. To measure self-perceived stress in children, the Perceived Stress Scale for Children (PSS-C) is one of the most common assessment tools [23]. Despite being validated, these questionnaires gather only self-reported outcomes and thus rely on subjective measurements that can be greatly influenced by multiple sorts of bias (i.e., response, acquiescence, cognitive bias). Moreover, some of these scales (e.g., PSS-C) only assess psychological distress in the past (i.e., in the past week), thus potentially incurring recall bias and not necessarily correlating to the present situation.
The development of fear, anxiety, and stress in children results as a physiological activation of the sympathetic nervous system, specifically, the sympathetic adrenal medullary system and the hypothalamic–pituitary–adrenal (HPA) axis [24]. Cortisol and alpha-amylase are considered reliable stress-related biomarkers, reflecting sympathetic activation and HPA functioning [25,26]. Because these molecules are found in physiological levels in saliva, they can be promptly quantified through salivary collection. Due to its non-invasive nature, saliva collection is not expected to increase stress in the patient. [26]. In addition, saliva collection provides means for an easy, hygienic, and cost-effective diagnostic method [27]. Accordingly, the quantification of stress-related biomarkers can be proposed in substitution of or in combination with self-reported psychological outcomes. Ideally, from a diagnostic standpoint, it would be advantageous if psychological and physiological stress-related outcomes were concordant and positively associated.
Therefore, the current study aimed to comprehensively review available psychological and salivary physiological measurements assessing dental fear, dental anxiety, and stress in pediatric patients undergoing dental procedures, highlighting the advantages and disadvantages of each assessment. A secondary aim was to summarize the existing literature on the relationship between psychological and physiological assessment of distress in pediatric patients undergoing dental treatment.
Understanding the factors contributing to dental anxiety and stress in this population is crucial to helping develop effective interventions and improving overall oral health during childhood, with important long-term oral health consequences in later years.
2. Materials and Methods
A comprehensive literature review was conducted from April to August 2024 on 4 databases (PubMed® via MEDLINE, PsycInfo, Dentistry & Oral Sciences Source, and Web of Science) for articles published in English language on psychological and physiological distress in the pediatric population (<18 years old) before dental procedures. Only articles that assessed both physiological and psychological measurements in the pediatric population without any further intervention and examined their correlation were included in this literature review. Studies conducted on adults, not quantifying nor assessing the relationship between psychological (stress, anxiety, fear) and acute physiological stress as measured by saliva samples, not related to any dental procedures, nor in English language were excluded. We also excluded case reports, systematic reviews, meta-analyses, and literature reviews. The search strategy utilized a combination of MeSH terms and keywords, including “dental fear” OR “dental anxiety” OR “stress” AND “pediatric patients” OR “pediatric population” OR “children” OR “adolescents” AND “dental procedure” OR “dental care” AND “salivary biomarkers” OR “salivary cortisol” OR “salivary alpha-amylase”. Boolean operators (“AND”, “OR”) were utilized to refine the search. Reference list of included articles, existing reviews, systematic reviews, and meta-analyses were manually screened to ensure comprehensiveness. Many of the available studies investigated other factors, such as the type of dental procedure, salivary flow volume, the presence and number of caries lesions. However, these factors are outside the scope of this review.
3. Results
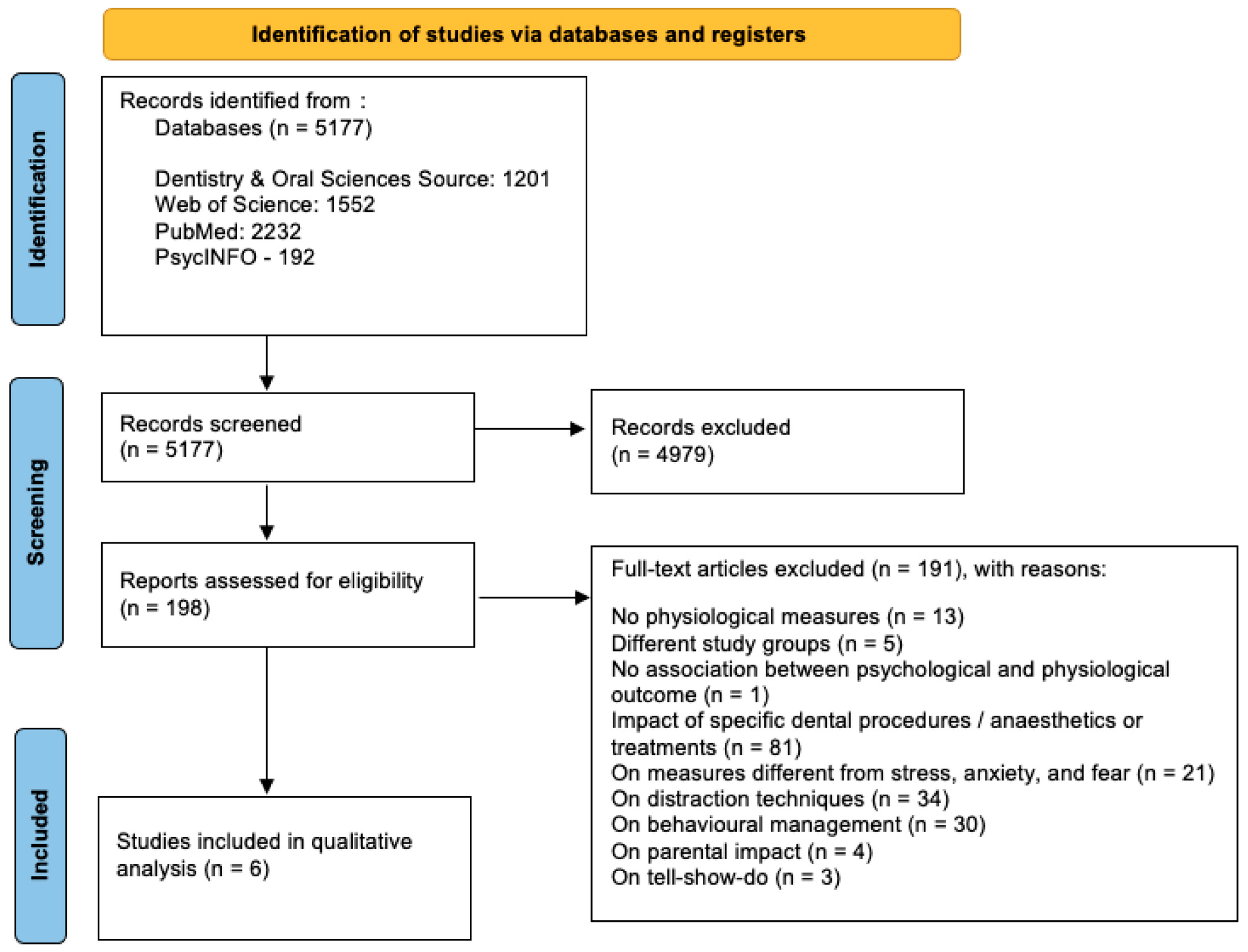
The study selection process is summarized in Figure 1.
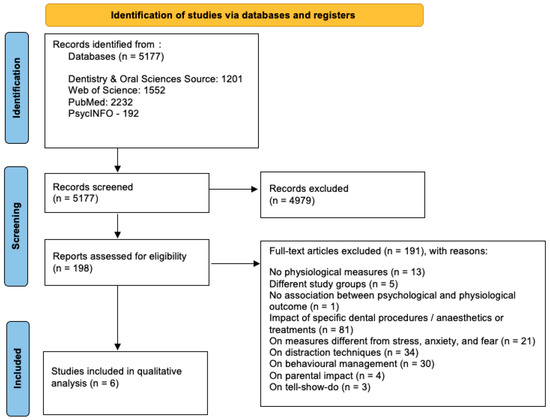
Figure 1.
Study selection process.
