Advancing Pediatric Surgery with Indocyanine Green (ICG) Fluorescence Imaging: A Comprehensive Review
Abstract
1. Introduction
2. Methods
Search Strategy
- Studies published between January 2014 and July 2024;
- Papers written in English;
- Studies involving pediatric patients (aged 0–18 years);
- Studies describing the use of indocyanine green (ICG) fluorescence imaging in pediatric surgical procedures.
- Non-clinical studies (animal models, in vitro research);
- Studies outside the field of pediatric surgery, including those focused exclusively on cardiac or neurosurgical procedures;
- Non-peer-reviewed publications (conference abstracts, editorials, letters);
- Studies lacking relevant data on ICG administration or surgical applications.
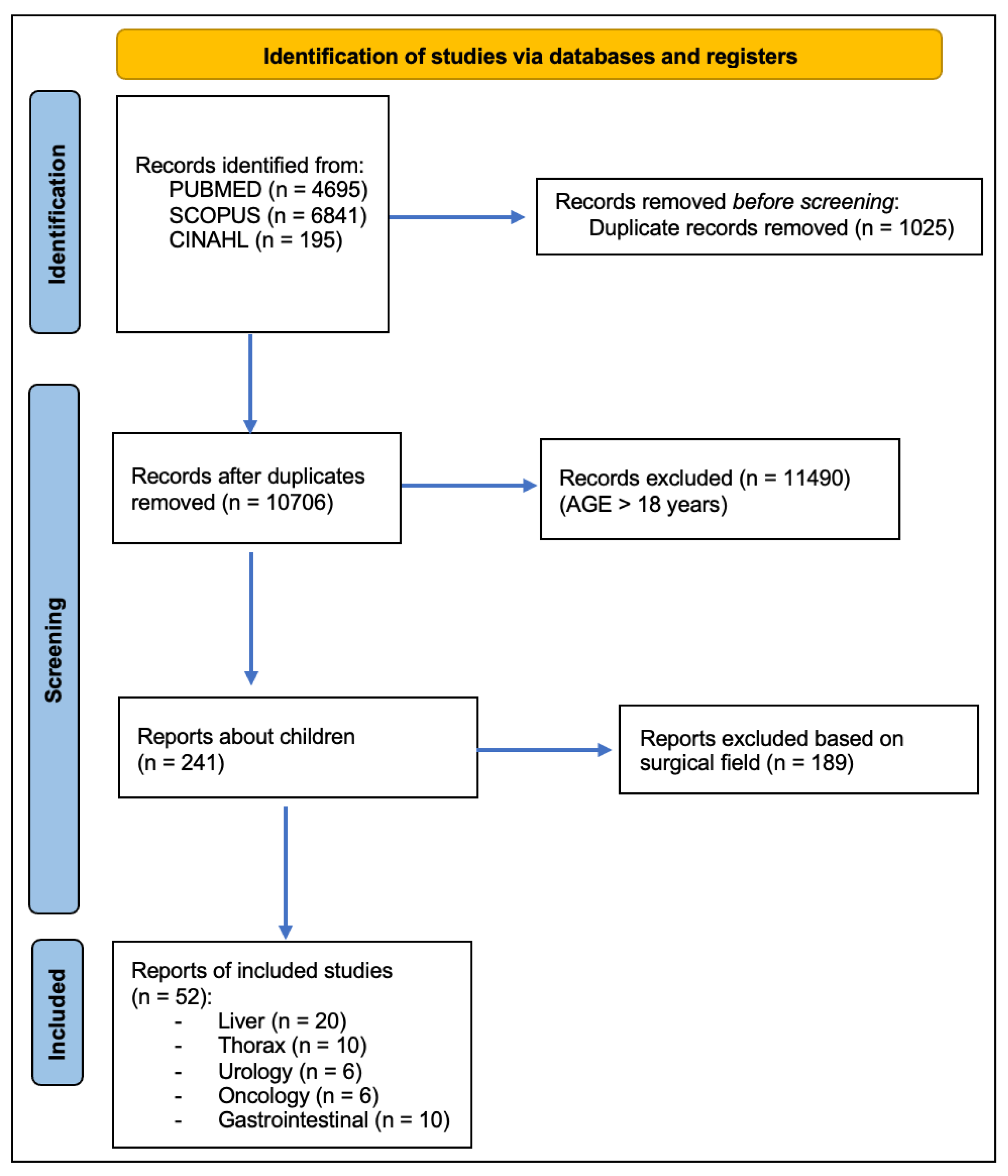
3. Results
3.1. Liver
3.1.1. Cholecystectomy
3.1.2. Hepatoblastoma
3.1.3. Biliary Atresia
3.1.4. Liver Transplant
3.1.5. Miscellanea
3.2. Thorax
3.2.1. Chylothorax
3.2.2. Pulmonary Surgery
3.3. Urology
3.3.1. Varicocelectomy
3.3.2. Urology Miscellanea
3.4. Oncology
Nodes
3.5. Gastrointestinal Surgery
Miscellanea
4. Discussion
4.1. Complications and Adverse Effects of ICG Fluorescence
4.2. Limitations
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morales-Conde, S.; Licardie, E.; Alarcón, I.; Balla, A. Indocyanine green (ICG) fluorescence guide for the use and indications in general surgery: Recommendations based on the descriptive review of the literature and the analysis of experience. Cir. Esp. 2022, 100, 534–554. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Majlesara, A.; Golriz, M.; Hafezi, M.; Saffari, A.; Stenau, E.; Maier-Hein, L.; Müller-Stich, B.P.; Mehrabi, A. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagnosis Photodyn. Ther. 2017, 17, 208–215. [Google Scholar] [CrossRef]
- Di Mitri, M.; Thomas, E.; Di Carmine, A.; Manghi, I.; Cravano, S.M.; Bisanti, C.; Collautti, E.; Ruspi, F.; Cordola, C.; Vastano, M.; et al. Intraoperative Ultrasound in Minimally Invasive Laparoscopic and Robotic Pediatric Surgery: Our Experiences and Literature Review. Children 2023, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.E.; Farooqui, Z.A.; Nagarajan, R.; Pressey, J.G.; Turpin, B.; Dasgupta, R. Fluorescent-guided surgery and the use of indocyanine green sentinel lymph node mapping in the pediatric and young adult oncology population. Cancer 2023, 129, 3962–3970. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Masieri, L.; Cerulo, M.; Castagnetti, M.; Del Conte, F.; Di Mento, C.; Esposito, G.; Tedesco, F.; Carulli, R.; Continisio, L.; et al. Indocyanine green (ICG) fluorescence technology in pediatric robotic surgery. J. Robot. Surg. 2024, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Clinical Applications of Indocyanine Green (ICG) Enhanced Fluorescence in Laparoscopic Surgery. Available online: https://pubmed.ncbi.nlm.nih.gov/25303914/ (accessed on 3 April 2025).
- Calabro, K.A.; Harmon, C.M.; Vali, K. Fluorescent Cholangiography in Laparoscopic Cholecystectomy and the Use in Pediatric Patients. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 586–589. [Google Scholar] [CrossRef]
- Esposito, C.; Alberti, D.; Settimi, A.; Pecorelli, S.; Boroni, G.; Montanaro, B.; Escolino, M. Indocyanine green (ICG) fluorescent cholangiography during laparoscopic cholecystectomy using RUBINATM technology: Preliminary experience in two pediatric surgery centers. Surg. Endosc. 2021, 35, 6366–6373. [Google Scholar] [CrossRef]
- Esposito, C.; Corcione, F.; Settimi, A.; Farina, A.; Centonze, A.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Twenty-Five Year Experience with Laparoscopic Cholecystectomy in the Pediatric Population-From 10 mm Clips to Indocyanine Green Fluorescence Technology: Long-Term Results and Technical Considerations. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 1185–1191. [Google Scholar] [CrossRef]
- Esposito, C.; Settimi, A.; Cerulo, M.; Escolino, M. Efficacy of indocyanine green (ICG) fluorescent cholangiography to improve intra-operative visualization during laparoscopic cholecystectomy in pediatric patients: A comparative study between ICG-guided fluorescence and standard technique. Surg. Endosc. 2022, 36, 4369–4375. [Google Scholar] [CrossRef]
- Daskalaki, D.; Fernandes, E.; Wang, X.; Bianco, F.M.; Elli, E.F.; Ayloo, S.; Masrur, M.; Milone, L.; Giulianotti, P.C. Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: Results of 184 consecutive cases in a single institution. Surg. Innov. 2014, 21, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Broderick, R.C.; Lee, A.M.; Cheverie, J.N.; Zhao, B.; Blitzer, R.R.; Patel, R.J.; Soltero, S.; Sandler, B.J.; Jacobsen, G.R.; Doucet, J.J.; et al. Fluorescent cholangiography significantly improves patient outcomes for laparoscopic cholecystectomy. Surg. Endosc. 2021, 35, 5729–5739. [Google Scholar] [CrossRef]
- Pavel, M.C.; Boira, M.A.; Bashir, Y.; Memba, R.; Llácer, E.; Estalella, L.; Julià, E.; Conlon, K.C.; Jorba, R. Near infrared indocyanine green fluorescent cholangiography versus intraoperative cholangiography to improve safety in laparoscopic cholecystectomy for gallstone disease—A systematic review protocol. Syst. Rev. 2022, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, T.; Oue, T.; Yonekura, T.; Owari, M.; Nakahata, K.; Umeda, S.; Nara, K.; Ueno, T.; Uehara, S.; Usui, N. Clinical application of indocyanine green (ICG) fluorescent imaging of hepatoblastoma. J. Pediatr. Surg. 2015, 50, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, X.; Pan, S.; Li, T.; Zhou, J. Effectiveness of indocyanine green fluorescence imaging in resection of hepatoblastoma. Pediatr. Surg. Int. 2023, 39, 181. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Ren, Q.; Qin, H.; Yang, W.; Cheng, H.; Yao, X.; Xu, J.; Han, J.; Chang, S.; et al. Evaluating the clinical efficacy and limitations of indocyanine green fluorescence-guided surgery in childhood hepatoblastoma: A retrospective study. Photodiagnosis Photodyn. Ther. 2023, 44, 103790. [Google Scholar] [CrossRef]
- Yanagi, Y.; Yoshimaru, K.; Matsuura, T.; Shibui, Y.; Kohashi, K.; Takahashi, Y.; Obata, S.; Sozaki, R.; Izaki, T.; Taguchi, T. The outcome of real-time evaluation of biliary flow using near-infrared fluorescence cholangiography with Indocyanine green in biliary atresia surgery. J. Pediatr. Surg. 2019, 54, 2574–2578. [Google Scholar] [CrossRef]
- Hirayama, Y.; Iinuma, Y.; Yokoyama, N.; Otani, T.; Masui, D.; Komatsuzaki, N.; Higashidate, N.; Tsuruhisa, S.; Iida, H.; Nakaya, K.; et al. Near-infrared fluorescence cholangiography with indocyanine green for biliary atresia. Real-time imaging during the Kasai procedure: A pilot study. Pediatr. Surg. Int. 2015, 31, 1177–1182. [Google Scholar] [CrossRef]
- Lim, Y.Z.; Mutore, K.; Bradd, M.V.; Pandya, S.; Corbitt, N. A Pilot Study for Biliary Atresia Diagnosis: Fluorescent Imaging of Indocyanine Green in Stool. J. Pediatr. Surg. 2024, 59, 1362–1368. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; Ye, Z.; Wu, Q.; Luo, Y. Role of indocyanine green-guided near-infrared fluorescence imaging in identification of the cause of neonatal cholestasis. Medicine 2024, 103, e38757. [Google Scholar] [CrossRef]