3.1. Psychological Measures of Dental Fear, Self-Perceived Stress, and Dental Anxiety
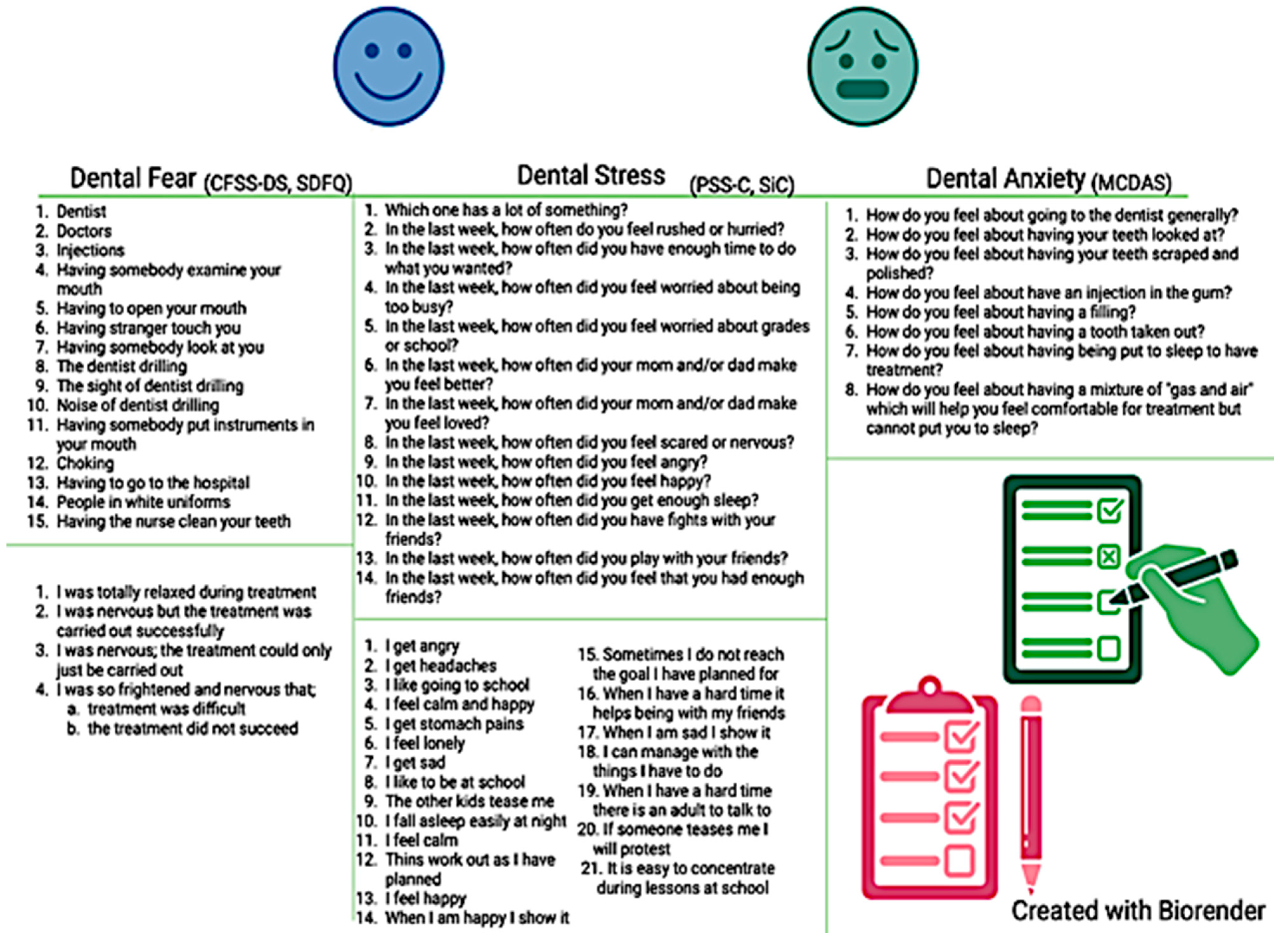
Questionnaires specifically validated for the pediatric population have been used by some clinicians to measure psychological functioning at baseline so that they can anticipate and better manage potential behaviors driven by psychological distress in their young patients [28]. In this instance, the dental care provider is required to invest more time explaining the purpose and the steps of each dental procedure and consequently address concerns of a fearful or anxious patient [14]. In the presence of moderate-to-severe psychological distress, the clinician could also personalize the procedure and adopt certain considerations to decrease dental anxiety. These strategies include the use of a calming voice, “tell–show–do” procedures, or distracting techniques (including music or movies during the dental treatment), among others [29]. Repeating the administration of the questionnaire at later appointments allows for monitoring longitudinal changes of psychological distress over time [30]. Some of the most utilized questionnaires to assess psychological functioning in children are presented in Figure 2. Three of the included studies utilized the scales illustrated in Figure 2 to assess self-perceived stress and dental anxiety; two studies utilized similar scales assessing dental anxiety, while one study used ad hoc questions.
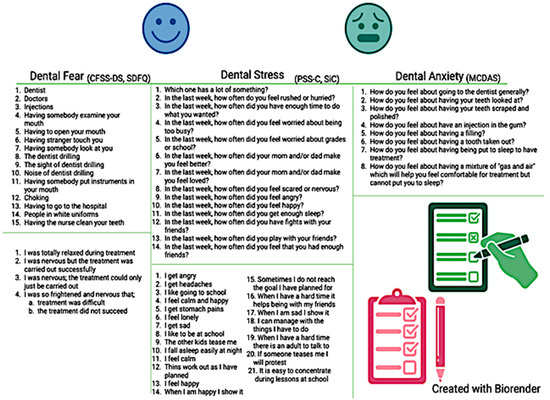
Figure 2.
Common questionnaires assessing psychological distress used in clinical pediatric research.
3.1.1. Assessment of Dental Fear
The most commonly used instrument to quantify dental fear in pediatric patients is the Children’s Fear Survey Schedule—Dental Subscale (CFSS-DS) [21]. The CFSS-DS consists of 15 items, each representing hypothetical scenarios occurring during a dental procedure, with Likert-like scale answers ranging from 1 = “Not afraid at all” to 5 = “Very much afraid” (Figure 2). The final score ranges between 15 and 75, with values ≥ 38 being consistent with dental fear [22]. Its use has been validated in children ages 7–12 [22]. Contradictory results emerge from the existing literature on whether dental fear may differ across age groups, with a study revealing no statistically significant difference between mean dental fear score across various age groups within adolescents [31]. Validity, time completion, and ease of use of the CFSS-DS make this questionnaire one of the most reliable. In relation to sex, one study showed significantly higher dental fear in girls compared to boys [32,33]. Another available instrument to measure dental fear is the Short Dental Fear Question (SDFQ) [34], which has been tested on teenagers, and consists of queries investigating how the dental procedure was completed [34]. The SDFQ is quick, easy to answer, and requires minimal advanced knowledge for interpretation [35].
3.1.2. Assessment of Self-Perceived Stress
Self-perceived stress has been commonly measured in the pediatric literature through several validated questionnaires. The most common is the Perceived Stress Scale for Children (PSS-C), a 13-item questionnaire with option choices ranging from 0 = “Never” to 3 = “A lot” (Figure 2). The final score ranges from 0 to 39, with higher values indicating greater symptomatology [23]. The items present different scenarios that could potentially evoke a state of mental stress. Specifically, items 2, 4, 5, 8, and 12 examine stressor sensitivity related to everyday scenarios; items 8, 9, 10, and 12 assess emotional state; items 6 and 7 investigate security; and items 2, 3, and 4 are considered time pressure questions. This questionnaire has been validated among various populations including dentists, healthcare students, and pediatric patients [23,36,37]. Another questionnaire developed and validated to assess self-reported stress is the Perceived Stress Scale for Kids (PeSSKi) [38], a 10-item assessment investigating feelings and thoughts a child remembers within the past month. Similarly, the 21-item Stress in Children (SiC) presents several factors regarding emotions and physical health (e.g., headaches, stomach pain) [39]. Another validated questionnaire indirectly assessing stress and mental well-being is the Child Perception Questionnaire (CPQ), which intrinsically examines the children’s quality of life and mental well-being [40].
3.1.3. Assessment of Dental Anxiety
Several measures have been validated in clinical research to assess dental anxiety. One of the most utilized assessment tools is the five-item Dental Anxiety Scale (DAS), which has been modified over time to the Modified Child Dental Anxiety Scale (MCDAS, Figure 2) [41]. Found to be most valid in children ages 8–12, this questionnaire consists of eight items, characterized by five-point Likert scale answers and anchors ranging from 1 = “Relaxed/not worried” to 5 = “Very worried” [41]. A final score is obtained by summing all item scores, and it ranges from 8 to 40. A cut-off of 19 has been established to detect the presence of anxiety symptomatology, with categories of 8–10 = no anxiety, 19–30 = presence of anxiety, and 31–40 = severe anxiety. Dental anxiety has been positively correlated with dental fear in a study conducted on children 8–12, regardless of the type of questionnaire utilized [41].