- Li, H.; Wei, L.; Zeng, Z.; Qu, W.; Zhu, Z. Laparoscopic Anatomic Segment III Procurement in Pediatric Living Donor Liver Transplantation Using Real-Time ICG Fluorescence In Situ Reduction by the Glissonean Approach. Transplant. Proc. 2023, 55, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.P.; Lautz, T.B.; Superina, R. Indocyanine green fluorescence imaging as an adjunct for the localization of a bile leak after split liver transplantation. Pediatr. Transplant. 2023, 27, e14431. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, W.W.; Shen, C.H.; Tao, Y.-F.; Wang, Z.-X.; Chen, J.-H.; Qin, L.-X. The application of real-time indocyanine green fluorescence cholangiography in laparoscopic living donor left lateral sectionectomy. Hepatobiliary Surg. Nutr. 2024, 13, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Shimizu, T.; Mori, M.; Okamatsu, C.; Nakamura, N.; Watanabe, T. Rim-type indocyanine green fluorescence pattern in a child with undifferentiated embryonal sarcoma of the liver treated with navigation surgery. Pediatr. Blood Cancer 2022, 69, e29799. [Google Scholar] [CrossRef] [PubMed]
- Masuya, R.; Matsukubo, M.; Nakame, K.; Kai, K.; Hamada, T.; Yano, K.; Imamura, N.; Hiyoshi, M.; Nanashima, A.; Ieiri, S. Using indocyanine green fluorescence in laparoscopic surgery to identify and preserve rare branching of the right hepatic artery in pediatric congenital biliary dilatation. Surg. Today 2022, 52, 1510–1513. [Google Scholar] [CrossRef]
- Tan, I.C.; Balaguru, D.; Rasmussen, J.C.; Guilliod, R.; Bricker, J.T.; Douglas, W.I.; Sevick-Muraca, E.M. Investigational lymphatic imaging at the bedside in a pediatric postoperative chylothorax patient. Pediatr. Cardiol. 2014, 35, 1295–1300. [Google Scholar] [CrossRef]
- Yokoyama, S.; Nakaoka, T. Successful use of intraoperative ICG fluorescence lymphography and fibrin sealant with PGA felt for refractory chylous ascites in an infant: A novel procedure. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2020, 62, 862–863. [Google Scholar] [CrossRef]
- Shibasaki, J.; Hara, H.; Mihara, M.; Adachi, S.; Uchida, Y.; Itani, Y. Evaluation of lymphatic dysplasia in patients with congenital pleural effusion and ascites using indocyanine green lymphography. J. Pediatr. 2014, 164, 1116–1120.e1. [Google Scholar] [CrossRef]
- Novel Thoracoscopic Navigation Surgery for Neonatal Chylothorax Using Indocyanine-Green Fluorescent Lymphography. Available online: https://pubmed.ncbi.nlm.nih.gov/29486888/ (accessed on 10 December 2024).
- Intraoperative Indocyanine Green Fluorescence Lymphography to Detect Chylous Leakage Sites After Congenital Heart Surgery. Available online: https://pubmed.ncbi.nlm.nih.gov/24731796/ (accessed on 10 December 2024).
- Chen-Yoshikawa, T.F.; Hatano, E.; Yoshizawa, A.; Date, H. Clinical application of projection mapping technology for surgical resection of lung metastasis. Interact Cardiovasc. Thorac. Surg. 2017, 25, 1010–1011. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Mothi, S.S.; Pio, L.; Mori, M.; Santiago, T.C.; McCarville, M.B.; Kaste, S.C.; Pappo, A.S.; Talbot, L.J.; Murphy, A.J.; et al. Feasibility of indocyanine green-guided localization of pulmonary nodules in children with solid tumors. Pediatr. Blood Cancer 2023, 70, e30437. [Google Scholar] [CrossRef]
- Hamaji, M.; Chen-Yoshikawa, T.F.; Minami, M.; Date, H. Near-Infrared Imaging Using Intravenous Indocyanine Green at a Conventional Dose to Locate Pulmonary Metastases: A Pilot Study. Thorac. Cardiovasc. Surg. 2019, 67, 688–691. [Google Scholar] [CrossRef]
- Kitagawa, N.; Shinkai, M.; Mochizuki, K.; Usui, H.; Miyagi, H.; Nakamura, K.; Tanaka, M.; Tanaka, Y.; Kusano, M.; Ohtsubo, S. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr. Surg. Int. 2015, 31, 407–411. [Google Scholar] [CrossRef]
- Yoshida, M.; Tanaka, M.; Kitagawa, N.; Nozawa, K.; Shinkai, M.; Goto, H.; Tanaka, Y. Clinicopathological study of surgery for pulmonary metastases of hepatoblastoma with indocyanine green fluorescent imaging. Pediatr. Blood Cancer 2022, 69, e29488. [Google Scholar] [CrossRef]