3.1.4. Advantages and Disadvantages of Psychological Assessment in Children
The use of validated questionnaires to assess and quantify psychological distress in children has been shown to provide additional means to assess children’s cognitive perspective with good reliability [42]. For instance, they allow for the assessment of the presence and degree of severity of psychological distress in a cost-effective manner. However, while useful, validated questionnaires are insufficient for establishing a conclusive diagnosis. A proper diagnosis requires meeting criteria as established by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) or the International Classification of Disease (ICD-10) [43], which is normally provided by a licensed psychologist, social worker, or psychiatrist. Moreover, these questionnaires rely solely on subjective self-reported data. Another potential issue is the reliability of the answers of the pediatric patients, who could potentially provide false responses to avoid showing signs of fear or based on social desirability. Given these limitations, it is likely that using objective measures of psychological distress in conjunction with self-reported questionnaires could greatly enhance the identification, quantification, and monitoring of symptoms in a reliable manner, to ultimately improve treatment care.
3.2. Psychological Measures Through Salivary Collection
3.2.1. Salivary Diagnostics
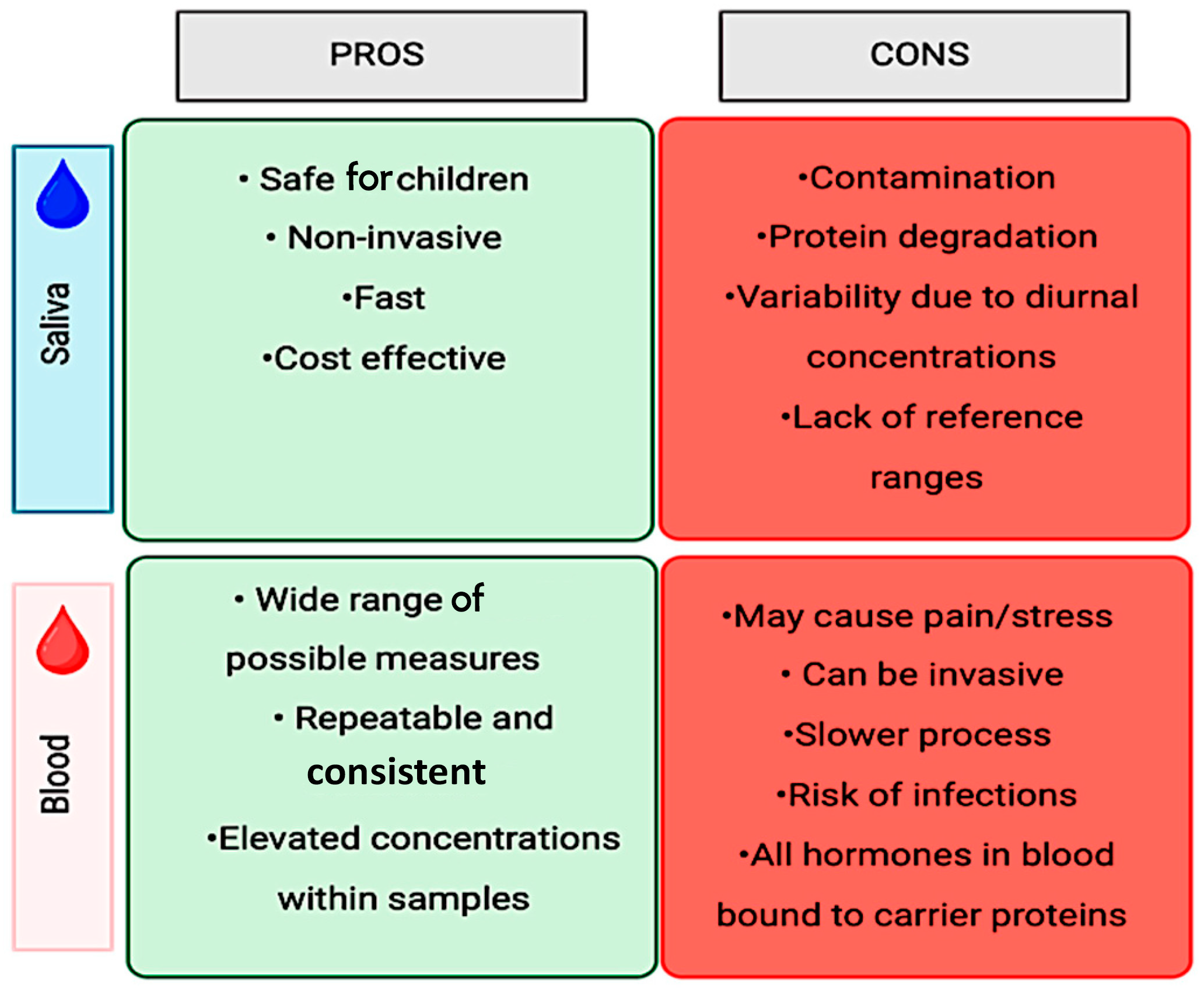
The most common methods proposed to objectively measure stress involve the collection of stress markers from bodily fluids, such as blood and saliva. Traditionally, clinical and experimental studies have utilized blood tests as a standard method of collection to identify and measure markers, make diagnoses, and monitor the progression of the condition. However, blood collection is not only an invasive method, but, especially in young patients, it is perceived as causing more discomfort and pain [44], in addition to being associated with a greater risk of possible infections depending on the volume extracted [44] and being potentially stressful [45]. Thus, the use of saliva as a diagnostic method to quantify molecules associated with stress offers a substantial advantage for the advancement of this field of research. Salivary diagnostics has proven to be a valid alternative for diagnosing and monitoring disease progression [46]. Among other benefits, salivary diagnostics is considered to be a minimally invasive, cost-effective, and less uncomfortable approach compared to blood diagnostics, especially for the pediatric population. The advantages and disadvantages of both quantification methods are summarized in Figure 3.
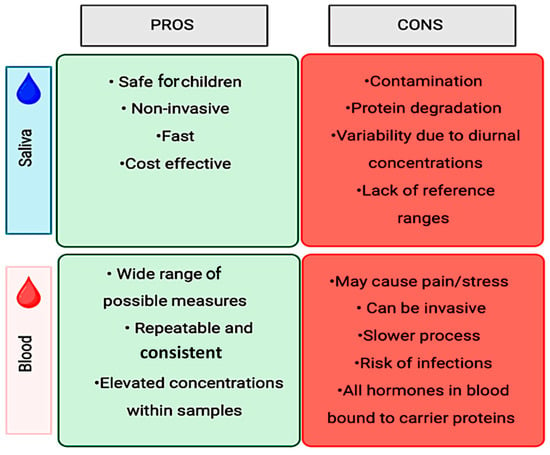
Figure 3.
Salivary vs. blood sample collection.
Salivary diagnostics consists of the collection of either the whole or salivary-gland-specific saliva and the quantification of the molecules within this biological fluid. The salivary glands are divided between the major and minor glands, which all perform the function of producing and secreting saliva into the oral cavity [47]. The three pairs of major salivary glands, from largest to smallest, are the parotid, submandibular, and sublingual. They are responsible for secreting the greatest volume of salivary fluid (~90%), which differs substantially between them in terms of composition and fluidity [47]. The different types of acinar cells in each major salivary gland result in different types of saliva production. While the parotid gland has serous acini and produces a watery serous saliva, the submandibular and sublingual glands are mixed glands, containing mucous and serous acini in different proportions. Consequently, since most of the submandibular acinar cells are serous, they produce more fluid saliva in contrast to the sublingual glands, which are composed primarily of mucous acinar cells [47,48,49]. Due to their proximity to blood vessels, salivary glands are a rich source of metabolite exchange between the oral cavity and the circulatory system [49]. In fact, many proteins found in human serum can also be detected in saliva, supporting its use as a proxy for the measurement of circulating biomarkers related to diseases [50].
Under normal physiological conditions, children produce on average 1.5 L of saliva per day [51]. The saliva can be secreted under unstimulated and stimulated conditions, where unstimulated salivary flow will result from salivary gland secretions in a resting state (~66% of the whole saliva) [51], while stimulated saliva (the remaining ~33%) is obtained by activating the entire multitude of salivary glands, major and minor, through the use of chemical substances or mechanical stimuli (e.g., chewing). Normal levels of stimulated and unstimulated saliva are shown in Table 1. Saliva production is susceptible to overproduction, a condition known as sialorrhea particularly seen in children [52]. The largest heterogeneity among saliva in individuals is the result of the sympathetic and parasympathetic effects of the salivary gland from diseases, medications, and pharmacological interventions, which can significantly influence the secretion of saliva volume and additionally lead to alterations in fluid composition [49,53].