- Delgado-Miguel, C.; Estefanía, K.; San Basilio, M.; Hernández Oliveros, F. Indocyanine green navigation in minimally invasive resection of multiple metachronous pulmonary metastases of hepatoblastoma. Thorac. Cancer 2023, 14, 528–532. [Google Scholar] [CrossRef]
- Fung, C.H.; Lau, C.T.; Wong, K.K.Y. Indocyanine green fluorescence-guided pulmonary wedge resection in a child: A case report. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2020, 26, 345–347. [Google Scholar] [CrossRef]
- Zundel, S.; Szavay, P. Para-testicular injection of indocyanine green for laparoscopic immunofluorescence-guided lymphatic-sparing Palomo procedure: Promising preliminary results. J. Pediatr. Urol. 2024, 20, 530–532. [Google Scholar] [CrossRef]
- Esposito, C.; Turrà, F.; Del Conte, F.; Izzo, S.; Gargiulo, F.; Farina, A.; Severino, G.; Cerulo, M.; Escolino, M. Indocyanine Green Fluorescence Lymphography: A New Technique to Perform Lymphatic Sparing Laparoscopic Palomo Varicocelectomy in Children. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 564–567. [Google Scholar] [CrossRef]
- Tomita, K.; Kageyama, S.; Hanada, E.; Yoshida, T.; Okinaka, Y.; Kubota, S.; Nagasawa, M.; Johnin, K.; Narita, M.; Kawauchi, A. Indocyanine Green Angiography-assisted Laparoendoscopic Single-site Varicocelectomy. Urology 2017, 106, 221–225. [Google Scholar] [CrossRef]
- Esposito, C.; Soria-Gondek, A.; Castagnetti, M.; Cerulo, M.; Del Conte, F.; Esposito, G.; Pecoraro, C.; Cicala, D.; Farina, A.; Escolino, M. Laparoscopic or Robotic Deroofing Guided by Indocyanine Green Fluorescence and Perirenal Fat Tissue Wadding Technique of Pediatric Simple Renal Cysts. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 471–476. [Google Scholar] [CrossRef]
- Esposito, C.; Autorino, G.; Coppola, V.; Esposito, G.; Paternoster, M.; Castagnetti, M.; Cardone, R.; Cerulo, M.; Borgogni, R.; Cortese, G.; et al. Technical standardization of ICG near-infrared fluorescence (NIRF) laparoscopic partial nephrectomy for duplex kidney in pediatric patients. World J. Urol. 2021, 39, 4167–4173. [Google Scholar] [CrossRef] [PubMed]
- Indocyanine Green (ICG)-Guided Onlay Preputial Island Flap Urethroplasty for the Single-Stage Repair of Hypospadias in Children: A Case Report. Available online: https://pubmed.ncbi.nlm.nih.gov/37444094/ (accessed on 14 December 2024).
- Fadel, M.G.; Rauf, S.; Mohamed, H.S.; Yusuf, S.; Hayes, A.J.; Power, K.; Smith, M.J. The Use of Indocyanine Green and Near-Infrared Fluorescence Imaging Versus Blue Dye in Sentinel Lymph Node Biopsy in Cutaneous Melanoma: A Retrospective, Cohort Study. Ann. Surg. Oncol. 2023, 30, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.W.; Couto, R.A.; Gastman, B. Indocyanine green fluorescence imaging with lymphoscintigraphy for sentinel node biopsy in head and neck melanoma. J. Surg. Res. 2018, 228, 77–83. [Google Scholar] [CrossRef]
- Jeremiasse, B.; van Scheltinga, C.E.J.T.; Smeele, L.E.; Tolboom, N.; Wijnen, M.H.W.A.; van der Steeg, A.F.W. Sentinel Lymph Node Procedure in Pediatric Patients with Melanoma, Squamous Cell Carcinoma, or Sarcoma Using Near-Infrared Fluorescence Imaging with Indocyanine Green: A Feasibility Trial. Ann. Surg. Oncol. 2023, 30, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Hirche, C.; Engel, H.; Hirche, Z.; Doniga, S.; Herold, T.; Kneser, U.; Lehnhardt, M.; Hünerbein, M. Real-time lymphography by indocyanine green fluorescence: Improved navigation for regional lymph node staging. Ann. Plast. Surg. 2014, 73, 701–705. [Google Scholar] [CrossRef]
- Pio, L.; Zaghloul, T.; Abdelhafeez, A.H. Indocyanine green fluorescence-guided lymphadenectomy with single site retroperitoneoscopy in children. J. Pediatr. Urol. 2023, 19, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Y.; Zhang, Y.; Liao, J.; Hua, K.; Gu, Y.; Wang, D.; Tian, J.; Huang, J. Indocyanine green localization for laparoscopic duodenal web excision. Photodiagnosis Photodyn. Ther. 2022, 38, 102842. [Google Scholar] [CrossRef]