Table 1.
Reference ranges of salivary flow/salivary concentration in children.
Table 1.
Reference ranges of salivary flow/salivary concentration in children.
| Measures | Unstimulated Whole Saliva | Stimulated Whole Saliva | Stimulated Parotid Saliva |
|---|---|---|---|
| Normal Reference Range in Saliva | 0.08–3.30 mL/min [54] | 0.25–5.58 mL/min [54] | 0.04–0.69 mL/min [54] |
| Reference Range under Psychological/ Physiological Stress | INCREASE [55] Range of values differs upon maximum volume of saliva in individuals. | INCREASE [56] Range of values differs upon maximum volume of saliva in individuals. | DECREASE [51] Range of value differs upon maximum volume of saliva in individuals. |
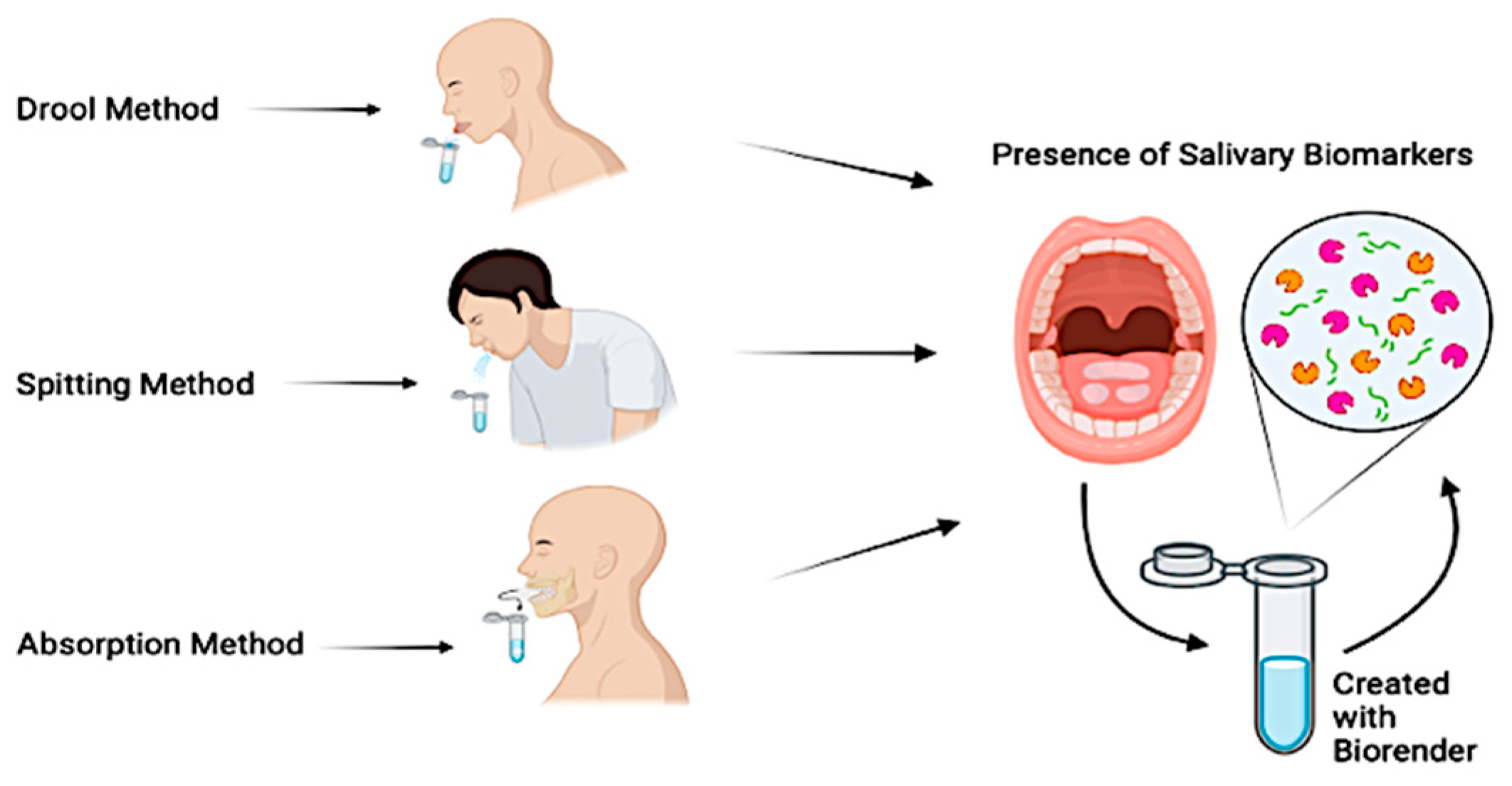
The most common saliva collection techniques consist of drooling, absorption, or spitting (Figure 4).
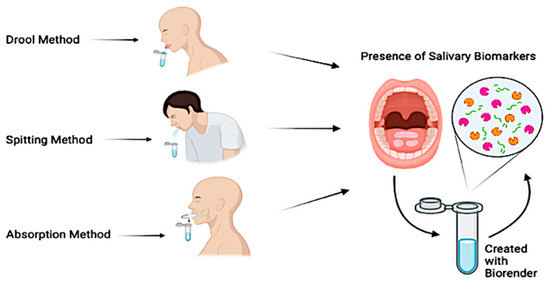
Figure 4.
Saliva collection techniques.
The drooling technique, while not commonly used in children, is considered the gold standard for unstimulated saliva collection [57]. In an upright position with the head tilted forward, the patient is instructed to collect the saliva and to empty it into a collection container. Given its ability to provide a high salivary flow rate with minimum effort required by the patient, it has consistently been selected as the primary collection method across several studies. The absorption technique collects both stimulated and unstimulated saliva and is often considered the method of choice within pediatric patients, thanks to the ease of collection and minimal time required. This technique consists of providing the patient with a dedicated tool that is often chewed on for a period from 60 s to 2 min, to allow for saliva absorption [58]. The spitting technique can be used both in children and adults and collects both stimulated and unstimulated saliva. This method consists of instructing the patient to spit every 30 s directly into a collection device below the chin, for a 2-to-5 min collection period [59]. Multiple studies have also proposed the combination of multiple collection techniques to either compare methods or assess the efficacy of stimulated vs. unstimulated saliva [60,61]. Each of these techniques offers advantages and disadvantages, while collecting different types of saliva. The researcher is ultimately expected to determine which technique is the most suitable for their patients and compare the results to established reference values [62,63] (Table 1).
3.2.2. Salivary Molecules
With the advance of salivary collection methods and analyses, clinicians and researchers can measure saliva volume and its biochemical composition [64]. This is particularly useful for those molecules that can be quantified within human body tissues and fluids in short response times (e.g., cortisol, alpha-amylase, immunoglobulins, C-reactive protein, and cytokines), all being produced across different physiological stages within the body. Notably, the concentration and volume of some of these molecules have been suggested to differ according to sex, age group, systemic medications and conditions, and diet [64]. Table 2 shows the normal values of the stress-related salivary molecules for pediatric populations and level changes according to the collection method used. The most common molecules quantifiable through salivary samples and related to stress in the existing literature are cortisol and alpha-amylase, which have been frequently assessed in children [59,65,66,67,68,69].
Salivary cortisol: Salivary cortisol is a well-known and reliable molecule released as a result of hypothalamic activation [70]. This hormone is produced within the adrenal cortex and can be measured both within the plasma and saliva. Cortisol is responsible for many human functions, including restoring the body’s homeostasis and regulating blood pressure and circadian rhythm, along with many others [71]. The highest levels of cortisol are present early in the morning, which then progressively decrease throughout the day. As cortisol levels are influenced by the circadian rhythm cycle, its collection needs to be performed at a consistent time during the day, to allow for within- and between-subject comparison [71]. Sex is also known to influence reference ranges of cortisol, although the literature has shown contradictory findings. For example, while older studies observed a 20% higher cortisol levels in girls between the ages of 8–16 years compared to age-matched boys [72], others have failed to reveal any significant differences across sexes [73]. Other studies showed a correlation between cortisol and social behaviors in male adolescents, but not in girls [74].
Alpha-amylase: Alpha-amylase (sAA) is a protein found in saliva whose levels are oppositely correlated to salivary cortisol [75]. The normal expected values of salivary alpha-amylase are suggested to progressively increase throughout the day [26]. Its function consists of hydrolysis and carbohydrate breakdown, secondary to caloric intake [25]. It is mainly produced and excreted by the pancreas and salivary glands [26]. In recent years, salivary alpha-amylase has been extensively studied and has been suggested as an effective biomarker of dental fear [76] and dental anxiety [77]; persistently inconclusive findings relate sAA with pain intensity [68,78,79].
Immunoglobulins: Among the primary immunoglobulins (IgA, IgG, IgM) found in saliva, IgA is the class with the highest concentration in saliva (sIgA). According to Alaki et al., sIgA can be used as a biomarker of stress in individuals [69]. Nevertheless, other studies showed that perceived stress and anxiety were correlated with low salivary IgA, which further decreased during the body’s response to stress [80].
Less common stress-related salivary molecules: There are few other stress-related salivary molecules that have been less commonly studied in children. For example, C-reactive protein (CRP) is an acute non-specific immune inflammatory biomarker that is commonly measured in saliva. The total protein concentration is affected by both CRP and immunoglobulin concentrations, specifically IgA [69]. Levels of CRP have been correlated with cardiovascular diseases; thus, quantification of CRP has also been utilized for diagnostic reasons and follow-ups [81]. Other alternative molecules are catecholamines, such as norepinephrine (NE) and epinephrine. Salivary NE levels were correlated with stress due to the sympathetic response caused by restorative dental procedures with local anesthesia [82]. Interestingly, NE returned to baseline levels after treatment. Conversely, salivary epinephrine levels were not correlated with dental anxiety [82].

Table 2.
Reference ranges of concentrations of salivary molecules in children.
Table 2.
Reference ranges of concentrations of salivary molecules in children.
| Salivary Molecule | Salivary Cortisol | Salivary Alpha-Amylase (sAA) | Salivary Immunoglobulin A (sIgA) | C-Reactive Protein (CRP) | Cytokines |
|---|---|---|---|---|---|
| Normal Reference Range in Saliva | AM: 3–19 mcg/dL PM: 1–11 mcg/dL | <10 mg/L [83] | 1–220 mg/dL [84] | 0.1 mg/L–6 mg/L (levels should not exceed 10 mg/L) [85] | IL-2: 11.91 ± 1.70 (pg/mL) IL-1β: 64.5 ± 89.6 (pg/mL) IL-6: 5.2 ± 2.8 (pg/mL) IL-8: 210.096 ± 142.302 (pg/mL) IL-10: 12.02 ± 7.23 (pg/mL) [86] |
| Reference Range under Psychological/ Physiological Stress | 159.83 ± 460.36 [87] | >10 mg/L [83] | Increase from existing studies. Pre-treatment = 1116.1–1121.7 µg/mL; Post treatment = 1200.6–1210.8 µg/mL [88] | Increase in the presence of psychological stressor [89] | Increase in the presence of psychological stressor [89] |
3.2.3. Advantages and Disadvantages of Salivary Stress-Related Physiological Measures
Salivary diagnostics and the quantification of pathogens and protein content can be utilized for the diagnosis and monitoring of the onset and progression of certain conditions [90]. Nevertheless, these findings should be used in conjunction with clinical considerations to achieve the most appropriate diagnosis.
While salivary diagnostics is a cost-effective, non-invasive procedure, especially for pediatric patients, the collection technique and timing, the protocol in place for storage, and the participant inclusion should be standardized and carefully planned, as they may contribute to errors and variations across study participants. For example, inconsistencies in results can arise from several factors, including the timing of salivary collection, as some of the salivary molecules fluctuate throughout the day due to circadian rhythms. Salivary samples need to be stored in ice soon after their collection, as the improper handling of samples can lead to variations in the salivary proteome [91]. Failure to use the recommended storing of samples based on the brand used can lead to an increased rate of degradation and bacterial growth [92].
3.3. Relationship Between Psychological and Physiological Distress in Pediatric Patients
While many studies in adults suggest that changes in the levels of salivary molecules may be related to stress and anxiety related to dental procedures [65,93,94,95], limited research has explored this correlation in children using validated questionnaires. The use of validated questionnaires is necessary to quantify measures of psychological distress with established validity and reliability.
Table 3 summarizes the correlation, or the lack thereof, between psychological and physiological stress-related outcomes in the pediatric literature.

Table 3.
Correlation between psychological outcomes and acute physiological stress in the included studies.
Most of the literature exploring the relationship between psychological and physiological distress in children has been published in the last decade [58,69,88,96,97,98]. All of these studies consistently used salivary cortisol as the primary physiological measure, to such an extent that cortisol can indeed be considered a reliable salivary biomarker. Salivary cortisol levels were found to be significantly and positively correlated with dental fear [96], stress [69], and anxiety symptoms [88,98]. Three studies found that the concentration of salivary cortisol was significantly greater in returning patients compared to new patients [69,88,97]. For example, Aksoy et al. investigated the salivary cortisol and anxiety levels in children starting an orthodontic treatment with multibracket fixed appliances. The highest level of cortisol was observed at the end of the second appointment compared to the first appointment, with values of 0.57 ± 0.07 and 0.59 ± 0.03 (according to the type of archwire utilized) vs. 0.39 ± 0.03 and 0.41 ± 0.04 at the beginning of the first appointment [97]. Similarly, the participants exhibited the highest levels of anxiety at the end of the second appointment, reflecting cortisol levels [97]. In another study, Dhinsa et al. found that cortisol levels measured at the end of the second appointment (0.32 ± 0.06) were significantly greater compared to those measured at the end of the first appointment (0.13 ± 0.02, p < 0.0001) [88]. Likewise, another study confirmed that, compared to new patients, returning patients exhibited 0.20% greater levels of salivary cortisol [69]. This potential difference between new and returning patients may not have been observed by those studies characterized by a cross-sectional design, thus not allowing for a longitudinal evaluation over different timepoints (e.g., [58,98]). Conflicting findings were found on the influence of sex on salivary cortisol levels, with Alaki et al. observing higher levels in male patients [69], while other studies supporting no significant difference between female and male patients [97,98]. Interestingly, Alaki et al. observed that the children whose saliva was collected by a male provider presented with greater stress levels (p = 0.05) than when a female provider conducted the procedure [69]. This factor is often normalized across the studies by using one experimenter, which certainly reduces confounding factors related to the provider, but could overlook some other important considerations such as the influence of the experimenter’s sex on the results. Within the adult population, increased levels of salivary cortisol have been associated with greater self-perceived stress and periodontitis severity. For example, a case–control study conducted on 120 adults revealed that those with periodontitis exhibited on average >50% higher salivary cortisol and stress levels compared to healthy controls (p < 0.001) [99], results which were confirmed by a meta-analysis of observational studies [100]. Conversely, Sadi et al. failed to reveal significant correlations between dental anxiety and salivary biomarkers, including salivary cortisol and sAA [77].
Four of the six studies also included sAA, with contradictory findings on its reliability as a stress- or fear-related salivary biomarker, and with differences across collection times. For example, overall sAA levels were found to be significantly and positively correlated with dental fear, especially in children undergoing dental procedures with local anesthetics [58]. Differences were found between the first and second appointments, in that sAA levels significantly correlated with dental fear at pre-intervention during the first appointment, while they correlated with dental fear at the second appointment only after the intervention [88]. Similar conclusions have been suggested by Alaki et al., who confirmed significantly higher levels of sAA in returning patients compared to new patients [69]. Moreover, in new patients, sAA was greater when measured in the dental chair (i.e., immediately prior to starting the intervention) compared to when it was measured in the waiting room [69]. This fluctuation may be attributed to expectations and prior dental experience. Levels of sAA were also found to positively correlate with dental anxiety [96]. In the adult literature, a study claimed that sAA levels were positively correlated with dental anxiety before the dental procedure, which decreased at post-treatment [101]. Conversely, some others failed to reveal any correlation between sAA levels and dental anxiety, although dental anxiety was positively correlated with intraoperative pain [102] and a history of traumatic events [77].
The overall literature in adults does not seem to attribute any influence of dental fear, dental anxiety, and stress on the change of sIgA. However, in studies conducted in the pediatric population, sIgA seemed to follow a trend similar to sAA [88]. For example, sIgA levels were observed to be higher in returning patients compared to new patients [69]. Moreover, sIgA was also proposed to be correlated with dental anxiety at pre-intervention during the first appointment, and at post-intervention during the second appointment [88].