- Hall, L.A.; Jackson, R.; Soccorso, G.; Lander, A.D.; Pachl, M.J. Assessment of jejunal interposition perfusion using indocyanine green. Photodiagnosis Photodyn. Ther. 2023, 43, 103687. [Google Scholar] [CrossRef]
- Le-Nguyen, A.; Bourque, C.J.; Trudeau, M.O.; Ducruet, T.; Faure, C.; Piché, N. Indocyanine green fluorescence angiography in pediatric intestinal resections: A first prospective mixed methods clinical trial. J. Pediatr. Surg. 2023, 58, 82–88. [Google Scholar] [CrossRef]
- Meisner, J.W.; Kamran, A.; Staffa, S.J.; Mohammed, S.; Yasuda, J.L.; Ngo, P.; Manfredi, M.; Zurakowski, D.; Jennings, R.W.; Hamilton, T.E.; et al. Qualitative features of esophageal fluorescence angiography and anastomotic outcomes in children. J. Pediatr. Surg. 2023, 58, 1359–1367. [Google Scholar] [CrossRef]
- Rentea, R.M.; Halleran, D.R.; Ahmad, H.; Sanchez, A.V.; Gasior, A.C.; McCracken, K.; Hewitt, G.D.; Alexander, V.; Smith, C.; Weaver, L.; et al. Preliminary Use of Indocyanine Green Fluorescence Angiography and Value in Predicting the Vascular Supply of Tissues Needed to Perform Cloacal, Anorectal Malformation, and Hirschsprung Reconstructions. Eur. J. Pediatr. Surg. 2020, 30, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, Z.; Zhang, Y.; Zhao, J.; Zhao, Y.; Liao, J.; Li, S.; Huang, J. Indocyanine green fluorescence imaging localization: A helpful addition to laparoscopic dissection and division of rectourethral fistulae. Photodiagnosis Photodyn. Ther. 2023, 42, 103335. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Onishi, S.; Kedoin, C.; Matsui, M.; Murakami, M.; Yano, K.; Harumatsu, T.; Yamada, K.; Yamada, W.; Matsukubo, M.; et al. A safe and effective laparoscopic Ladd’s procedure technique involving the confirmation of mesenteric vascular perfusion by fluorescence imaging using indocyanine green: A case report of an infant. Asian J. Endosc. Surg. 2022, 15, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Muto, M.; Yamada, K.; Murakami, M.; Kedoin, C.; Nagano, A.; Matsui, M.; Sugita, K.; Yano, K.; Harumatsu, T.; et al. Feasibility of delayed anastomosis for long gap esophageal atresia in the neonatal period using internal traction and indocyanine green-guided near-infrared fluorescence. Asian J. Endosc. Surg. 2022, 15, 877–881. [Google Scholar] [CrossRef]
- Esposito, C.; De Luca, U.; Cerulo, M.; Del Conte, F.; Bagnara, V.; Coppola, S.; Corcione, F.; Lepore, B.; Settimi, A.; Escolino, M. Twenty-Five-Year Experience with Minimally Invasive Splenectomy in Children: From Minilaparotomy to Use of Sealing Devices and Indocyanine Green Fluorescence Technology: Tips and Tricks and Technical Considerations. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 1010–1015. [Google Scholar] [CrossRef]
- Breuking, E.A.; Varsseveld, O.C.; van Harms, M.; Tytgat, S.H.A.J.; Hulscher, J.B.F.; Ruiterkamp, J. Safety and Feasibility of Indocyanine Green Fluorescence Angiography in Pediatric Gastrointestinal Surgery: A Systematic Review. J. Pediatr. Surg. 2023, 58, 1534–1542. [Google Scholar] [CrossRef]
- Chu, W.; Chennamsetty, A.; Toroussian, R.; Lau, C. Anaphylactic Shock After Intravenous Administration of Indocyanine Green During Robotic Partial Nephrectomy. Urol. Case Rep. 2017, 12, 37–38. [Google Scholar] [CrossRef]
- Sigley, K.; Hope, P.; Laird, R. Subcutaneous Infiltration of Indocyanine Green From a Malpositioned Intravenous Catheter. Cureus 2021, 13, e16378. [Google Scholar] [CrossRef]
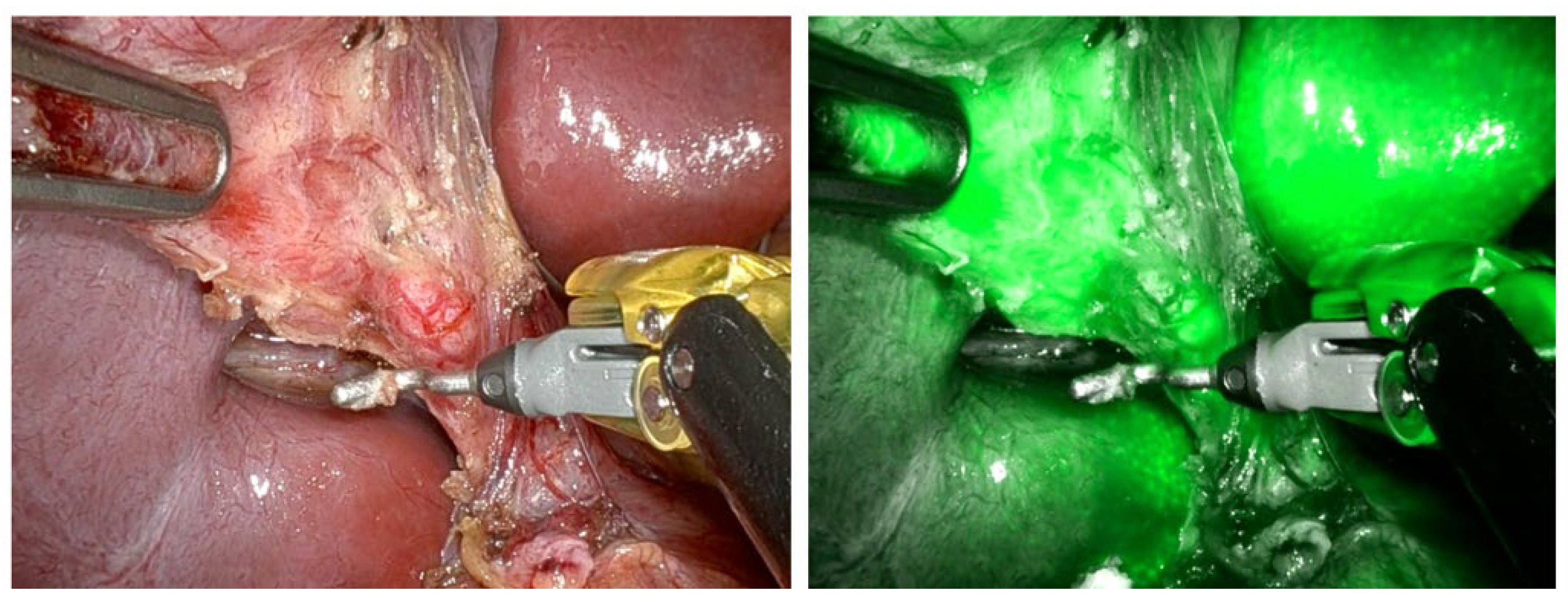
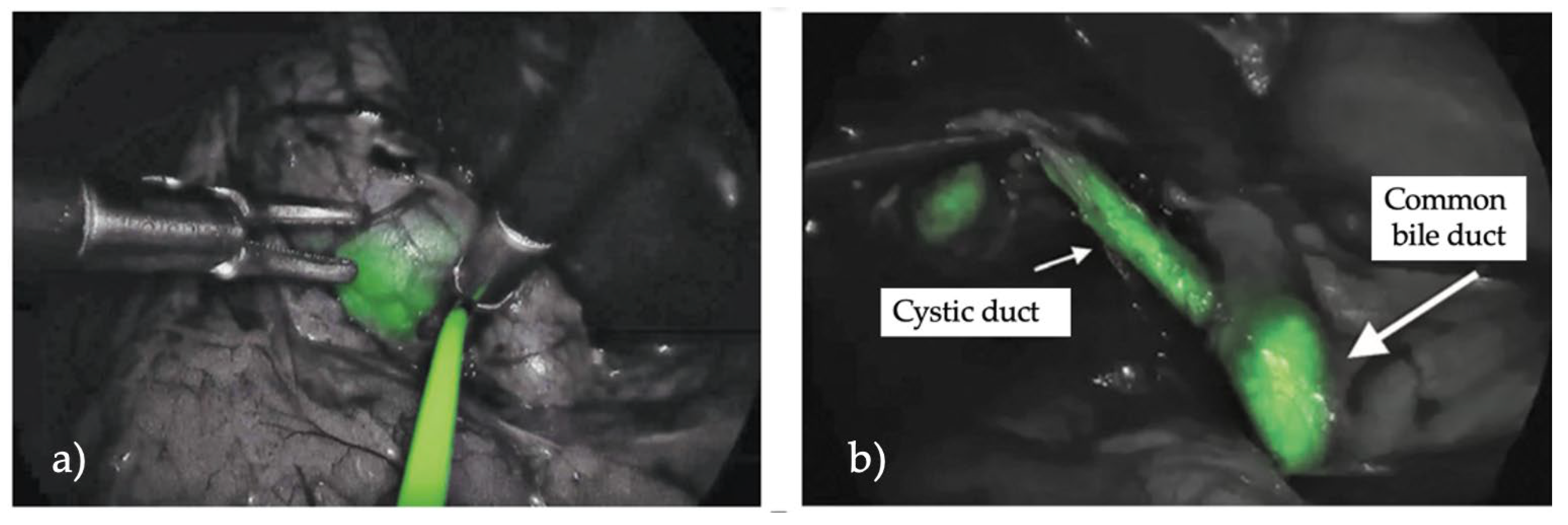

| Liver | ||||
|---|---|---|---|---|
| Cholecystectomy | ||||
| Title | Authors | ICG Dosing | Time | Visualization |
| Indocyanine green (ICG) fluorescent cholangiography during laparoscopic cholecystectomy using RUBINA™ technology: preliminary experience in two pediatric surgery centers | Esposito et al. [9] | 0.35 mg/kg | 15.6 h | Biliary structures |
| Fluorescent cholangiography significantly improves patient outcomes for laparoscopic cholecystectomy | Broderick et al. [13] | 7.5 mg | 45 min | Cystic duct Hepatic ducts |
| Fluorescent cholangiography in laparoscopic cholecystectomy and the use in pediatric patients | Calabro et al. [8] | 2.5 mg | Before surgical incision | Cystic duct Common hepatic duct Common bile duct |