Altogether, this suggests that past dental experience may be a contributing factor. Approximately 50% of the studies suggested that results fluctuate depending on the sex of the patients [58,69,88,96,97,98].
4. Discussion
The current study aimed to summarize psychological assessment tools available in the pediatric literature to evaluate the presence and intensity of perceived stress, anxiety, and fear related to dental procedures and to correlate these measures to acute physiological measures of stress. Despite contradictory evidence derived from studies conducted on adults, the available literature in pediatric patients showed a substantial agreement between measures of psychological and acute physiological distress.
The pediatric population is largely affected by fear and anxiety while facing dental procedures. These conditions play a major role in influencing poor oral health outcomes, as they lead to non-compliance, avoidance of dental care, and difficulties during procedures [103]. Moreover, social and ethnic minority groups, particularly low-income populations, often face greater barriers to preventive dental care, such as fewer visits and more missed appointments [104]. This impacts both overall health and economic productivity, with studies showing that poor oral health is correlated with lost workdays.
As seen throughout this review of the literature, there is an increasing interest in the scientific community in identifying useful stress-related molecules that could be utilized as stress biomarkers to assess dental anxiety, dental fear, and stress in pediatric patients. In this review, we have summarized existing physiological and psychological instrument tools available in the literature. Further research, using combinations of methods, is expected to continue providing evidence on the correlation of these assessment tools.
Overall, our findings suggested a relative concordance between psychological measures assessed through validated questionnaires and levels of acute physiological stress-related molecules. There was a consistent positive relationship between dental anxiety and dental fear and levels of salivary cortisol [58,88,96,98], which confirms salivary cortisol as a valuable biomarker of psychological distress in pediatric dental patients. Interestingly, differences were seen between new patients and returning patients. For example, the fact that returning patients may experience higher levels of cortisol, sIgA, and sAA (and, thus, of fear and anxiety) suggests that the same approaches adopted by the pediatric dentist during the first visit to create a comfortable and friendly environment may need to be replicated for returning patients. SAA was also consistently related to increased psychological distress, adding this as a reliable physiological marker of stress.
The findings have significant implications for pediatric dentistry, by highlighting the importance of managing dental anxiety and fear to improve overall patient outcomes and by contributing to the development and exploration of non-invasive diagnostic tools and targeted interventions to enhance patient comfort and cooperation during dental treatments. There are many preventive measures that could be included in all appointments, known to lessen children’s fear during their dental visits. These include scheduling dental appointments at a time that best accommodates the child, as well as increasing the frequency of visits for children to become more familiar with the environment [14] and enhancing the patient’s involvement and interactive approaches (i.e., tell–show–do) to increase the child’s sense of comfort [39].
Nevertheless, saliva collection and the detection of salivary molecules within a dental office would additionally require advanced technology [105] and access to laboratory spaces meeting biosafety requirements. While these analyses may be possible at university-affiliated dental clinics with interdisciplinary departments, a routine assessment of salivary biomarkers may not be a feasible option in private clinical practices. Yet, confirmation of self-reported psychological outcomes with physiological measurements could potentially help tailor the way dental procedures are completed [106], contributing to providing more comprehensive care and procedural explanation to children who present with fear. Additionally, clinicians could help parents develop routine dental habits at home based on their children’s behaviors and reactions in the office, which could likely promote positive responses of those children presenting with psychological distress [107].
Future studies should explore other physiological measurements of distress, such as heart rate variability, and investigate their potential associations with psychological outcomes. Additionally, biomarkers of pain, such as calcitonin gene-related peptide (CGRP) or substance P, could be measured to better understand the relationship between physiological stress responses and pain perception. Future research should also consider differences between returning and new patients, examining how past traumatic experiences or lack of prior dental treatment influence physiological and psychological responses to dental care.
Limitations
Despite the authors’ efforts to thoroughly review the topic, this study should be regarded as a literature review and not a systematic review, which may limit the generalizability of its findings. Thus, potential limitations include the selection bias of the included studies, a lack of standardized methodology across sources, and the possibility of missing relevant literature.
5. Conclusions
Assessment of psychological distress with validated questionnaires and quantification of physiological molecule levels appears to be relatively consistent and positively correlated in the pediatric literature. Given the several contributing factors that can enhance dental fear, dental anxiety, and stress in the pediatric population, understanding the influence of such factors during dental procedures will ultimately enable the provider to enhance the quality of care and the well-being of the pediatric patients.
Author Contributions
Conceptualization, L.S., C.S., A.A.-B. and M.R.C.; methodology, S.M. and L.S.; investigation, S.M.; data curation, S.M. and L.S.; writing—original draft preparation, S.M.; writing—review and editing, S.M., L.S., M.R.C., J.R., S.H. and C.S.; supervision, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by both the College of Dental Medicine and the Biomedical Sciences Program at Midwestern University, Downers Grove campus.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created. All of the data summarized in this review are contained within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reich, S.M.; Hoeft, K.S. Disparities in the Quality of Pediatric Dental Care: New Research and Needed Changes. Soc. Polic. Rep. 2018, 31, 1–27. [Google Scholar] [CrossRef]
- Shim, Y.S.; Kim, A.H. Dental fear & anxiety and dental pain in children and adolescents; a systemic review. J. Dent. Anesth. Pain. Med. 2015, 15, 53–61. [Google Scholar] [PubMed]
- Armfield, J.M.; Stewart, J.F. The vicious cycle of dental fear: Exploring the interplay between oral health, service utilization and dental fear. BMC Oral. Health 2007, 7, 1. [Google Scholar] [CrossRef]
- Klinberg, G. Dental anxiety and behaviour management problems in paediatric dentistry--a review of background factors and diagnostics. Eur. Arch. Paediatr. Dent. 2008, 9, 11–15. [Google Scholar] [CrossRef]
- Mohammed, R.B.; Lalithamma, T. Prevalence of dental anxiety and its relation to age and gender in coastal Andhra (Visakhapatnam) population, India. J. Nat. Sci. Biol. Med. 2014, 5, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Chellappah, N.; Vignehsa, H. Prevalence of dental anxiety and fear in children in Singapore. Community Dent. Oral. Epidemiol. 1990, 18, 269–271. [Google Scholar] [CrossRef]
- Kakkar, M.; Wahi, A. Prevalence of dental anxiety in 10–14 years old children and its implications. J. Dent. Anesth. Pain. Med. 2016, 16, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Dadalti, M.T.; Cunha, A.J. Anxiety about dental treatment—A gender issue. Acta Odontol. Latinoam. 2021, 34, 195–200. [Google Scholar] [CrossRef]
- Gatchel, R.; Ingersoll, B.D. The prevalence of dental fear and avoidance: A recent survey study. J. Am. Dent. Assoc. 1983, 107, 609–610. [Google Scholar] [CrossRef]
- Hakeberg, M.; Berggren, U. Prevalence of dental anxiety in an adult population in a major urban area in Sweden. Community Dent. Oral. Epidemiol. 1992, 20, 97–101. [Google Scholar] [CrossRef]