| Twenty-five year experience with laparoscopic cholecystectomy in the pediatric population—from 10 mm clips to indocyanine green fluorescence technology: long-term results and technical considerations | Esposito et al. [10] | 0.4 mg/kg | 18 h | Biliary structures |
| Efficacy of indocyanine green (ICG) fluorescent cholangiography to improve intraoperative visualization during laparoscopic cholecystectomy in pediatric patients: a comparative study between ICG-guided fluorescence and standard technique | Esposito et al. [11] | 0.35 mg/kg | 15.6 h | Biliary structures |
| Near-infrared indocyanine green fluorescent cholangiography versus intraoperative cholangiography to improve safety in laparoscopic cholecystectomy for gallstone disease—a systematic review protocol | Pavel et al. [14]. | 0.5 mg/kg | 2 h | Biliary structures |
| Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution | Despoina Daskalaki [12] | 2.5 mg | 45 min | Biliary structures |
| Hepatoblastoma | ||||
| Clinical application of indocyanine green fluorescent imaging of hepatoblastoma | Yamamichi et al. [15] | 0.5 mg/kg | 3–4 days | Metastatic lesions |
| Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients | Souzaki et al. [16] | 0.5 mg/kg | 90.5 ± 33 h | Residual tumors near the diaphragm and vena cava |
| Effectiveness of indocyanine green fluorescence imaging in resection of hepatoblastoma | Shen et al. [17] | 0.1 mg/kg | 24–48 h | Tumor margins |
| Evaluating the clinical efficacy and limitations of indocyanine green fluorescence-guided surgery in childhood hepatoblastoma: a retrospective study | Liu et al. [18] | 0.1 mg/kg | 24h | Tumor margins |
| Biliary Atresia | ||||
| The outcome of real-time evaluation of biliary flow using near-infrared fluorescence cholangiography with indocyanine green in biliary atresia surgery | Yanagi et al. [19] | 0.5 mg/kg | 24 h | Hilar micro-bile ducts |
| Near-infrared fluorescence cholangiography with indocyanine green for biliary atresia. Real-time imaging during the Kasai procedure: a pilot study | Hirayama et al. [20] | 0.1 mg/kg | 24 h | Bile flow and the dissection level. |
| A pilot study for biliary atresia diagnosis: fluorescent imaging of indocyanine green in stool | Zou Lim et al. [21] | 0.1 mg/kg | Variable timing | Biliary system patency |
| Role of indocyanine green-guided near-infrared fluorescence imaging in identification of the cause of neonatal cholestasis | Zhang et al. [22] | 0.3 mg/kg | 12 h | Distinguished biliary atresia from other causes of cholestasis |
| Liver Transplant | ||||
| Laparoscopic anatomic segment III procurement in pediatric living donor liver transplantation using real-time ICG fluorescence in situ reduction by the Glissonean approach | Li et al. [23] | 2.5 mg/kg | During surgery | Graft vascularization |
| Indocyanine green fluorescence imaging as an adjunct for the localization of a bile leak after split liver transplantation | Lemoine et al. [24] | 0.5 mg/kg | During surgery | Bile leak |
| The application of real-time indocyanine green fluorescence cholangiography in laparoscopic living donor left lateral sectionectomy | Lu et al. [25] | 0.004–0.05 mg/kg | During surgery | Biliary anatomical structures and bile leak |
| Miscellanea Liver Surgery | ||||
| Rim-type indocyanine green fluorescence pattern in a child with undifferentiated embryonal sarcoma of the liver treated with navigation surgery | Yamamoto et al. [26] | 0.5 mg/kg | 4 days before surgery | Tumor margins |
| Using indocyanine green fluorescence in laparoscopic surgery to identify and preserve rare branching of the right hepatic artery in pediatric congenital biliary dilatation | Masuya et al. [27] | 0.6 mg/kg | During surgery | Aberrant right hepatic artery |
| Thorax | ||||
|---|---|---|---|---|
| Chylothorax | ||||
| Title | Authors | ICG Dosing | Time | Visualization |
| Investigational lymphatic imaging at the bedside in a pediatric postoperative chylothorax patient | Tan et al. [28] | 0.05 mg | During surgery | Lymphatic leakage |