- Porritt, J.; Buchanan, H. Assessing children’s dental anxiety: A systematic review of current measures. Community Dent. Oral. Epidemiol. 2013, 41, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Locker, D.; Liddell, A.; Dempster, L.; Shapiro, D. Age of Onset of Dental Anxiety. J. Dent. Res. 1999, 78, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, J.M.; Berg, R.G. The relationship of age and gender to fear and anxiety in response to dental care. Spec. Care Dentist. 1997, 17, 82–87. [Google Scholar] [CrossRef]
- Carrillo-Díaz, M.; Migueláñez-Medrán, B.C. How can we reduce dental fear in children? The importance of the first dental visit. Children 2021, 8, 1167. [Google Scholar] [CrossRef]
- Berggren, U.; Meynert, G. Dental fear and avoidance: Causes, symptoms, and consequences. J. Am. Dent. Assoc. 1984, 109, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.; Macpherson, L. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Flores, G.; Lin, H. Trends in racial/ethnic disparities in medical and oral health, access to care, and use of services in US children: Has anything changed over the years? Int. J. Equity Health 2013, 12, 10. [Google Scholar] [CrossRef]
- Mouradian, W.; Wehr, E. Disparities in children’s oral health and access to dental care. JAMA 2000, 284, 2625–2631. [Google Scholar] [CrossRef]
- Huh, H.J.; Kim, K.H. Perceived Stress, Positive Resources and Their Interactions as Possible Related Factors for Depressive Symptoms. Psychiatry Investig. 2021, 18, 59–68. [Google Scholar] [CrossRef]
- Basudan, S.; Binanzan, N. Depression, anxiety and stress in dental students. Int. J. Méd. Educ. 2017, 8, 179–186. [Google Scholar] [CrossRef]
- Beena, J.P. Dental subscale of children’s fear survey schedule and dental caries prevalence—PMC. Eur. J. Dent. 2013, 7, 181–185. [Google Scholar]
- Leko, J.; Škrinjarić, T. Reliability and validity of scales for assessing child dental fear and anxiety. Acta Stomatol. Croat. 2020, 54, 22–31. [Google Scholar] [CrossRef]
- Meng, R.; Li, J. The Chinese version of the Perceived Stress Questionnaire: Development and validation amongst medical students and workers. Health Qual. Life Outcomes 2020, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, S.; McGrath, J.J. Inter-relation between autonomic and HPA axis activity in children and adolescents. Biol. Psychol. 2016, 117, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Nater, U.M. Salivary alpha-amylase as a biomarker of stress in behavioral medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef]
- Blomqvist, M.; Holmberg, K. Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur. J. Oral. Sci. 2007, 115, 1–6. [Google Scholar] [CrossRef]
- Yfanti, K.; Kitraki, E. Psychometric and biohormonal indices of dental anxiety in children. A Prospect. Cohort Study. Stress 2014, 17, 296–304. [Google Scholar]
- Anthonappa, R.P.; Ashley, P.F. Non-pharmacological interventions for managing dental anxiety in children. Cochrane Database Syst. Rev. 2017, 6, CD012676. [Google Scholar] [CrossRef]
- Cohen, S.M.; Fiske, J. The impact of dental anxiety on daily living. Br. Dent. J. 2000, 189, 385–390. [Google Scholar] [CrossRef]
- Singh, A.; Palshikar, A. Prevalence of dental fear in children of 3–14 years visiting the OPD in Dental College, Lucknow, India. MGM J. Méd. Sci. 2021, 8, 15. [Google Scholar] [CrossRef]
- Slabšinskienė, E.; Kavaliauskienė, A. Dental Fear and Associated Factors among Children and Adolescents: A School-Based Study in Lithuania. Int. J. Environ. Res. Public. Health 2021, 18, 8883. [Google Scholar] [CrossRef]
- Helkimo, A.N.; Rolander, B. Dental fear in school children and young adults attending public dental health care: Prevalence and relationship to gender, oral disease and dental treatment; trends over 40 years. BMC Oral. Health 2022, 22, 146. [Google Scholar]
- Alshoraim, M.; El-Housseiny, A. Effects of child characteristics and dental history on dental fear: Cross-sectional study. BMC Oral. Health 2018, 18, 33. [Google Scholar] [CrossRef]
- Jaakkola, S.; Rautava, P. Dental Fear: One Single Clinical Question for Measurement. Open Dent. J. 2009, 3, 161–166. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, H. Validation of the Chinese version of the Perceived Stress Scale-10 integrating exploratory graph analysis and confirmatory factor analysis. Gen. Hosp. Psychiatry 2023, 84, 194–202. [Google Scholar] [CrossRef] [PubMed]
- White, B.P. The perceived stress scale for children: A pilot study in a sample of 153 children. Int. J. Pediatr. Child. Health 2014, 2, 45–52. [Google Scholar] [CrossRef]
- Kechter, A.; Black, D.S. Factors in the perceived stress scale differentially associate with mindfulness disposition and executive function among early adolescents. J. Child. Fam. Stud. 2019, 28, 814–821. [Google Scholar] [CrossRef]
- Frauches, M.; Monteiro, L. Association between children’s perceptions of the dentist and dental treatment and their oral health-related quality of life. Eur. Arch. Paediatr. Dent. 2018, 19, 321–329. [Google Scholar] [CrossRef]
- Barbosa, T.S.; Tureli, M.C.M. Validity and reliability of the Child Perceptions Questionnaires applied in Brazilian children. BMC Oral. Health 2009, 9, 13. [Google Scholar] [CrossRef]
- Howard, K.E.; Freeman, R. Reliability and validity of a faces version of the Modified Child Dental Anxiety Scale. Int. J. Clin. Pediatr. Dent. 2007, 17, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Yon, M.J.Y.; Chen, K.J. An Introduction to Assessing Dental Fear and Anxiety in Children. Healthcare 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Sabuncuoglu, O.; Taser, H. Relationship between traumatic dental injuries and attention-deficit/hyperactivity disorder in children and adolescents: Proposal of an explanatory model. Dent. Traumatol. 2005, 21, 249–253. [Google Scholar] [CrossRef]
- Hofman, L.F. Human saliva as a diagnostic specimen. J. Nutr. 2001, 131, 1621S–1625S. [Google Scholar] [CrossRef]
- Chojnowska, S.; Ptaszynska-Sarosiek, I. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef]
- Wong, D.T.W. Salivaomics. J. Am. Dent. Assoc. 2012, 143, 19S–24S. [Google Scholar] [CrossRef]
- Ghannam, M.G.; Singh, P. Anatomy, Head and Neck, Salivary Glands. [Updated 2023 May 29]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538325/ (accessed on 28 February 2025).
- Holmberg, K.V.; Hoffman, M.P. Anatomy, biogenesis, and regeneration of salivary glands. Monogr. Oral. Sci. 2014, 24, 1–13. [Google Scholar] [PubMed]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Yan, W.; Apweiler, R. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin. Appl. 2009, 3, 116–134. [Google Scholar] [CrossRef]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. life 2009, 2, 303–307. [Google Scholar]
- Leung, A.K.; Kao, C.P. Drooling in children. Paediatr. Child. Health 1999, 4, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.W. The salivary glands. Embryology, anatomy, and surgical applications. Surg. Clin. N. Am. 2000, 80, 261–273. [Google Scholar]
- Percival, R.S.; Challacombe, S.J. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J. Dent. Res. 1994, 73, 1416–1420. [Google Scholar] [CrossRef]
- Martínez-Borrás, R.; Navarrete, J. Changes in Salivary Immunoglobulin A, Stress, and Burnout in a Workplace Mindfulness Intervention: A Pilot Study. Int. J. Environ. Res. Public. Health 2022, 19, 6226. [Google Scholar] [CrossRef]
- Mizuhashi, F.; Koide, K. Salivary secretion and salivary stress hormone level changes induced by tongue rotation exercise. J. Adv. Prosthodont. 2020, 12, 204–209. [Google Scholar] [CrossRef]
- Williamson, S.; Munro, C. Comparison of biomarkers in blood and saliva in healthy adults. Nurs. Res. Pr. 2012, 2012, 246178. [Google Scholar] [CrossRef] [PubMed]
- Noorani, H.; Joshi, H.V. Salivary alpha amylase as a noninvasive biomarker for dental fear and its correlation with behavior of children during dental treatment. Int. J. Clin. Pediatr. Dent. 2014, 7, 18–22. [Google Scholar] [CrossRef]
- Fey, J.M.H.; Bikker, F.J. Saliva collection methods among children and adolescents: A scoping review. Mol. Diagn. Ther. 2024, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.F.; Salgado, P.A. Saliva sampling methods. Cariogenic streptococci count using two different methods of saliva collection in children. AOL 2022, 35, 51–57. [Google Scholar] [CrossRef]
- Hiremath, G.; Olive, A. Comparing methods to collect saliva from children to analyze cytokines related to allergic inflammation. Ann. Allergy Asthma Immunol. 2015, 114, 63–64. [Google Scholar] [CrossRef]