| Successful use of intraoperative ICG fluorescence lymphography and fibrin sealant with PGA felt for refractory chylous ascites in an infant: a novel procedure | Yokoyama et al. [29] | 0.1 mL | During surgery | Lymphatic leakage |
| Evaluation of lymphatic dysplasia in patients with congenital pleural effusion and ascites using indocyanine green lymphography | Shibasaki et al. [30] | 0.25 mg | During lymphography | Lymphatic leakage |
| Novel thoracoscopic navigation surgery for neonatal chylothorax using ICG fluorescent lymphography | Shirotsuki et al. [31] | 0.025 mg | 1 h | Lymphatic leakage |
| Intraoperative ICG fluorescence lymphography to detect chylous leakage sites after congenital heart surgery | Chang et al. [32] | 0.5 mg | During surgery | Lymphatic leakage |
| Pulmonary Surgery | ||||
| Clinical application of indocyanine green in pediatric pulmonary metastases surgery | Yoshikawa et al. [33] | 0.5 mg/kg | 24 h | Metastasis localization |
| Feasibility of indocyanine green-guided localization of pulmonary nodules in children with solid tumors | Abdelhafeez et al. [34] | 1.5 mg/kg | 24 h | Metasasis localization |
| Near-infrared imaging using intravenous indocyanine green at a conventional dose to locate pulmonary metastases: a pilot study | Hamaji et al. [35] | 0.25–0.5 mg/kg | 12–24 h | Metastasis localization |
| Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery | Kitagawa et al. [36] | 0.5 mg/kg | 24 h | Metastasis localization |
| Clinicopathological study of surgery for pulmonary metastases of hepatoblastoma with indocyanine green fluorescent imaging | Yosida et al. [37] | 0.5 mg/kg | 24 h | Metastasis localization |
| Indocyanine green navigation in minimally invasive resection of multiple metachronous pulmonary metastases of hepatoblastoma | Carlos Delgado-Miguel [38] | 0.5 mg/kg | 24 h | Metastasis localization |
| ICG fluorescence-guided pulmonary wedge resection in a child | Fung et al. [39] | 0.5 mL | 1 h | Margins of a pulmonary nodule |
| Urology | ||||
|---|---|---|---|---|
| Varicocelectomy | ||||
| Title | Authors | ICG Dosing | Time | Visualization |
| Para-testicular injection of indocyanine green for laparoscopic immunofluorescence-guided lymphatic-sparing Palomo procedure | Zundel et al. [40] | 6.25 mg | During surgery | Lymphatic vessels |
| Indocyanine green fluorescence lymphography: a new technique to perform lymphatic sparing laparoscopic Palomo varicocelectomy in children | Esposito et al. [41] | 2 mL | During surgery | Lymphatic vessels |
| Indocyanine green angiography-assisted laparoendoscopic single-site varicocelectomy | Tomita et al. [42] | 2.5 mg | During surgery | Artery and vein |
| Miscellanea Urology Surgery | ||||
| Laparoscopic or robotic deroofing guided by indocyanine green fluorescence and perirenal fat tissue injection in simple renal cysts | Esposito et al. [43] | 0.35 mg/kg | During surgery | Margins of the cyst |
| Technical standardization of ICG-NIRF laparoscopic partial nephrectomy for duplex kidney | Esposito et al. [44] | 0.3 mg/kg (intravenous) 2.5 mg (ureteral) | During surgery | Ureteral and vessel visualization |
| ICG-guided onlay preputial island flap urethroplasty for the single-stage repair of hypospadias in children: a case report | Paraboschi et al. [45] | 0.15 mg/kg | During surgery | Flap vascularization |
| Oncologic Surgery | ||||
|---|---|---|---|---|
| Title | Authors | ICG Dosing | Time | Visualization |
| The use of indocyanine green and near-infrared fluorescence imaging in sentinel lymph node biopsy in cutaneous melanoma | Fadel et al. [46] | 2.5 mg | During surgery | Visualization of the sentinel lymph node |
| Indocyanine green fluorescence imaging with lymphoscintigraphy improves the accuracy of sentinel lymph node biopsy in melanoma | Knackstedt et al. [47] | 0.2–0.3 mL | During surgery | Visualization of the sentinel lymph node |
| Sentinel lymph node procedure in pediatric patients with melanoma, squamous cell carcinoma, or sarcoma using near-infrared fluorescence imaging with indocyanine green: a feasibility trial | Jeremiasse et al. [48] | 0.25 mg | 4–24 h | Visualization of the sentinel lymph node |
| Real-time lymphography by indocyanine green fluorescence: improved navigation for regional lymph node staging | Hirche et al. [49] | 7 mg | During surgery | Visualization of the sentinel lymph node |
| Fluorescent-guided surgery and the use of indocyanine green sentinel lymph node mapping in the pediatric and young adult oncology population | Johnston et al. [5] | 1.25 mg | During surgery | Visualization of the sentinel lymph node |
| Indocyanine green fluorescence-guided lymphadenectomy with single site retroperitoneoscopy in children | Pio et al. [50] | - | During surgery | Visualization of the sentinel lymph node |
| Gastrointestinal Surgery | ||||
|---|---|---|---|---|
| Title | Authors | ICG Dosing | Time | Visualization |
| Indocyanine green localization for laparoscopic duodenal web excision | Li et al. [51] | 1.25 mg | During surgery | Localization of the web |
| Assessment of jejunal interposition perfusion using indocyanine green | Hall et al. [52] | 0.2 mg/kg | During surgery | Perfusion evaluation |
| Indocyanine green fluorescence angiography in pediatric intestinal resections: a first prospective mixed methods clinical trial | Le-Nguyen et al. [53] | 0.14 mg/kg | During surgery | Resection margins based on perfusion |
| Qualitative features of esophageal fluorescence angiography and anastomotic outcomes in children | Meisner et al. [54] | - | During surgery | Perfusion evaluation |
| Preliminary use of indocyanine green fluorescence angiography and value in predicting the vascular supply of tissues needed to perform cloacal, anorectal malformation, and Hirschsprung reconstructions | Rentea et al. [55] | 0.1–0.3 mg/kg | During surgery | Perfusion evaluation |
| Indocyanine green fluorescence imaging localization: a helpful addition to laparoscopic dissection and division of rectourethral fistulae | Li et al. [56] | 1.25 mg | During surgery | Localization of the rectourethral fistula |
| A safe and effective laparoscopic Ladd’s procedure technique involving the confirmation of mesenteric vascular perfusion by fluorescence imaging using indocyanine green: a case report of an infant | Sugita et al. [57] | - | During surgery | Perfusion evaluation |
| Feasibility of delayed anastomosis for long gap esophageal atresia in the neonatal period using internal traction and indocyanine green-guided near-infrared fluorescence | Onishi et al. [58] | 0.5 mg/kg | During surgery | Perfusion evaluation |
| Twenty-five year experience with Minimally Invasive Splenectomy in Children: From Minilaparotomy to Use of Sealing Devices and Indocyanine Green Fluorescence Technology: Tips and tricks and Technical Considerations | Esposito et al. [59] | 0.3 mg/kg/mL | During surgery | Vascular anatomy identification |
| Safety and feasibility of indocyanine green fluorescence Angiography in pediatric gastrointestinal surgery: a systematic review | Breuking et al. [60] | 0.1–0.5 mg/kg | During surgery | Perfusion evaluation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mitri, M.; Di Carmine, A.; Zen, B.; Collautti, E.; Bisanti, C.; D’Antonio, S.; Libri, M.; Gargano, T.; Lima, M. Advancing Pediatric Surgery with Indocyanine Green (ICG) Fluorescence Imaging: A Comprehensive Review. Children 2025, 12, 515. https://doi.org/10.3390/children12040515
Di Mitri M, Di Carmine A, Zen B, Collautti E, Bisanti C, D’Antonio S, Libri M, Gargano T, Lima M. Advancing Pediatric Surgery with Indocyanine Green (ICG) Fluorescence Imaging: A Comprehensive Review. Children. 2025; 12(4):515. https://doi.org/10.3390/children12040515
Chicago/Turabian StyleDi Mitri, Marco, Annalisa Di Carmine, Benedetta Zen, Edoardo Collautti, Cristian Bisanti, Simone D’Antonio, Michele Libri, Tommaso Gargano, and Mario Lima. 2025. "Advancing Pediatric Surgery with Indocyanine Green (ICG) Fluorescence Imaging: A Comprehensive Review" Children 12, no. 4: 515. https://doi.org/10.3390/children12040515
APA StyleDi Mitri, M., Di Carmine, A., Zen, B., Collautti, E., Bisanti, C., D’Antonio, S., Libri, M., Gargano, T., & Lima, M. (2025). Advancing Pediatric Surgery with Indocyanine Green (ICG) Fluorescence Imaging: A Comprehensive Review. Children, 12(4), 515. https://doi.org/10.3390/children12040515