- Neyraud, E.; Bult, J.H.F. Continuous analysis of parotid saliva during resting and short-duration simulated chewing. Arch. Oral. Biol. 2009, 54, 449–456. [Google Scholar] [CrossRef]
- Acquier, A.B.; Pita, A.K.D.C. Comparison of salivary levels of mucin and amylase and their relation with clinical parameters obtained from patients with aggressive and chronic periodontal disease. J. Appl. Oral. Sci. 2015, 23, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Kim, H.R. Compliance with Saliva Collection Protocol in Healthy Volunteers: Strategies for Managing Risk and Errors. Int. J. Méd. Sci. 2018, 15, 823–831. [Google Scholar] [CrossRef]
- Chaturvedi, Y.; Chaturvedy, S. Salivary cortisol and alpha-amylase—Biomarkers of stress in children undergoing extraction: An in vivo study. Int. J. Clin. Pediatr. Dent. 2018, 11, 214–218. [Google Scholar] [PubMed]
- Padmanabhan, V.; Islam, M.S.; Habib, M.; Abdulaziz, Z.; Goud, M.; Chaitanya, N.C.; Haridas, S.; Rahman, M.M. Association between salivary cortisol levels, dental anxiety, and dental caries in children: A cross-sectional study. Dent. J. 2023, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Špiljak, B.; Vilibić, M. A Review of Psychological Stress among Students and Its Assessment Using Salivary Biomarkers. Behav. Sci. 2022, 12, 400. [Google Scholar] [CrossRef]
- Brummelte, S.; Chau, C.M.Y. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology 2015, 51, 151–163. [Google Scholar] [CrossRef]
- Alaki, S.; Safi, A. Comparing dental stress in new child patients and returning patients using salivary cortisol, immunoglobulin-A and alpha- amylase. J. Clin. Pediatr. Dent. 2017, 41, 462–466. [Google Scholar] [CrossRef]
- Kalman, D.S.; Feldman, S. Effect of a proprietary Magnolia and Phellodendron extract on stress levels in healthy women: A pilot, double-blind, placebo-controlled clinical trial. Nutr. J. 2008, 7, 7–11. [Google Scholar] [CrossRef]
- Törnhage, C.J. Reference values for morning salivary cortisol concentrations in healthy school-aged children. J. Pediatr. Endocrinol. Metab. 2002, 15, 197–204. [Google Scholar] [CrossRef]
- Netherton, C.; Goodyer, I. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology 2004, 29, 125–140. [Google Scholar] [CrossRef]
- Groschl, M.; Rauh, M.; Dorr, H. Circadian Rhythm of Salivary Cortisol 17a-Hydroxy-progesterone and Progesterone in Healthy Children. Clin. Chem. 2003, 49, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Tout, K.; Haan, M.d. Social behavior correlates of cortisol activity in child care: Gender differences and time-of-day effects. Child. Dev. 1998, 69, 1247–1262. [Google Scholar] [PubMed]
- Petersen, K.E. ACTH in normal children and children with pituitary and adrenal diseases. II. Plasma ACTH (cortisol and growth hormone) values during insulin hypoglycaemia--patients with idiopathic hypopituitarism and intracranial tumour. Acta Paediatr. Scand. 1984, 73, 372–378. [Google Scholar] [CrossRef]
- Koh, D.; Ng, V. Alpha amylase as a salivary biomarker of acute stress of venepuncture from periodic medical examinations. Front. Public. Health 2014, 2, 121. [Google Scholar] [CrossRef]
- Sadi, H.; Finkelman, M. Salivary cortisol, salivary alpha amylase, and the dental anxiety scale. Anesthesia Prog. 2013, 60, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Christidis, N.; Baghernejad, P. Salivary alpha-amylase in experimentally-induced muscle pain. Diagnostics 2020, 10, 722. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Duran, P. Green light exposure in children with autism spectrum disorder: A pilot study. J. Clin. Pediatr. Dent. 2024, 48, 99. [Google Scholar]
- Engeland, C.G.; Hugo, F.N. Psychological distress and salivary secretory immunity. Brain, Behav. Immun. 2016, 52, 11–27. [Google Scholar]
- Nam, K.; Jeon, Y. Intraoperative transfusion and an increased preoperative C-reactive protein level are associated with higher mortality after off-pump coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2020, 159, 558–565. [Google Scholar] [CrossRef]
- Mitome, M.; Shirakawa, T. Salivary catecholamine assay for assessing anxiety in pediatric dental patients. J. Clin. Pediatr. Dent. 1997, 21, 255–259. [Google Scholar]
- Arhakis, A.; Karagiannis, V. Salivary Alpha-Amylase Activity and Salivary Flow Rate in Young Adults. Open Dent. 2013, 7, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kardar, G.; Oraei, M. Reference Intervals for Serum Immunoglobulins IgG, IgA, IgM and Complements C3 and C4 in Iranian Healthy Children. Iran. J. Public Health 2011, 41, 59–63. [Google Scholar]
- Fuligni, A.J.; Telzer, E.H. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosom. Med. 2009, 71, 329–333. [Google Scholar] [CrossRef]
- Riderknecht, C.; Filippi, C. Associations between salivary cytokines and oral health, age, and sex in healthy children. Sci. Rep. 2022, 12, 15991. [Google Scholar]
- Cay, M.; Ucar, C. Effect of increase in cortisol level due to stress in healthy young individuals on dynamic and static balance scores. North. Clin. Istanb. 2018, 5, 295–301. [Google Scholar] [PubMed]
- Dhinsa, G.; Singh, G. Assessment and comparison of dental anxiety by measuring physiological, psychological and immune responses in pediatric patients undergoing noninvasive dental treatment. J. S. Asian Assoc. Pediatr. Dent. 2019, 2, 14–21. [Google Scholar]
- Parent, C.; Pokhvisneva, I. Salivary cytokine cluster moderates the association between caregivers perceived stress and emotional functioning in youth. Brain Behav. Immun. 2021, 94, 125–137. [Google Scholar] [CrossRef]
- Paqué, P.N.; Herz, C. Salivary biomarkers for dental caries detection and personalized monitoring. J. Pers. Med. 2021, 11, 235. [Google Scholar] [CrossRef]
- Thomadaki, K.; Helmerhorst, E.J. Whole-saliva proteolysis and its impact on salivary diagnostics. J. Dent. Res. 2011, 90, 1325–1330. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Caruso, S.; Gatto, R. Association between salivary cortisol level and caries in early childhood. Eur. J. Paediatr. Dent. 2018, 19, 10–15. [Google Scholar]
- Khanduri, N.; Singhal, N. The prevalence of dental anxiety and fear among 4-13-year-old nepalese children. J. Indian Soc. Pedod. Prev. Dent. 2019, 37, 345–349. [Google Scholar] [CrossRef]
- Souza, P.M.; Magalhães, P.d.O. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- AlMaummar, M.; AlThabit, H. The impact of dental treatment and age on salivary cortisol and alpha-amylase levels of patients with varying degrees of dental anxiety. BMC Oral. Health 2019, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Cesur, M. Assessment of pain, anxiety, and cortisol levels during the initial aligning phase of fixed orthodontic treatment. Turk. J. Orthod. 2019, 32, 34–40. [Google Scholar] [CrossRef]
- Vlad, R.; Pop, A.M. The evaluation of dental anxiety in primary school children: A cross-sectional study from Romania. Children 2020, 7, 158. [Google Scholar] [CrossRef]
- Hingorjo, M.; Owais, M. The impact of psychological stress on salivary cortisol levels in periodontitis patients: A case-control study. BMC Oral. Health 2025, 25, 276. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V. Stress, salivary cortisol and periodontitis: A systematic review and meta-analysis of observational studies. Arch. Oral. Biol. 2018, 96, 58–65. [Google Scholar] [CrossRef]
- Jafari, A.; Pouramir, M. Evaluation of Salivary Alpha Amylase as a Biomarker for Dental Anxiety. Iran. J. Psychiatry Behav. Sci. 2018, 12, e9350. [Google Scholar] [CrossRef]
- Lee, K.; Bassiur, J. Salivary Alpha Amylase, Dental Anxiety, and Extraction Pain: A Pilot Study. Anesth. Prog. 2017, 64, 22–28. [Google Scholar] [CrossRef]
- Gragoll, I.; Schumann, L. Healthcare avoidance: A qualitative study of dental care avoidance in Germany in terms of emergent behaviours and characteristics. BMC Oral. Health 2021, 21, 563. [Google Scholar] [CrossRef] [PubMed]
- Northridge, M.E.; Kumar, A. Disparities in Access to Oral Health Care. Annu. Rev. Public. Health 2020, 41, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, D.; Johannsen, B. A chair-side compatible molecular platform using whole saliva for monitoring oral health at the dental practice. Biosensors 2021, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Ge, C.L.H. Factors associated with dental behaviour management problems in children aged 2–8 years in Beijing, China. Int. J. Paediatr. Dent. 2011, 21, 200–209. [Google Scholar] [CrossRef]
- Pop-Jordanova, N.; Sarakinova, O. Anxiety, stress and coping patterns in children in dental settings. J. Méd. Sci. 2018, 6, 692–697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).